This study aimed to determine whether the administration of antiepileptic drugs (AED) alters the likelihood of detecting epileptiform abnormalities in electroencephalographies (EEG) performed early after a first epileptic seizure.
MethodsWe performed a retrospective, observational study including patients with a first seizure attended at our centre’s emergency department between July 2014 and November 2019. We collected clinical data, as well as technical data on the acquisition and interpretation of the EEG performed within the first 72 hours after the seizure, and the factors related with seizure recurrence.
ResultsWe recruited 155 patients with a mean (SD) age of 48.6 (22.5) years; 61.3% were men. Regarding seizure type, 51% presented tonic-clonic seizures of unknown onset and 12% presented focal to bilateral tonic-clonic seizures. Thirty-nine patients (25.2%) received AED treatment before the EEG was performed: 33 received a non-benzodiazepine AED and 6 received a benzodiazepine. Epileptiform abnormalities were observed in 29.7% of patients. Previous administration of AEDs was not significantly associated with the probability of detecting interictal epileptiform abnormalities (P = .25) or with the risk of recurrence within 6 months (P = .63).
ConclusionsAdministration of AEDs before an early EEG following a first seizure does not decrease the likelihood of detecting epileptiform abnormalities. These findings suggest that starting AED treatment immediately in patients with a high risk of early recurrence does not imply a reduction in the diagnostic accuracy of the test.
Determinar si la administración de fármacos antiepilépticos (FAE) puede alterar la probabilidad de encontrar anomalías epileptiformes en EEG realizados de forma precoz tras una primera crisis epiléptica (CE).
MétodoEstudio observacional retrospectivo en el que se incluyeron los pacientes atendidos en urgencias de nuestro centro por una primera CE entre julio de 2014 y noviembre de 2019. Se recogieron los datos clínicos, las características técnicas de adquisición e interpretación de los EEG efectuados durante las primeras 72 horas tras la CE y los factores relacionados con la recurrencia.
ResultadosSe recogieron 155 pacientes; edad media 48,6 ± 22,5 años; 61,3% hombres. El 51% presentaron crisis tónico-clónicas (TC) de inicio desconocido y el 12 % focales con progresión a tónico-clónica bilateral. El 25,2% (39/155) recibieron tratamiento con FAE antes de la realización del EEG; en 33 pacientes se administró un FAE no benzodiacepínico y en 6 una benzodiacepina. Se observaron anomalías epileptiformes en 29,7% de los pacientes. La administración previa de FAE no se asoció de forma significativa ni con la probabilidad de detectar anomalías epileptiformes (p = 0,25) ni con el riesgo de recurrencia a los 6 meses (p = 0,63).
ConclusionesLa administración de un FAE previo a la realización del EEG precoz tras una primera CE no disminuye la probabilidad de detectar anomalías epileptiformes. Estos hallazgos sugieren que iniciar un FAE de forma inmediata en aquellos pacientes con alto riesgo de recurrencia precoz no implica un menor rendimiento diagnóstico de dicha prueba.
Electroencephalography (EEG) is one of the most important diagnostic tools after a first epileptic seizure. Detection of interictal epileptiform activity in EEG after a seizure is associated with increased risk of recurrence1; this risk has been studied by several authors and varies slightly between adult and paediatric populations, but it is considered significantly high in 64%-77% of these cases.2–4 In 2014, the International League Against Epilepsy (ILAE)5 proposed a new definition of epilepsy that helps establish a diagnosis of epilepsy after a first unprovoked seizure, which includes patients whose EEG shows interictal epileptiform activity.
The effect of antiepileptic drugs (AED) when interictal epileptiform activity is present is still a subject of debate. Although some classical studies have shown an inverse relationship between the level of AEDs and the frequency of epileptiform discharges,6–9 other studies show a higher presence of activity after administration of AEDs,10 or observe no association between frequency of interictal activity and levels of AEDs.11–14 A recent study showed a decrease in interictal epileptiform activity during EEG monitoring after suspension of AEDs.15
Thus, starting AED treatment after a first unprovoked seizure and before performing an EEG may affect the likelihood of detecting epileptiform activity and therefore hinder differential diagnosis. No strong evidence is currently available on this matter; therefore, the aim of the present study is to determine whether the administration of an AED affects the identification of diagnostic patterns in the EEG performed early in this group of patients.
Material and methodsThis is a retrospective, observational study that included all patients attended consecutively at the emergency department of Hospital Vall d’Hebron due to first unprovoked seizure between July 2014 and November 2019, and who underwent an EEG study in the first 72 hours after seizure.
All patients were assessed by an on-call neurologist, and EEG was performed if episodes were highly suggestive of epilepsy, which included paroxysmal episodes with involuntary tonic, clonic, or tonic-clonic movements; with or without altered level of consciousness, versive seizure, automatism, sensory aura, or characteristic speech or behavioural disorders. Semiological classification was based on the clinical description of seizures at the patient’s arrival at the emergency department, when no neuroimaging or EEG data were available. Semiological groups were adapted to the most recent ILAE classification: tonic-clonic seizures of unknown onset, focal seizures progressing to bilateral tonic-clonic seizures, and focal seizures not progressing to bilateral tonic-clonic seizures.16
Our centre is equipped to perform and interpret EEG studies 365 days per year. AEDs were administered before the EEG when considered appropriate by the responsible physician. Our centre’s protocol for onset of AED treatment after a first unprovoked seizure does not establish whether AEDs should be administered before or after the EEG. In everyday clinical practice, identification of a structural lesion in a neuroimaging study or in patients considered to be at higher risk due to their clinical characteristics (older age, comorbidities, pregnancy, certain cases of generalised tonic-clonic seizures, etc) frequently leads to the decision to start antiepileptic treatment before performing the EEG.
In all cases, a detailed medical interview was performed to identify any relevant family history, peripartum alterations, psychomotor retardation, febrile seizures, head trauma, or infections of the central nervous system. We also took into consideration history of subtle paroxysmal events or of episodes suggestive of previous absence seizures or myoclonus.
All patients underwent CT or MRI studies; imaging findings were considered pathological when lesions were potentially epileptogenic (eg, tumours, cerebrovascular disease, encephalomalacia areas, etc).5 Finally, based on clinical data and complementary examinations, patients were diagnosed by an on-call neurologist with a first unprovoked seizure, which was subsequently confirmed by an expert epileptologist from the epilepsy unit. For the analysis, diagnoses were retrospectively adapted to the most recent ILAE classification.16
We gathered demographic data, classification of seizure symptoms, date and time of seizure onset, date and time of EEG performance, administration of the AED before the EEG, and whether long-term antiepileptic treatment was started. Clinical records were followed up for at least 6 months.
Acute symptomatic seizures were excluded, as were patients with previous history of seizures or with suspected status epilepticus. We also excluded those patients whose EEG recording revealed epileptic activity.
The minimum duration of the EEG recording was 20 minutes, and we used a Deltamed Coherence® EEG system (64 channels, Natus Europe GmbH; Munich, Germany). Electrodes were placed according to the international 10-20 EEG system, using bipolar and referential montages (average). Activation procedures were applied in all cases, with the exception of those patients whose baseline level of consciousness or comorbidities made this impossible. EEG recordings were independently assessed by 2 neurophysiologists, and were classified into 3 categories17:
- a)
Interictal epileptiform abnormalities, including focal or generalised paroxysmal activity in the form of spikes, polyspikes, or sharp waves
- b)
Non-epileptiform abnormalities, including focal or generalised slowing
- c)
Lack of significant abnormalities (normal EEG recordings).
To simplify the statistical analysis and focus it on the initial aim of the study, patients with non-epileptiform abnormalities or with lack of significant abnormalities were included in the same group.
Statistical analysisStatistical analysis was conducted using the SPSS statistics software, version 26.0 (IBM Corp; Armonk, NY, USA).
To compare the groups of patients who did and did not receive AEDs before the EEG and the different characteristics and recurrence at 6 months, we used the chi-square test or the Fisher exact test for categorical variables, and the t-test for continuous variables.
P-values < .05 were considered statistically significant.
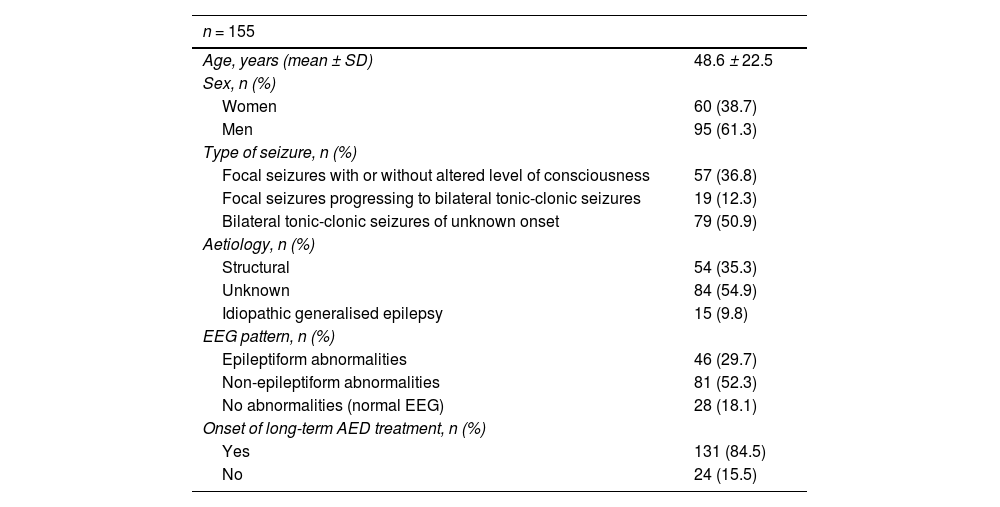
ResultsThe study sample included a total of 155 individuals. Table 1 shows the baseline characteristics of the study population. Mean age was 48 years, and 38.7% were women. Regarding the type of seizures, 37% presented focal seizures, 12% focal seizures progressing to bilateral tonic-clonic seizures, and 51% tonic-clonic seizures of unknown onset. According to the aetiological study, most cases were of unknown cause (55%), followed by structural cause (identified in 35% of cases). We observed interictal epileptiform abnormalities in 30% of the total EEG recordings. AED treatment was continued at discharge in 84.5% of cases.
Demographic and clinical characteristics of the sample.
| n = 155 | |
|---|---|
| Age, years (mean ± SD) | 48.6 ± 22.5 |
| Sex, n (%) | |
| Women | 60 (38.7) |
| Men | 95 (61.3) |
| Type of seizure, n (%) | |
| Focal seizures with or without altered level of consciousness | 57 (36.8) |
| Focal seizures progressing to bilateral tonic-clonic seizures | 19 (12.3) |
| Bilateral tonic-clonic seizures of unknown onset | 79 (50.9) |
| Aetiology, n (%) | |
| Structural | 54 (35.3) |
| Unknown | 84 (54.9) |
| Idiopathic generalised epilepsy | 15 (9.8) |
| EEG pattern, n (%) | |
| Epileptiform abnormalities | 46 (29.7) |
| Non-epileptiform abnormalities | 81 (52.3) |
| No abnormalities (normal EEG) | 28 (18.1) |
| Onset of long-term AED treatment, n (%) | |
| Yes | 131 (84.5) |
| No | 24 (15.5) |
AED: antiepileptic drug; EEG: electroencephalography; SD: standard deviation.
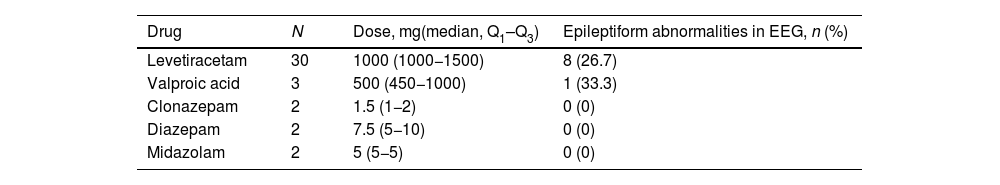
AED treatment was started before the EEG recording in 39 patients (25.2%). The most frequently used AED was levetiracetam (LEV) (30 cases). The other drugs used included valproic acid (VPA) (3), clonazepam (2), diazepam (2), and midazolam (2). Benzodiazepines were used according to the judgement of the responsible physician, always in a single dose, to avoid risk of early recurrence during the patient’s stay at the emergency department. Table 2 shows the AED doses administered, as well as the percentage of pathological EEG recordings identified for each drug. The doses of LEV and VPA employed were those typically used to start antiepileptic treatment; in these patients, epileptiform abnormalities were observed in 26.7% and 33.3% of cases, respectively. None of the 6 patients treated with benzodiazepines showed epileptiform activity in the EEG.
Antiepileptic drugs used before EEG.
| Drug | N | Dose, mg(median, Q1–Q3) | Epileptiform abnormalities in EEG, n (%) |
|---|---|---|---|
| Levetiracetam | 30 | 1000 (1000−1500) | 8 (26.7) |
| Valproic acid | 3 | 500 (450−1000) | 1 (33.3) |
| Clonazepam | 2 | 1.5 (1−2) | 0 (0) |
| Diazepam | 2 | 7.5 (5−10) | 0 (0) |
| Midazolam | 2 | 5 (5−5) | 0 (0) |
We report the number of patients prescribed each AED, the dose administered, and the number presenting epileptiform abnormalities in the EEG.
AED: antiepileptic drug; EEG: electroencephalography; Q1–Q3: quartiles 1 and 3.
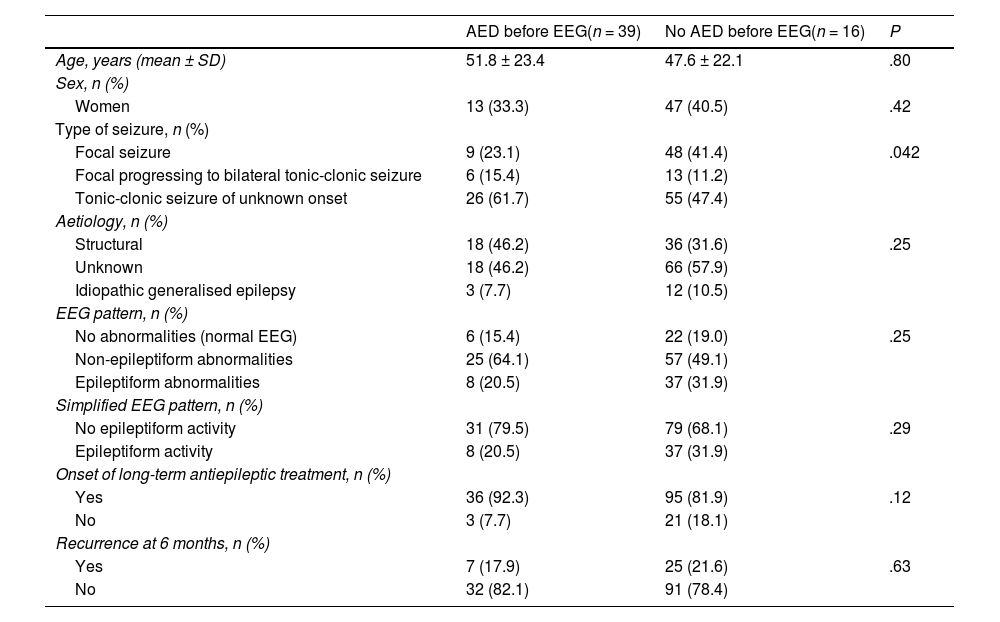
No significant differences in age, sex, or aetiology were observed when comparing the patients who received AED treatment before or after the EEG (Table 3). Early prescription of AEDs was significantly more frequent (P = .042) in patients attended due to tonic-clonic seizures of unknown onset (32.1% [26/81]) and focal seizures progressing to bilateral tonic-clonic seizures (31.6% [6/19]) than in patients with isolated focal seizures (15.8% [9/57]).
Comparison between patients who did and who did not receive an antiepileptic drug before the EEG.
| AED before EEG(n = 39) | No AED before EEG(n = 16) | P | |
|---|---|---|---|
| Age, years (mean ± SD) | 51.8 ± 23.4 | 47.6 ± 22.1 | .80 |
| Sex, n (%) | |||
| Women | 13 (33.3) | 47 (40.5) | .42 |
| Type of seizure, n (%) | |||
| Focal seizure | 9 (23.1) | 48 (41.4) | .042 |
| Focal progressing to bilateral tonic-clonic seizure | 6 (15.4) | 13 (11.2) | |
| Tonic-clonic seizure of unknown onset | 26 (61.7) | 55 (47.4) | |
| Aetiology, n (%) | |||
| Structural | 18 (46.2) | 36 (31.6) | .25 |
| Unknown | 18 (46.2) | 66 (57.9) | |
| Idiopathic generalised epilepsy | 3 (7.7) | 12 (10.5) | |
| EEG pattern, n (%) | |||
| No abnormalities (normal EEG) | 6 (15.4) | 22 (19.0) | .25 |
| Non-epileptiform abnormalities | 25 (64.1) | 57 (49.1) | |
| Epileptiform abnormalities | 8 (20.5) | 37 (31.9) | |
| Simplified EEG pattern, n (%) | |||
| No epileptiform activity | 31 (79.5) | 79 (68.1) | .29 |
| Epileptiform activity | 8 (20.5) | 37 (31.9) | |
| Onset of long-term antiepileptic treatment, n (%) | |||
| Yes | 36 (92.3) | 95 (81.9) | .12 |
| No | 3 (7.7) | 21 (18.1) | |
| Recurrence at 6 months, n (%) | |||
| Yes | 7 (17.9) | 25 (21.6) | .63 |
| No | 32 (82.1) | 91 (78.4) | |
AED: antiepileptic drug; EEG: electroencephalography; SD: standard deviation.
Statistical significance: P < .05.
The probability of detecting epileptiform abnormalities did not significantly vary according to whether AEDs were administered prior to the EEG study: interictal epileptiform abnormalities were detected in 31.9% of patients not receiving AEDs prior to EEG, as compared to the 20.5% of the patients who did receive the drugs (P = .3).
After assessing whether epileptiform abnormalities more frequently disappeared after the administration of an AED before EEG in patients with different seizure aetiologies, we found no significant differences (P = .41) between patients with structural lesions (31.3%), idiopathic generalised epilepsy (18.2%), and unknown aetiology (11.1%).
Furthermore, we observed no significant differences in the risk of recurrence at 6 months between patients who did and who did not receive AEDs prior to EEG (17.9% vs 21.6%, P = .063).
DiscussionWe observed that the administration of an AED before the EEG does not significantly affect the detection of interictal epileptiform activity in patients with a first unprovoked seizure.
Epilepsy can currently be diagnosed in patients with a single unprovoked seizure and risk of recurrence above 60% at 10 years, or with a diagnosis of a specific epileptic syndrome.5 Therefore, it is highly important to detect epileptiform activity for this definition, especially in cases of genetically determined generalised epilepsy and in patients not presenting structural lesions. This requires us to optimise the use of this diagnostic tool. There is considerable evidence that early EEG is much more beneficial than late EEG, with this benefit being greatest in an initial period between 12 and 16 hours after seizure17–20; as neurologists, we will face the reasonable doubt as to whether AED treatment should be started immediately, before EEG, or after interpretation of the EEG. Our study provides data suggesting that starting early AED treatment does not decrease the diagnostic yield of EEG, especially in the case of LEV, one of the most frequently used drugs in the treatment of epilepsy at emergency departments.
Although a higher level of evidence is needed to confirm these findings, it seems prudent to begin AED treatment for seizures with high risk of recurrence according to the Spanish Society of Neurology’s consensus statement on the emergency management of epileptic seizures,21 such as seizures in pregnant women or in individuals with fever, severe psychiatric comorbidity, head trauma, or other lesions secondary to seizures. Seizures scoring > 1 on the ADAN scale may also be considered to present high risk.22
It should be noted that in this study, we were unable to establish differences as a function of the different AEDs used, as the drug of choice was LEV in 30 of the 39 patients who started treatment before EEG. In the light of this limitation, it is possible that this phenomenon does not occur with all AEDs, and especially in the case of benzodiazepines, as no epileptiform activity was observed in any of the 6 patients receiving these drugs. Several works analysing interictal activity after withdrawal of different AEDs report highly divergent findings on this question7,8,10–13; therefore, we may also assume that the use of different AEDs may have influenced these findings. Specifically, the effect of LEV on epileptiform activity is null; although several studies rule out a decrease in interictal activity associated with LEV treatment in monotherapy,14 others suggest that in the case of generalised epilepsy, the drug may better control the interictal epileptiform activity.9 Our findings are consistent with those of Pro et al.,14 who identified no significant decrease in interictal epileptiform activity in the group of patients receiving LEV in monotherapy. Regarding benzodiazepines, this group of drugs presents high potential in suppressing epileptiform activity, as has been demonstrated in experimental studies23,24 and clinical trials, which have shown them to be more effective in treating status epilepticus than other AEDs.25
The main limitation of the study is its retrospective design, which means that we were unable to control which group of patients underwent the intervention. However, we may assert that there were no differences between the 2 groups, apart from the type of seizures (generalised or focal). Another significant limitation is that despite the lack of significant differences, the percentage of patients presenting epileptiform activity was surprisingly lower among those receiving an AED before the EEG; this low percentage may be explained by the lack of epileptiform activity in the group of patients who received benzodiazepines. However, in the total sample, the lack of statistical significance may be due to the sample size. Future research may validate these findings through regulated randomised studies analysing the effect of the different types of AEDs and different types of seizures.
ConclusionImmediate AED treatment of patients with a first unprovoked seizure seems not to decrease the diagnostic yield of early EEG in the detection of epileptiform activity; therefore, treatment onset should not be delayed in patients at high risk of early recurrence, particularly in patients treated with LEV, and with the possible exception of benzodiazepines.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.
Conflicts of interestThe authors have no conflicts of interest to declare.