Multiple sclerosis (MS) is a chronic autoimmune disease predominantly affecting young women.1 Fingolimod is a sphingosine-1-phosphate receptor modulator used to treat very aggressive or relapsing-remitting forms of MS that are refractory to other types of treatment.2
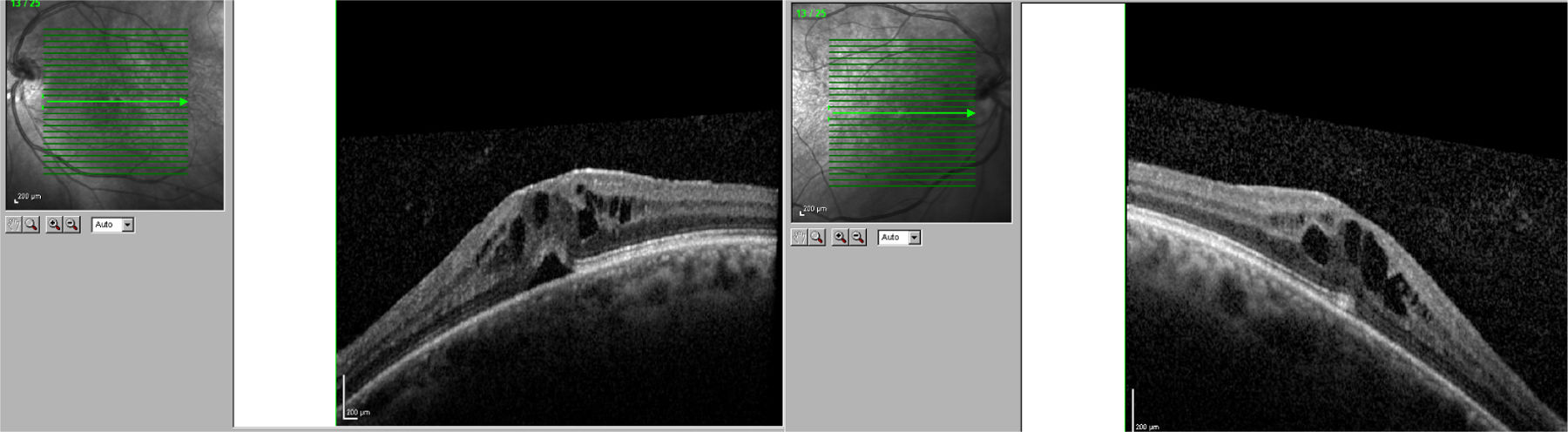
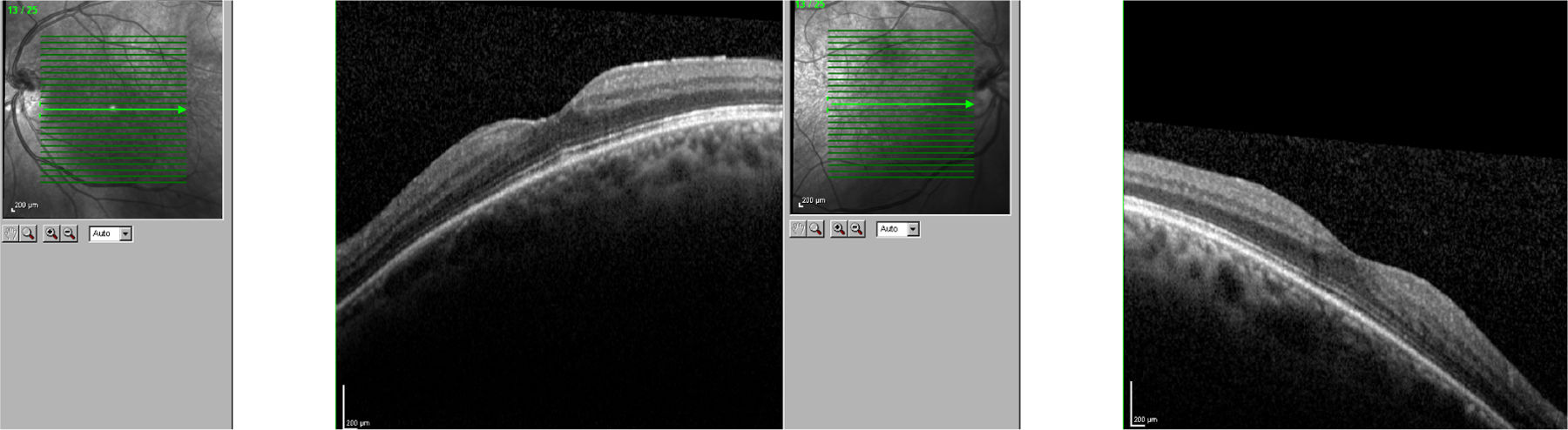
We present the case of a 56-year-old woman with a 20-year history of MS and no other systemic or ocular diseases. Due to poor control of relapses and the rapid progression of the disease, treatment was started with oral fingolimod (0.5mg/day). The patient was referred for evaluation at the neuro-ophthalmology department one week later due to blurred vision. The examination determined visual acuity (VA) of 0.6 in the right eye and 0.7 in the left. No alterations were observed in the anterior segment of the eye. Fundus examination revealed mild cystoid macular oedema bilaterally. Optical coherence tomography determined central macular thickness at 480μm in the right eye and 490μm in the left (Fig. 1). The patient was transferred to the neurology department, and treatment with fingolimod was withdrawn. At a follow-up visit to the neuro-ophthalmology department 20 days later, the macular oedema had fully resolved in both eyes (Fig. 2), and VA was restored bilaterally. Two months later, fingolimod was reintroduced to control MS. The patient was referred to the neuro-ophthalmology department 10 days later due to reduced VA (right eye: 0.7, left eye: 0.8). Cystoid macular oedema was present in both eyes; central macular thickness was 395μm in the right eye and 420μm in the left. Fingolimod was withdrawn once more, and neuro-ophthalmological and optical coherence tomography alterations resolved after a month. The macular oedema was not treated on either occasion; rather, it spontaneously and completely resolved following withdrawal of the drug.
In clinical trials, 0.5% of patients receiving fingolimod displayed macular oedema.3 The summary of product characteristics recommends that patients be evaluated before treatment is started, with an ophthalmological evaluation to be performed at 3-4 months.4 Fingolimod can cause vision loss due to macular oedema; this adverse reaction usually resolves following withdrawal of the drug, although cases have been described of visual impairment persisting months after withdrawal. Macular oedema is known to present more frequently in patients with diabetes mellitus and/or a history of uveitis. Our patient had no associated comorbidities; oedema developed less than 7 days after treatment onset. The literature includes other reports of macular oedema developing within 3-4 months of treatment onset,5 although not as early as in our patient. We consider it important to begin performing ophthalmological reviews after treatment is started, and to expedite referral of patients at greater risk of macular oedema (patients with diabetes mellitus or history of uveitis) or who report reduced VA or blurred vision.
Please cite this article as: Cifuentes-Canorea P, Nieves-Moreno M, Sáenz-Francés F, Santos-Bueso E. Edema macular precoz y recurrente en paciente en tratamiento con fingolimod. Neurología. 2019;34:206–207.