Oromandibular dystonia (OMD) is an involuntary, repetitive, and sustained or spasmodic contraction of the muscles of mastication in the face and tongue. It may give rise to involuntary opening or closing of the jaw and involuntary tongue movements.
Both primary and secondary causes of OMD have been described.1,2 Late-onset dystonia is one of the most common secondary causes; this entity is associated with the use of dopamine antagonists, especially neuroleptic drugs.3
Peripheral nerve injury has been suggested as a trigger of focal dystonia, as in type 1 complex regional pain syndrome or reflex sympathetic dystrophy syndrome.4–6 OMD has been linked to peripheral nerve injury, especially in cases caused by dental procedures.7
Today, there is a growing interest in providing an explanation for the potential pathophysiological mechanisms of focal dystonia triggered by or appearing after peripheral nerve damage.
We present 2 cases of OMD secondary to focal radiotherapy due to nasopharynx cancer and discuss current medical literature on this topic.
Case 1Case 1 was a 39-year-old man diagnosed with nasopharynx cancer in November 2010. His medical history included smoking with no alcohol abuse or other drug habits. He had no history of high blood pressure, diabetes, or dyslipidaemia. The patient had not experienced head trauma or any diseases affecting the temporomandibular joint. He had not taken dopamine agonists or any other similar drugs.
Doctors resected the tumour completely and the histological study showed it to be a squamous cell tumour with a wide surgical margin. The patient subsequently received an 8-week course of focal nasopharyngeal radiotherapy for a total dose of 54Gy.
Radiotherapy was well-tolerated initially, with no immediate signs of significant adverse effects. Three weeks after the last session, the patient began to experience intermittent, spasmodic, and painful closing of the jaw, resulting in repeated tongue-biting and difficulty speaking and eating.
Clinical examination revealed intermittent and very painful closing of the mouth secondary to spasmodic contractions of the resting masseter muscles; contractions were much more evident when the patient tried to open his mouth. These contractions were unsightly and caused the patient aesthetic, psychological, and social problems.
Neurological examination yielded normal results in all other areas. Doctors ruled out changes in the temporomandibular joint after a clinical and radiological assessment.
Cranial CT and brain MRI were normal. Electromyography (EMG) showed signs of co-contraction in the masseter muscles and platysma.
Routine blood tests, as well as tests for serum copper, ceruloplasmin, and acanthocytes, yielded normal results. Video-EEG monitoring did not detect abnormalities.
The patient was treated with antidystonic drugs including baclofen, benzodiazepines, biperiden, and amantadine without demonstrating significant improvement. The patient's symptoms began to improve 24hours after he underwent infiltration with botulinum toxin (BTX) type A (50IU to each masseter muscle for a total dose of 100IU). At 4 days after infiltration, he had reached the maximum level of improvement of dystonic symptoms, with full remission of masseter muscle contractions. Beneficial effects of the infiltration with botulinum toxin lasted 3 months. The patient has subsequently required BTX infiltrations in the masseter muscles (100IU) on 2 different occasions; each infiltration resulted in considerable improvement lasting 3 to 4 months.
Case 2A 42-year-old male patient was referred to our neurology department due to presenting involuntary jaw closure and tongue-biting.
The most relevant episode in his medical history was recent diagnosis with nasopharynx cancer. In the preceding months, the lesion had been resected and treated with local radiotherapy (55Gy in 15 sessions). The patient consumed one pack of cigarettes daily and cannabis on occasion. He was also a sporadic user of cocaine.
Three weeks after finishing his radiotherapy sessions, he experienced jaw discomfort, mild pain that grew more intense, and a tendency to bite his tongue. This initially occurred while he was eating and progressed to become spontaneous. He did not report bruxism while sleeping, but he did notice spasmodic, but not incapacitating, movements of the masseter muscles.
A maxillofacial specialist found no anomalies in the temporomandibular joint.
Cranial MRI was normal and the EMG showed spontaneous activity in the masseter muscles with simultaneous activity in the platysma. The analysis yielded normal results in all other areas.
Physical examination revealed brief, spontaneous spasms in both masseter muscles appearing in 4 to 5 second clusters and increasing when the patient tried to speak or chew. The patient maintained that they did not bother him, but he did express concern about biting his tongue.
As the patient did not accept the proposed treatment with botulinum toxin, we prescribed clonazepam 0.5mg/12hours, which produced modest improvements. The patient has been experiencing these symptoms for 6 months with no exacerbations or improvements.
In the last 10 years, authors have published several cases of patients with focal dystonia related to peripheral trauma and provided lists of possible pathophysiological mechanisms for this type of dystonia.6 To the best of our knowledge, information in the literature is scarce but it does link dystonias to radiotherapy.
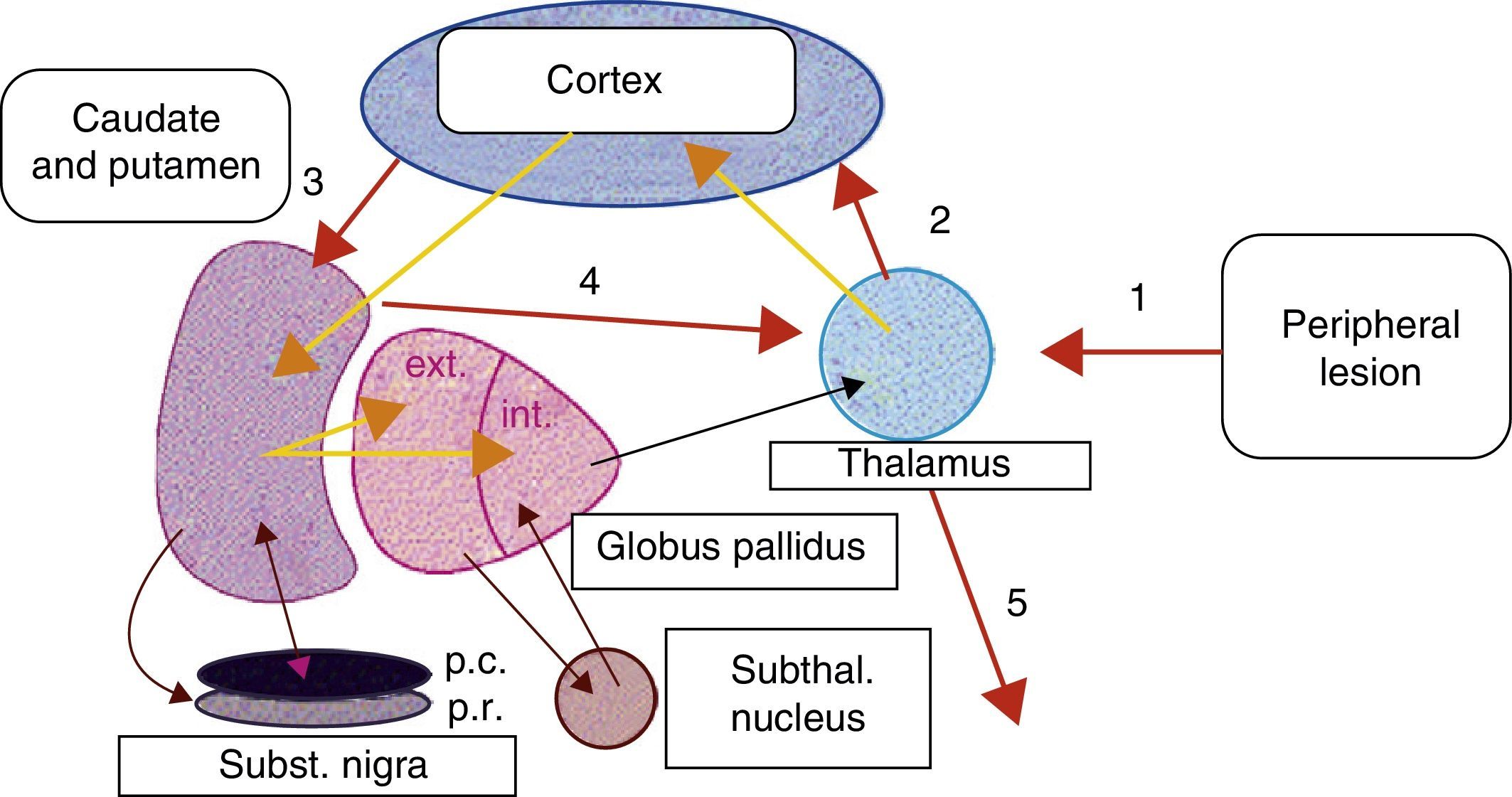
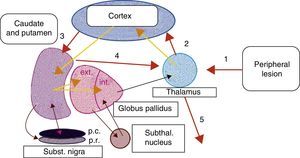
Peripheral lesions have been highlighted as possible trigger factors of focal dystonias, for example, in type I complex regional pain syndrome (reflex sympathetic dystrophy). In such cases, a history of mild focal trauma has been linked to the appearance of focal dystonia (frequently in arms and legs), local autonomic dysfunction, and pain.8 Some authors suggest that peripheral nerve injury or trauma may generate abnormal stimuli in the basal ganglia, which could give rise to anomalies in the voluntary control of muscle groups near the focal traumatic injury.9,10 As we support the hypothesis described above, we feel that peripheral injuries, such as those caused by dental procedures and possibly by focal radiotherapy, may induce fundamental reorganisation of somatic sensory representation in the thalamus. This change in cortical–subcortical neural circuits could produce altered synaptic transmission in basal ganglia. We could not say whether this change would result from an anomaly in the basal ganglia circuits that could be triggered by changes in sensory information, or if it would constitute a direct response to peripheral trauma (Fig. 1). We would also like to point out that the proximity between the nasopharynx and brainstem structures could enable peripheral stimuli, such as radiotherapy, to become distorted in such structures as the pons. The pons houses the well-described central pattern generator (CPG), and from this point, the ‘distorted’ impulse may enter higher structures.
Proposed model of basal ganglia disruption following a peripheral lesion. 1, peripheral trauma; 2, ‘altered’ thalamocortical stimulus; 3, cortical-putamenal stimulus; 4, putamenal-thalamic stimulus; 5, output stimulus from the thalamus towards the somatic sensory representation of the origin of the peripheral stimulus.
Sankhla et al.11 propose the following criteria for a diagnosis of peripheral-origin OMD: (a) onset of dystonia occurs within days or months (up to 1 year) of the lesion; (b) the original traumatic injury is well-documented in the patient's medical and/or dental records; and (c) onset of dystonia is anatomically related to the site of lesion (facial and oral).
We believe that focal radiotherapy may have caused a mild but repeated injury to the trigeminal nerve in these patients. This condition may have given rise to abnormal stimuli that elicited a change in somatic sensory representation in the thalamus. In such a case, the thalamus would transmit ‘altered’ nerve impulses to the sensory cerebral cortex, followed by involuntary impulses in the cortex, putamen, and thalamus that would lead to loss of control of mandibular movements due to spasmodic contractions of the masseter muscles. In 2002, Esteban et al. described follow-up on a patient with hemimasticatory spasm who displayed a possibly demyelinating trigeminal lesion according to neurophysiological tests. The authors proposed trigeminal hyperexcitability caused by a peripheral lesion of the trigeminal nerve.12 The hyperexcitability mentioned by these authors could refer to ‘distorted’ impulses that would modify the cortical-subcortical circuits, resulting in loss of voluntary control over mandibular movements. We propose this mechanism in our cases.
Radiotherapy is a common and widely accepted treatment for nasopharyngeal carcinoma and complications are infrequent. Some patients presenting OMD subsequent to focal radiotherapy may have a genetic susceptibility, given that many more patients do not; this topic requires further clarification. In summary, we believe that focal dystonia and peripheral lesions must be studied systematically in order to identify any changes in neurotransmitter regulation in the basal ganglia due to the abnormal stimuli caused by mild peripheral trauma.
In the 2 cases described here, we observed patients presenting clear cases of mandibular dystonia subsequent to focal radiotherapy at conventional doses for nasopharyngeal carcinoma. The first case, which was very disabling, showed an excellent response to treatment with botulism toxin; in the second case, the patient presented mild, non-disabling mandibular dystonia. The importance of this unusual condition lies in its potential for focusing and permitting future research on the association between peripheral lesions and focal dystonia.
FundingThis article was funded entirely by Hospital de Terrassa CST, Terrassa, Barcelona, Spain.
Please cite this article as: Salazar G, Español G, Fragoso M. Distonia oromandibular secundaria a radioterapia: Descripcion de 2 casos. Neurología. 2014;29:189–191.