Speed of administration conditions the effectiveness of intravenous fibrinolysis in treating acute ischaemic stroke. To reduce the risk of haemorrhagic complications, the intervention is contraindicated in certain cases, such as where the International Normalised Ratio (INR) is ≥1.7. This study aimed to determine the reliability of point-of-care INR readings (POC-INR) taken using the CoaguChek® XS portable coagulometer compared to laboratory results (L-INR).
MethodsWe conducted a retrospective observational study of consecutive patients admitted to our centre with acute ischaemic stroke and who were treated with intravenous fibrinolysis, over a period of 4 years. Patients’ INR was measured with a portable coagulometer and in the laboratory. Results were compared using the paired-sample t test; using L-INR results as a reference value, ROC analysis was performed to determine POC-INR with greater predictive value.
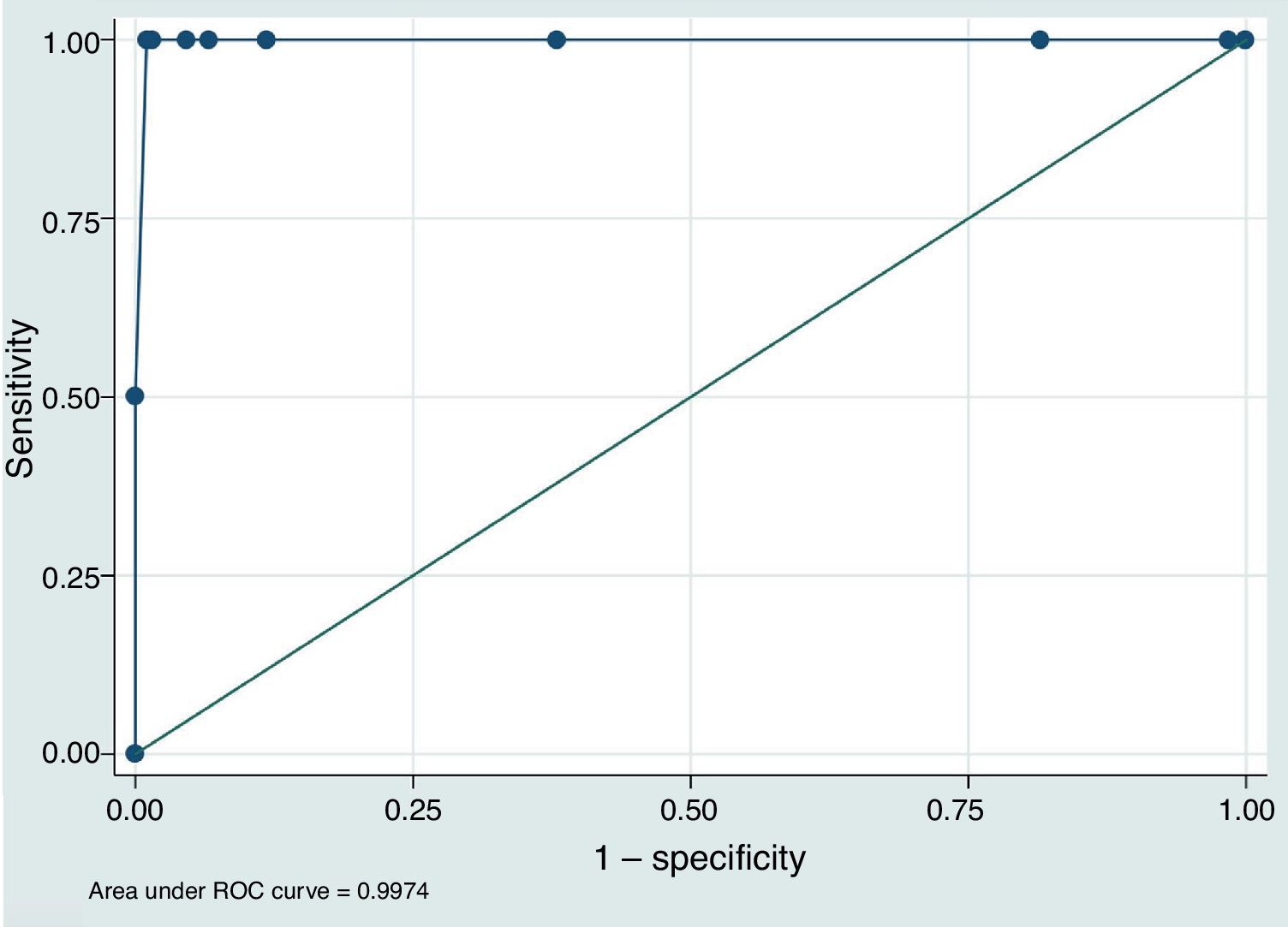
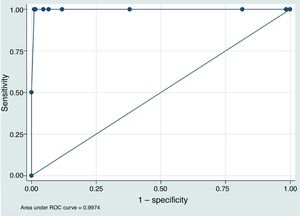
ResultsThe study included 210 patients with a mean age of 74.3±11.5 years old; 18 (8.6%) were taking vitamin K antagonist oral anticoagulants (OAC). There were no significant differences between the 2 INR measurements in the population as a whole (POC-INR–L-INR difference: 0.001±0.085; P=.82). In subgroup analysis, the results coincided for patients taking OACs (0.001±0.081; P=.42) and those with L-INR ≤ 1.2 (0.008±0.081; P=.16). For L-INR>1.2, however, the portable coagulometer underestimated INR (0.058±0.095; P=.01). Through ROC analysis, POC-INR <1.6 was found to be the cut-off point with greatest sensitivity (100%) and specificity (98.97%) for identifying patients eligible for intravenous fibrinolysis (L-INR <1.7).
ConclusionsPOC-INR shows a good correlation with L-INR. Our results suggest that the best threshold to predict an L-INR <1.7 is POC-INR <1.6. Internal validation studies for POC-INR should be considered in all treatment centres.
La eficacia de la fibrinólisis intravenosa como tratamiento en el ictus isquémico agudo depende de la rapidez en su administración. Para reducir el riesgo de complicaciones hemorrágicas existen contraindicaciones, como una INR ≥ 1,7. Nuestro objetivo fue determinar la fiabilidad del valor de INR obtenido mediante el coagulómetro portátil (CP) CoaguChek XS® (CPINR) frente al resultado del laboratorio (LINR).
MétodosEstudio retrospectivo observacional de pacientes consecutivos con ictus isquémico tratados con fibrinólisis intravenosa en nuestro centro durante 4 años. La INR fue medida con CP y en el laboratorio. Se compararon ambos valores mediante t de Student para datos apareados y, tomando como referencia la LINR, se realizó análisis ROC para determinar la CPINR con mayor valor predictivo.
ResultadosAnalizamos a 210 pacientes, con edad media 74,3±11,5 años, y 18 (8,6%) tomaban anticoagulantes orales antivitamina K. Se compararon LINR y CPINR sin evidenciarse diferencias significativas (diferencia LINR-CPINR −0,001±0,085; p=0,82). En el análisis por subgrupos: para pacientes con anticoagulantes orales (diferencia LINR-CPINR 0,001±0,081; p=0,42) y para LINR ≤ 1,2 (diferencia LINR-CPINR −0,008±0,081; p=0,16) ambas técnicas fueron concordantes, mientras que para LINR >1,2, CPINR infraestimó la INR (diferencia LINR-CPINR 0,058±0,095; p=0,01). Mediante análisis ROC una CPINR < 1,6 fue el punto de corte más sensible y específico para seleccionar pacientes tratables con fibrinólisis intravenosa (LINR < 1,7).
ConclusionesEl CP en el código ictus tiene una buena concordancia con el laboratorio. Este estudio indica que en nuestro centro una CPINR < 1,6 es el mejor umbral para predecir una LINR< 1,7. La validación de la CPINR en cada centro es recomendable para su uso protocolizado.
The effectiveness of fibrinolysis with recombinant tissue plasminogen activator (rtPA) for ischaemic stroke depends on prompt administration of the treatment.1–5 Before indicating rtPA, however, it is essential to rule out any potential contraindications for treatment. These include an international normalised ratio (INR) >1.7.1 Measuring this parameter before starting rtPA may be decisive. Patients with no suspected bleeding disorders and not taking anticoagulants may start rtPA before coagulation data are obtained.6 However, it is essential to measure INR before starting rtPA in patients receiving vitamin K antagonists.
Point-of-care coagulometers provide immediate INR readings (POC-INR), whereas central laboratory INR (L-INR) testing takes 45 to 60 minutes. Time is critical in the management of ischaemic stroke. Some studies have shown that portable coagulometers effectively and safely reduce door-to-needle time in patients taking vitamin K antagonists.7 Other studies have demonstrated the usefulness and reliability of these devices, although they note that point-of-care testing tends to return slightly lower values.8,9 However, the usefulness of portable coagulometers in clinical practice is unknown in most hospitals and many centres still rely on L-INR measurements only, which inevitably increases stroke management times.
This study aimed to determine the reliability of POC-INR testing in the acute phase of ischaemic stroke, placing special emphasis on patients receiving vitamin K antagonists.
Materials and methodsPatientsWe conducted a retrospective study of consecutive patients admitted to our centre between 2012 and 2015 due to ischaemic stroke and treated with intravenous fibrinolysis. We included patients receiving the treatment within 4.5hours after stroke onset and presenting no known contraindications for treatment according to Spanish and international guidelines for acute stroke care. For patients to be included, their clinical histories had to include both POC-INR and L-INR values.
We gathered the following data: (1) demographic data (age and sex), (2) vascular risk factors (arterial hypertension, dyslipidaemia, diabetes mellitus, drug habits, obesity, family history of cerebrovascular disease), (3) relevant medical history (including liver disease and other conditions potentially causing coagulation disorders), (4) previous treatment (antiplatelets, anticoagulants, antihypertensives, antidiabetics, diuretics, beta blockers), (5) POC-INR and L-INR measurements, and (6) coagulation profile and platelet count.
SamplesVenous blood samples were routinely collected upon patient arrival at the emergency department and analysed at our hospital's central laboratory. During blood collection, a drop of venous blood was obtained for analysis with the CoaguChek® XS system (Roche). The procedure was performed by emergency department nurses trained to use the device. POC-INR testing is included within our hospital's code stroke protocol.
Statistical analysisData are expressed as n (%) for qualitative variables, means (standard deviation) for normally distributed quantitative variables, and medians (Q1-Q3) for non-normally distributed or ordinal variables. We calculated a single variable to analyse the difference between POC-INR and L-INR measurements (L-INR – POC-INR). After testing for normality, we used the paired-sample t test. We also analysed the subgroup of patients receiving vitamin K antagonists.
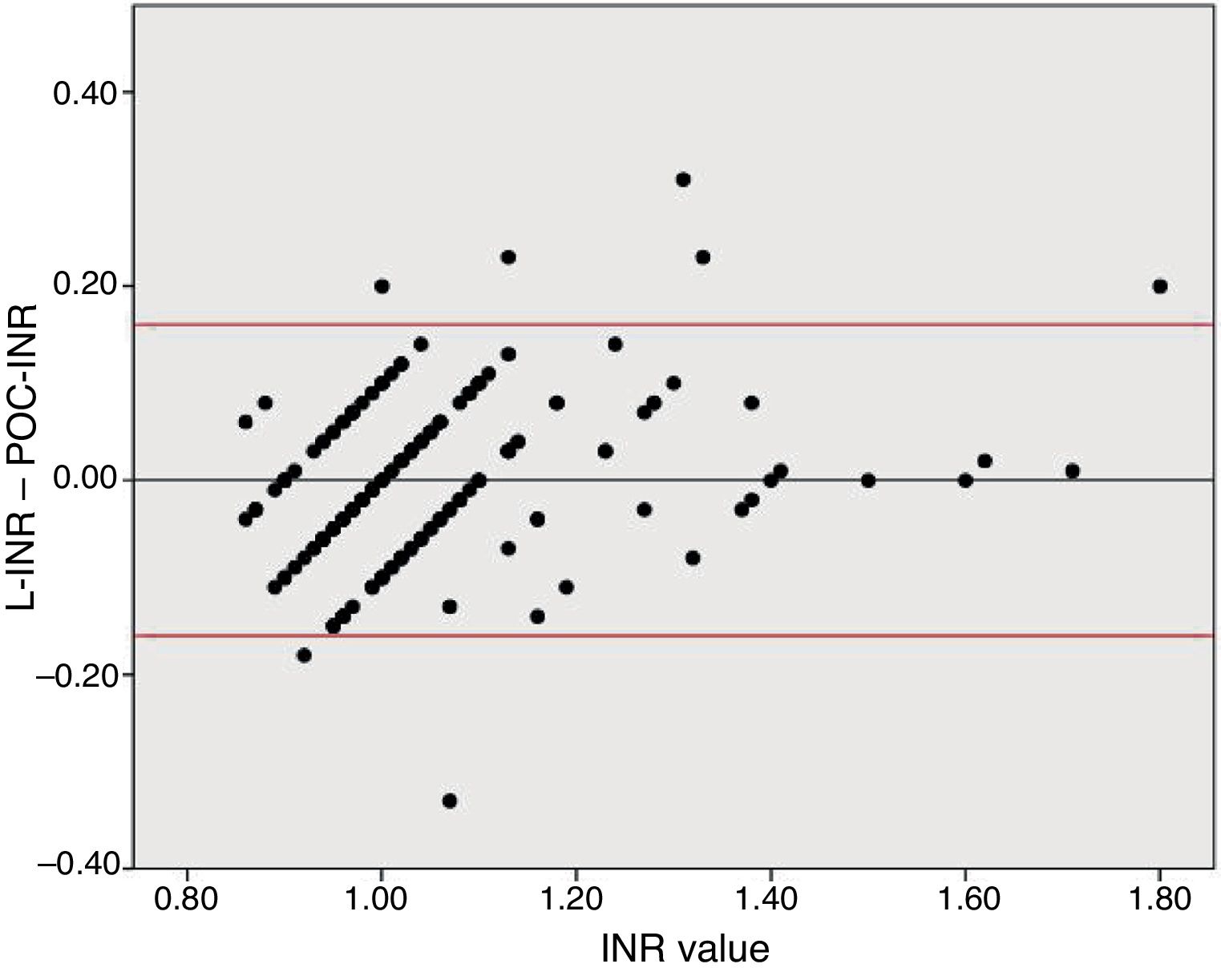
Agreement between both tests was analysed using a Bland–Altman plot to compare differences between INR values. Given that POC-INR testing tends to underestimate higher INR values, we arbitrarily chose an L-INR value of 1.2 to divide the sample into 2 subgroups (INR ≤ 1.2 and INR > 1.2). We then compared L-INR and POC-INR measurements between groups using the t test. Finally, we used a ROC curve to analyse the predictive value of POC-INR testing for an L-INR value < 1.7 in order to establish the cut-off point with the greatest possible sensitivity and specificity for detecting this INR value.
Statistical analysis and generation of graphics were performed using Stata® 14 (StataCorp, College Station, USA) and SPSS Statistics® 24.0 (IBM, Armonk, USA).
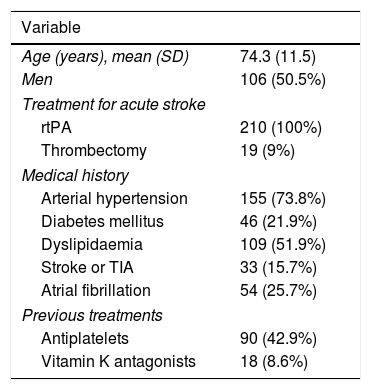
ResultsA total of 251 patients received intravenous rtPA during the study period; 28 patients were excluded due to incomplete clinical history and 13 due to problems in blood sample processing. The final sample included 210 patients, with a mean age of 74.3 years (11.5); 106 were men (50.5%). Eighteen patients (8.6%) were taking vitamin K antagonists at the time of stroke. All patients received intravenous rtPA and 19 (9%) underwent rescue mechanical thrombectomy. Table 1 summarises patients’ demographic and clinical characteristics.
Demographic and clinical characteristics of our sample.
| Variable | |
|---|---|
| Age (years), mean (SD) | 74.3 (11.5) |
| Men | 106 (50.5%) |
| Treatment for acute stroke | |
| rtPA | 210 (100%) |
| Thrombectomy | 19 (9%) |
| Medical history | |
| Arterial hypertension | 155 (73.8%) |
| Diabetes mellitus | 46 (21.9%) |
| Dyslipidaemia | 109 (51.9%) |
| Stroke or TIA | 33 (15.7%) |
| Atrial fibrillation | 54 (25.7%) |
| Previous treatments | |
| Antiplatelets | 90 (42.9%) |
| Vitamin K antagonists | 18 (8.6%) |
Data are expressed as n (%), unless otherwise indicated.
AHT: arterial hypertension; rtPA: recombinant tissue plasminogen activator; TIA: transient ischaemic attack.
The median L-INR was 1.02 (0.96-1.08) and the median POC-INR was 1 (1.0-1.1). The range of L-INR values in our sample was 0.86–1.8. No statistically significant differences were observed between INR measurements in the whole sample (mean L-INR–POC-INR difference, −0.001 [0.085]; P=.82) or in the subgroup of patients taking vitamin K antagonists (mean L-INR–POC-INR difference, 0.001 [0.081]; P=.42).
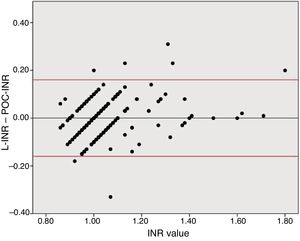
To analyse agreement between the tests for higher INR values, we divided the sample into 2 groups based on the sample distribution. Both tests showed similar INR values in the INR ≤ 1.2 group (mean L-INR–POC-INR difference, −0.008 [0.081]; P=0.16). In the INR > 1.2 group, however, POC-INR testing underestimated the INR (mean L-INR–POC-INR difference, 0.058 [0.095]; P=0.01); this difference was not clinically relevant, as it was not considered a contraindication for fibrinolysis in any patient.
To assess the value of POC-INR results for safely indicating intravenous rtPA (L-INR < 1.7), we built a predictive model based on the study population and plotted an ROC curve (Fig. 1). The area under the curve was 0.997 (95% CI, 0.991–1.000). The best cut-off point for predicting L-INR < 1.7 was POC-INR < 1.6, with 100% sensitivity and 98.97% specificity.
DiscussionOur results confirm that the CoaguChek® XS device is a fast, reliable tool for determining INR during the acute phase of stroke. Our study provides additional information about the predictive value of POC-INR measurements and the clinical relevance of the differences between POC-INR and L-INR values.
Management during the acute phase greatly determines prognosis in patients with ischaemic stroke. Any diagnostic tool contributing to faster treatment decision-making may have a direct impact on the effectiveness of reperfusion therapy. POC-INR testing, as compared to waiting for L-INR results, saves invaluable time during the acute phase of stroke. A recent study estimated the time gain at 28minutes.7 Confirming the concordance between POC-INR and L-INR measurements is therefore essential.
Interestingly, we found no significant differences between POC-INR and L-INR values in our sample. The same was true for the subgroup of patients receiving vitamin K antagonist. This is consistent with results from previous studies7–9 and confirms that the CoaguChek® XS device is a reliable diagnostic tool for acute stroke.
Disagreement between the 2 techniques was greater for high INR values than for lower values. Due to the previously reported tendency of POC-INR testing to underestimate the INR8,9 and the differences observed between POC- and L-INR values in the scatter and Bland–Altman plots (Fig. 2), we decided to divide the sample into 2 subgroups based on L-INR value (≤ 1.2 or > 1.2). The INR ≤ 1.2 subgroup showed no significant differences between techniques, but the INR > 1.2 subgroup did. The difference between both techniques did not represent a contraindication for fibrinolysis in any case. This means that the discrepancies between the 2 techniques for high INR values are not clinically relevant, at least in our sample. However, it is unclear whether the tendency to underestimate INR in our sample would remain stable in populations with higher INR values, or whether underestimation would be more marked.
Bland–Altman plot for the differences between L-INR and POC-INR values. The central line shows the mean difference, whereas the top and bottom lines indicate the 95% confidence interval. The plot indicates good agreement between techniques but reveals the influence of a measurement bias: POC-INR testing underestimates the INR when values are high.
Lastly, one of our most interesting findings is the observation that a cut-off point of POC-INR < 1.6 showed the greatest sensitivity and specificity for predicting L-INR < 1.7 in our predictive model. This may cause problems in identifying candidates for rtPA based on POC-INR measurements; each centre should validate these results internally before implementing POC-INR testing as part of the code stroke protocol.
Our study presents some limitations. Due to its retrospective design, some patients were not included in the analysis because their clinical histories were incomplete. Furthermore, data were gathered from our hospital's registry of patients receiving reperfusion therapy; as a result, very few patients had INR > 1.7. This constitutes a limitation to our predictive model and may introduce bias, since it is unclear whether discrepancies between both techniques would be greater in patients with L-INR > 1.7. Lastly, the time elapsed between POC-INR and L-INR testing was not recorded; therefore, we are unable to provide an estimation of the time gain associated with the use of portable coagulometers.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Guisado-Alonso D, Fayos-Vidal F, Martí-Fàbregas J, Prats-Sánchez L, Marín-Bueno R, Martínez-Domeño A, et al. Fiabilidad del coagulómetro portátil en pacientes con ictus isquémico agudo tratados con fibrinólisis intravenosa. Neurología. 2020;35:155–159.