We evaluated the consumption of specific medications for treating cognitive symptoms associated with AD and other types of dementia in individuals over 60 years of age between 2006 and 2011 in the Basque Country.
MethodsA retrospective descriptive study was conducted. The pharmacy division of the Basque Government Department of Health provided the prescribing data for the following drugs: donepezil, rivastigmine, galantamine, and memantine. The number of defined daily doses (DDDs) and the number of DDDs per 1000inhabitants per day (DHD) were calculated.
ResultsConsumption increased by 49.72% between 2006 and 2011. There were marked differences between drugs (13.02% donepezil; 93.18% rivastigmine; 37.79% galantamine; 70.40% memantine) and Basque provinces (16.34% in Álava; 50.49% in Bizkaia; 57.37% in Gipuzkoa). Likewise, expenditure increased from €11.5 million in 2006 to €18.1 million in 2011.
ConclusionsThis study shows increased consumption of these drugs, although there are also marked differences by province which may be due to differences in prescribing habits. Spending for these drugs rose parallel to this increase in consumption; drug prices remained stable throughout the study period.
Este estudio evalúa el consumo de medicamentos para el tratamiento cognitivo de la EA y otras demencias en personas mayores de 60 años entre los años 2006 y 2011 en el País Vasco.
MétodosSe realizó un estudio descriptivo retrospectivo. La Dirección de Farmacia del Departamento de Salud del Gobierno Vasco facilitó los datos de prescripción de donepezilo, rivastigmina, galantamina y memantina. Se obtuvieron el número de dosis diarias definidas (DDD) y el número de DDD por 1.000 habitantes/día (DHD).
ResultadosEl consumo se incrementó un 49,72% durante el periodo 2006-2011, aumento que varió en función del medicamento (donepezilo 13,02%; rivastigmina 93,18%; galantamina 37,79%; memantina 70,40%) y del TTHH (Álava 16,34%; Bizkaia 50,49%; Gipuzkoa 57,37%). El gasto aumentó de 11,5 millones de euros en 2006 a 18,1 millones en 2011.
ConclusionesSe observó un aumento en el consumo aunque existen diferencias entre TTHH que pueden deberse a hábitos de prescripción diferentes. El gasto farmacéutico se incrementó paralelamente al aumento en el consumo, ya que el precio de los medicamentos permaneció estable en ese periodo.
Alzheimer disease (AD), a progressive and irreversible condition, is the most common type of dementia and affects 60% to 80% of all dementia patients. According to numerous clinical and epidemiological studies, AD is a very common disease and its incidence is increasing mainly due to population ageing.1,2 In a recent review article conducted within the framework of ALCOVE (ALzheimer COoperative Valuation in Europe), the prevalence of dementia is estimated at 7.23% for the European population older than 65.2 However, a Spanish study conducted in the Basque Country reported a prevalence of 9.1% in the same age group.3 Dementia is therefore estimated to affect between 30000 and 38000 people in the Basque Country.
Although no curative or preventive treatments for AD are currently available, certain drugs can slow disease progression and manage the associated symptoms. Most patients with AD are treated with combination therapy, including specific drugs for cognitive symptoms (neuroleptics, antidepressants, antipsychotics, etc.). For the purposes of this study, however, we only included specific drugs for treating cognitive impairment associated with dementia provided that clinical practice guidelines show consensus on their use.4–8 The active ingredients included in our study are donepezil, rivastigmine, and galantamine for mild to moderate AD, and memantine for moderate to severe AD.
The purpose of our study was to analyse consumption of specific medications for treating cognitive symptoms in AD and other dementias in people older than 60 between 2006 and 2011 in the Basque Country, as well as to assess the economic impact of consumption patterns.
Material and methodsWe conducted a retrospective descriptive study of the consumption of medications for cognitive symptoms in patients with AD in the Basque Country between 2006 and 2011. We included pharmacological presentations classified by the WHO as ATC code ‘N06D’ (anti-dementia drugs), recommended by clinical practice guidelines, and approved for prescription in Spain. These drugs are further categorised in 2 groups: anticholinesterases or ACE (N06DA), comprising donepezil, rivastigmine, and galantamine; and other anti-dementia drugs (N06DX), which include memantine. Data were gathered from the Pharmacy Division of the Basque Government Department of Health, which records the number of packages dispensed by pharmacies in the Basque Country and invoiced to the Spanish Health System. Therefore, our study does not include data on prescriptions paid for by citizens or private entities such as insurance companies, or data from in-hospital drug consumption. Annual demographic data were gathered from the Statistics Institute of the Basque Country.9
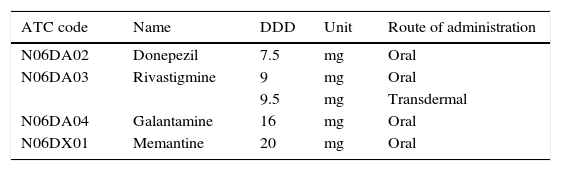
We based drug consumption estimates on the ‘defined daily dose’ (DDD), the technical unit of measurement recommended by the WHO as an international standard for pharmacoepidemiological studies. The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults, and it is normally expressed in grams of the active ingredient.10 DDDs have been established based on the literature and expert consensus.11 The DDD for each active ingredient was obtained from the WHO Collaborating Centre for Drug Statistics Methodology (Table 1).12 Drug consumption is expressed as the number of DDDs per 1000inhabitants per day (DDI); this unit provides an estimate of the population treated each day with the standard dose of a drug.10,13 We decided to analyse the population older than 60 so as to include patients with early onset dementia. In addition, we calculated the total cost of the specific AD medications used in the Basque Country during the study period.
DDD recommended by the WHO for each of the analysed drugs.
| ATC code | Name | DDD | Unit | Route of administration |
|---|---|---|---|---|
| N06DA02 | Donepezil | 7.5 | mg | Oral |
| N06DA03 | Rivastigmine | 9 | mg | Oral |
| 9.5 | mg | Transdermal | ||
| N06DA04 | Galantamine | 16 | mg | Oral |
| N06DX01 | Memantine | 20 | mg | Oral |
DDD, defined daily dose.
Data taken from the WHO Collaborating Centre for Drug Statistics Methodology.12
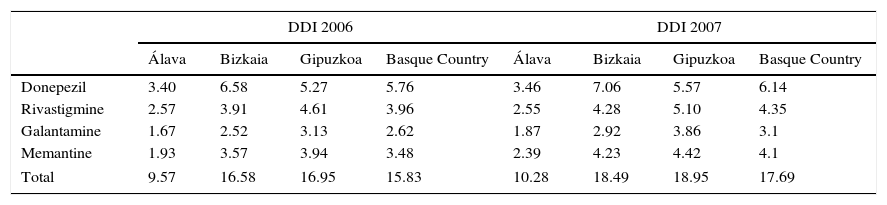
The analysis of dementia drug consumption in patients older than 60 between 2006 and 2011 in the Basque Country shows an overall increase for all the drugs included in our study. Consumption trends for each active ingredient are shown in Table 2.
ACE and memantine consumption in DDI (DDD per 1000inhabitants per day) in the Basque Country between 2006 and 2011.
| DDI 2006 | DDI 2007 | |||||||
|---|---|---|---|---|---|---|---|---|
| Álava | Bizkaia | Gipuzkoa | Basque Country | Álava | Bizkaia | Gipuzkoa | Basque Country | |
| Donepezil | 3.40 | 6.58 | 5.27 | 5.76 | 3.46 | 7.06 | 5.57 | 6.14 |
| Rivastigmine | 2.57 | 3.91 | 4.61 | 3.96 | 2.55 | 4.28 | 5.10 | 4.35 |
| Galantamine | 1.67 | 2.52 | 3.13 | 2.62 | 1.87 | 2.92 | 3.86 | 3.1 |
| Memantine | 1.93 | 3.57 | 3.94 | 3.48 | 2.39 | 4.23 | 4.42 | 4.1 |
| Total | 9.57 | 16.58 | 16.95 | 15.83 | 10.28 | 18.49 | 18.95 | 17.69 |
| DDI 2008 | DDI 2009 | |||||||
|---|---|---|---|---|---|---|---|---|
| Álava | Bizkaia | Gipuzkoa | Basque Country | Álava | Bizkaia | Gipuzkoa | Basque Country | |
| Donepezil | 3.49 | 7.87 | 5.79 | 6.65 | 3.11 | 7.92 | 5.70 | 6.66 |
| Rivastigmine | 2.65 | 4.50 | 5.25 | 4.53 | 3.39 | 5.59 | 7.00 | 5.87 |
| Galantamine | 2.11 | 3.24 | 4.60 | 3.55 | 1.88 | 3.33 | 4.32 | 3.53 |
| Memantine | 2.49 | 4.79 | 5.13 | 4.64 | 2.54 | 5.34 | 5.87 | 5.28 |
| Total | 10.75 | 20.40 | 20.77 | 19.38 | 10.93 | 22.18 | 22.89 | 21.33 |
| DDI 2010 | DDI 2011 | |||||||
|---|---|---|---|---|---|---|---|---|
| Álava | Bizkaia | Gipuzkoa | Basque Country | Álava | Bizkaia | Gipuzkoa | Basque Country | |
| Donepezil | 3.08 | 7.68 | 5.78 | 6.56 | 3.48 | 7.69 | 5.76 | 6.51 |
| Rivastigmine | 3.39 | 6.31 | 8.41 | 6.72 | 3.84 | 7.27 | 9.84 | 7.65 |
| Galantamine | 1.61 | 3.69 | 4.02 | 3.58 | 1.62 | 3.98 | 3.79 | 3.61 |
| Memantine | 2.10 | 5.64 | 6.50 | 5.57 | 2.20 | 6.01 | 7.29 | 5.93 |
| Total | 10.18 | 23.32 | 24.70 | 22.43 | 11.14 | 24.95 | 26.68 | 23.70 |
| Increase in DDI (%) 2006-2011 | ||||
|---|---|---|---|---|
| Álava | Bizkaia | Gipuzkoa | Basque Country | |
| Donepezil | 2.47 | 16.81 | 9.26 | 13.02 |
| Rivastigmine | 49.54 | 86.11 | 113.28 | 93.18 |
| Galantamine | –3.51 | 58.16 | 21.02 | 37.79 |
| Memantine | 13.81 | 68.19 | 85.16 | 70.40 |
| Total | 16.34 | 50.49 | 57.37 | 49.72 |
ACE use shows significant changes, with some drug prescriptions becoming increasingly common at the expense of the rest. Whereas donepezil use remains stable, consumption of both rivastigmine and galantamine has increased considerably. This increase is especially marked in the case of rivastigmine, which in 2011 reached 3.84, 7.27, and 9.84 DDI in Álava, Bizkaia, and Gipuzkoa, respectively; this represents an overall increase of 93.18% in the Basque Country between 2006 and 2011. In fact, rivastigmine became the most frequently used active ingredient in Álava and Gipuzkoa in 2009 when it overtook donepezil, which had been the ACE of reference until that time. Likewise, galantamine use has experienced substantial changes. The province of Bizkaia shows the most significant increase at 58.16%; consumption in Gipuzkoa has experienced a more discreet increase at 21.02%, whereas in Álava galantamine consumption has decreased by 3.51%. Differences for memantine are also noteworthy. In 2011, a total of 2.20, 6.01, and 7.29 DDI were dispensed in Álava, Bizkaia, and Gipuzkoa, respectively, which represents an increase of 13.81% in Álava, 68.91% in Bizkaia, and 85.16% in Gipuzkoa during the study period.
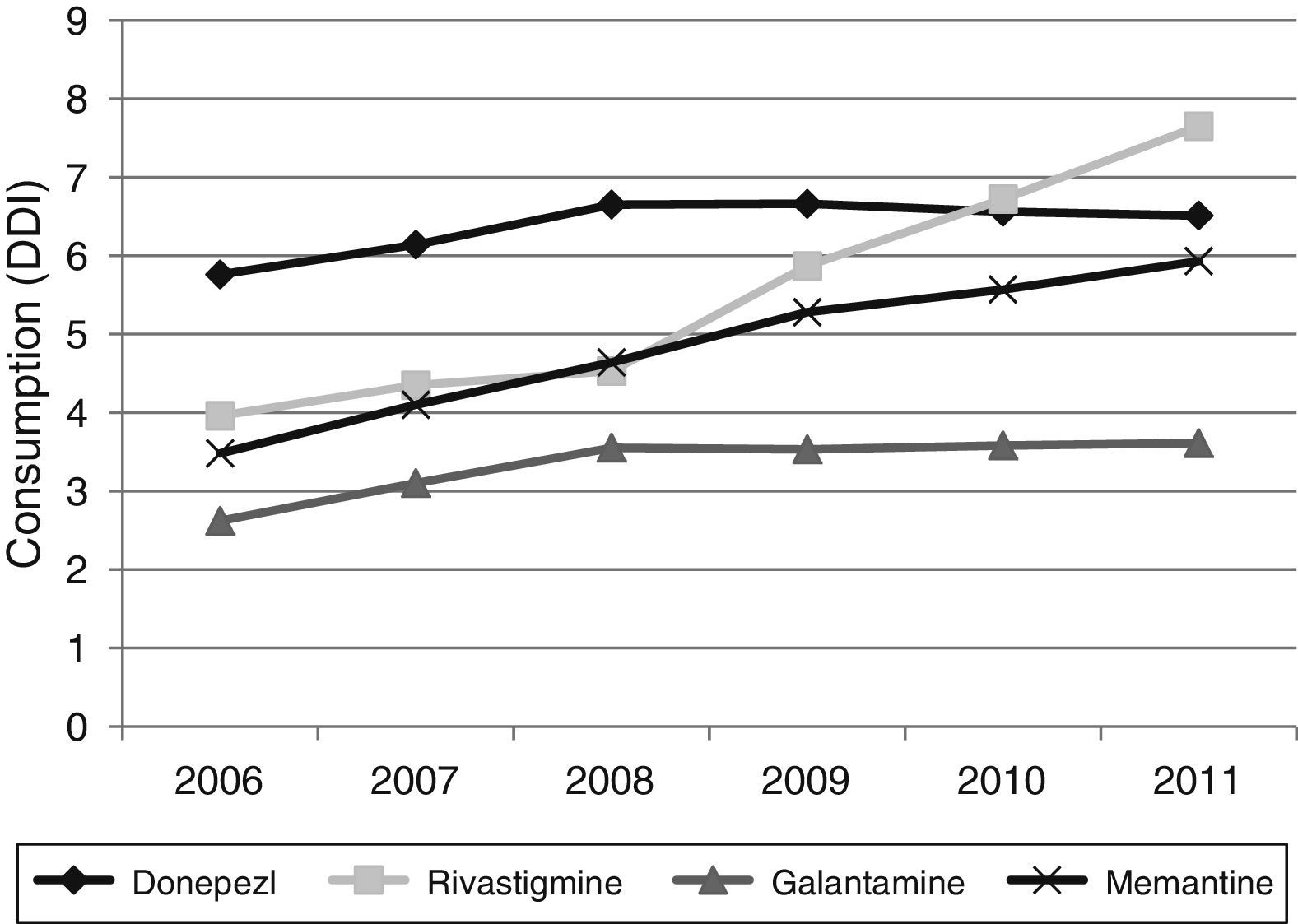
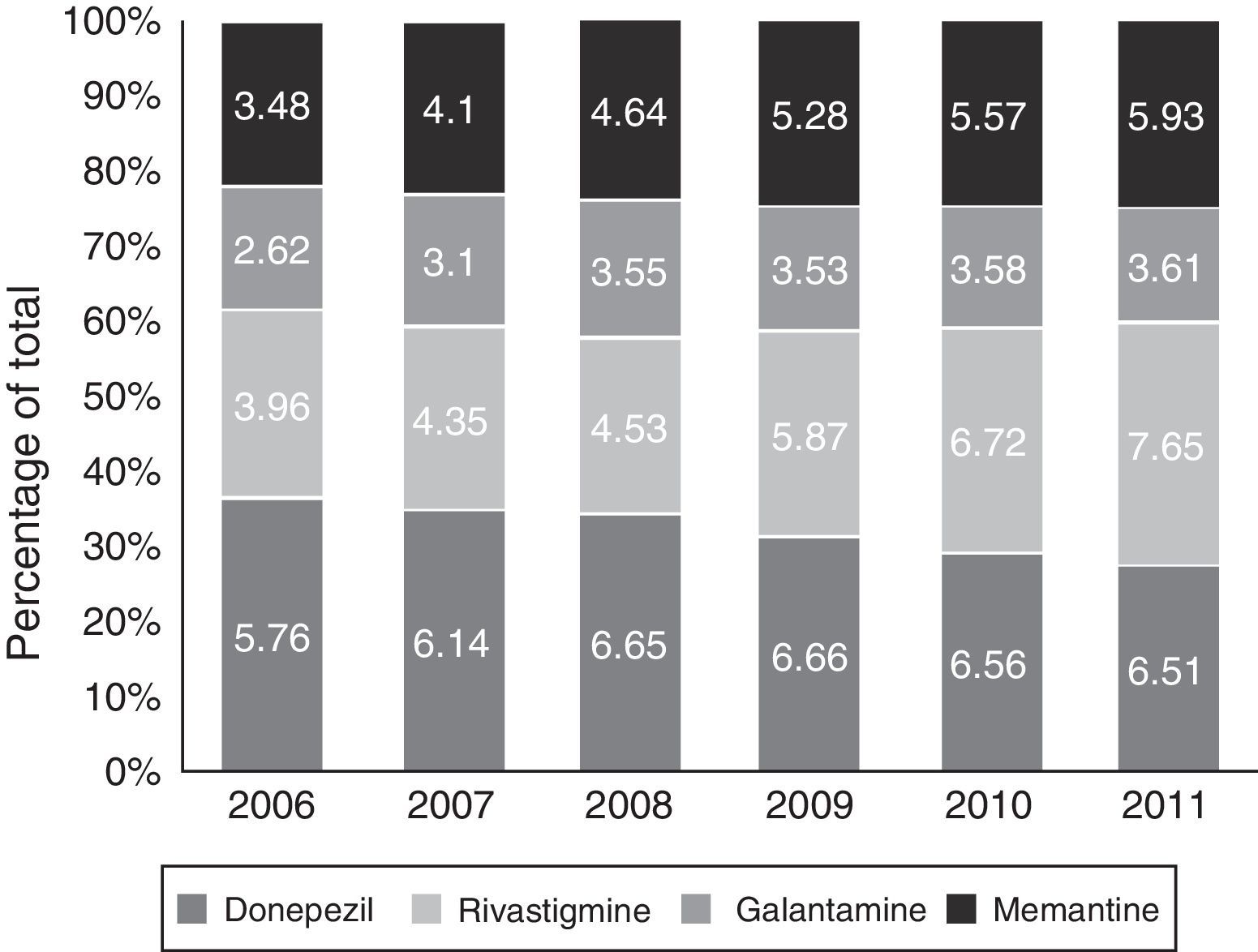
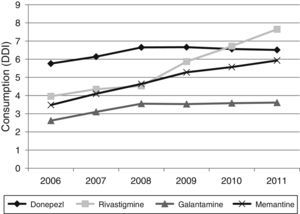
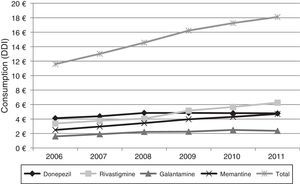
Figs. 1 and 2 show consumption trends for each of the active ingredients as well as the percentage of use of each active ingredient over total use in the Basque Country. We observed an overall increase in consumption, although patterns vary by drug. In 2006, the most frequently used drug was donepezil (5.76 DDI); consumption of this drug increased by 13.02% between 2006 and 2011 but has stabilised over time. We also observed a moderate increase in galantamine use, from 2.62 DDI in 2006 to 3.61 DDI in 2011, representing an increase of 37.79%. However, the most remarkable finding was the increase in rivastigmine and memantine use (by 93.18% and 70.40%, respectively).
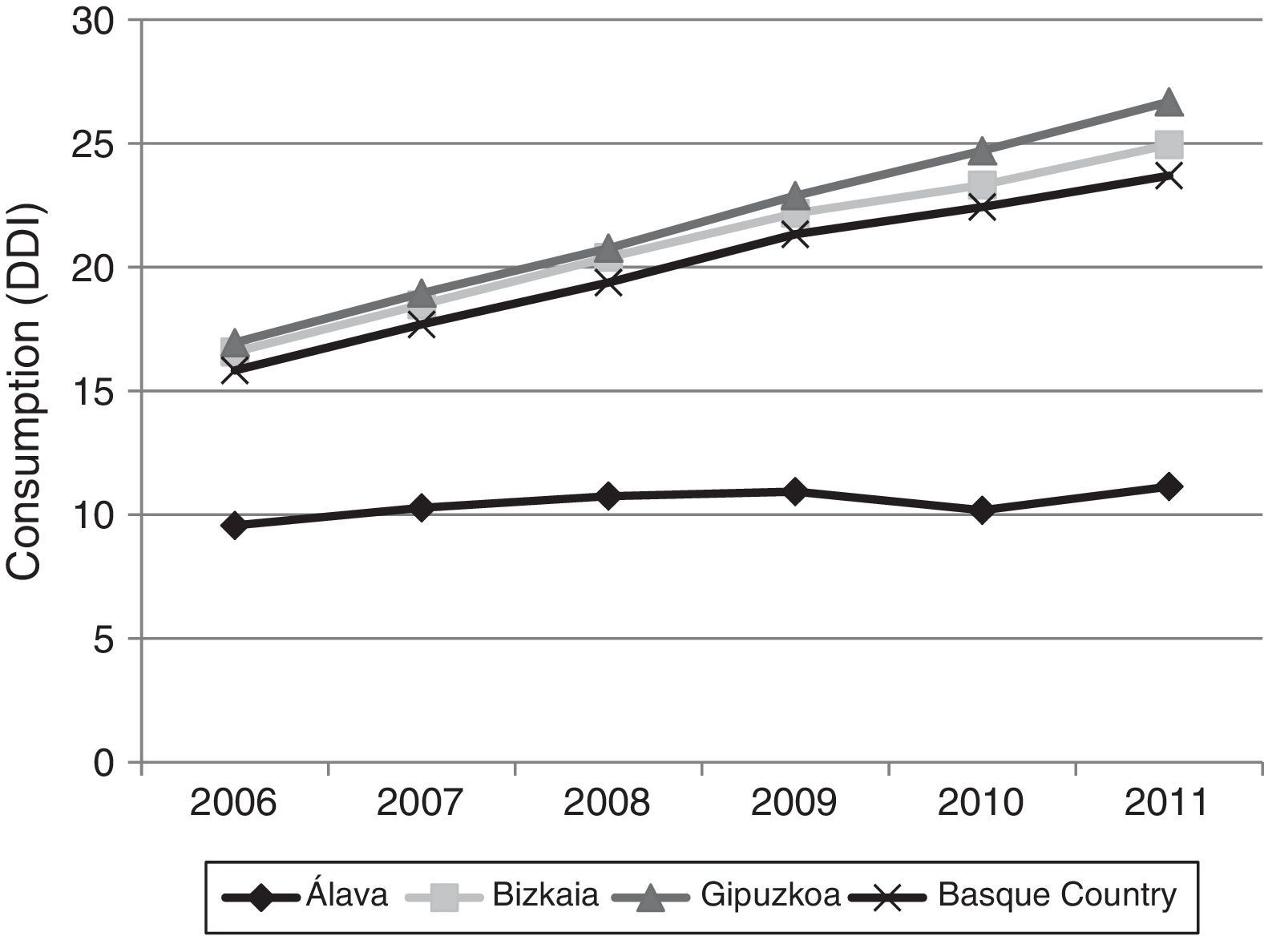
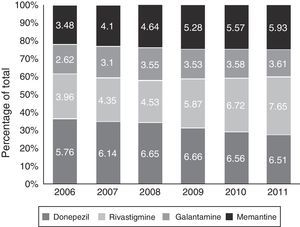
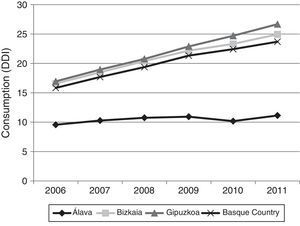
Fig. 3 shows consumption trends for each active ingredient and each province of the Basque Country. Although drug consumption has increased overall, consumption patterns for each of the analysed drugs vary from province to province with increases of 16.34% in Álava, 50.49% in Bizkaia, and 57.37% in Gipuzkoa.
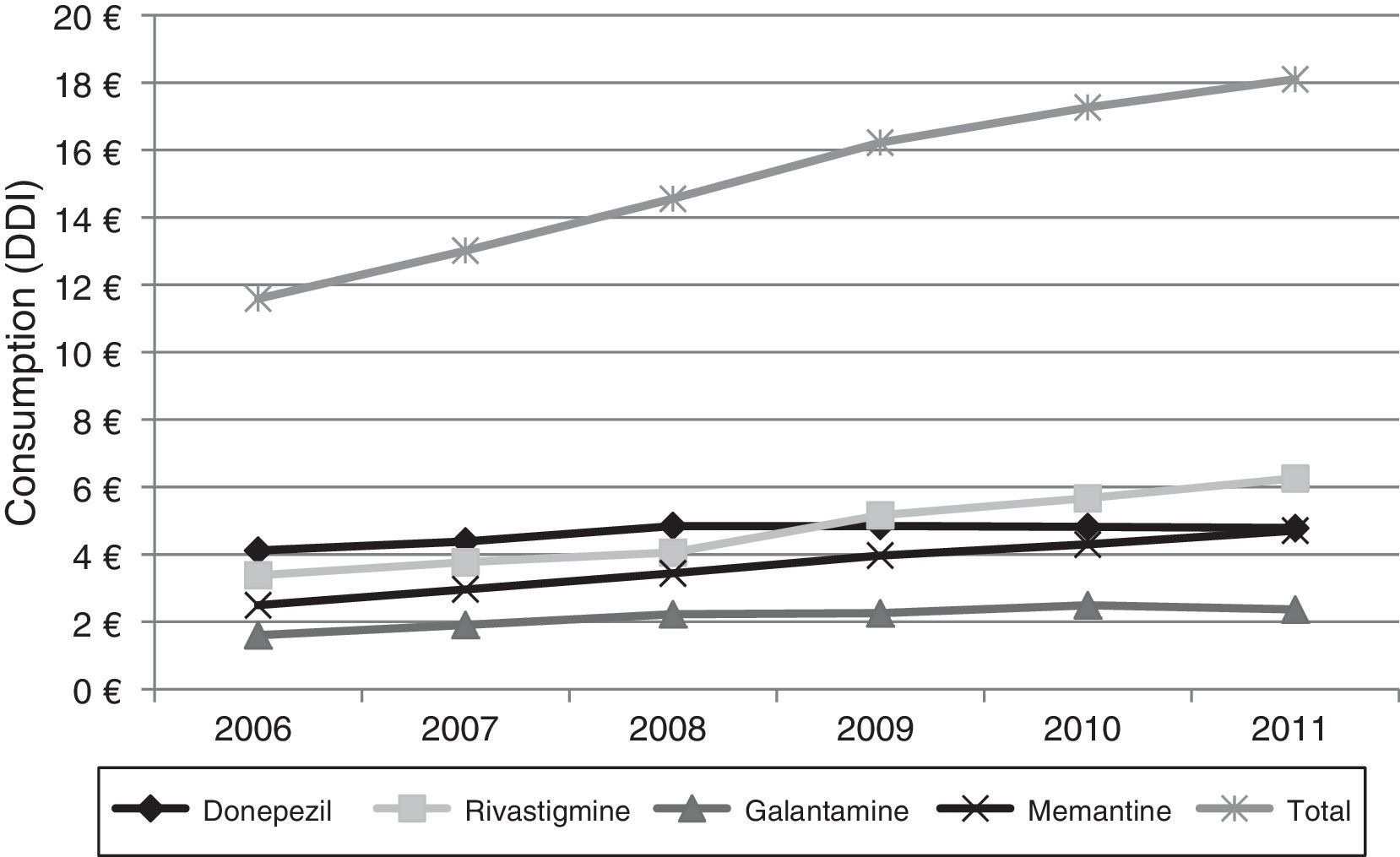
Lastly, Fig. 4 shows the increase in pharmaceutical costs resulting from increased prescription of these drugs. In 2006, pharmaceutical costs amounted to €11.5 million, but had risen to €18.1 million by 2011; this 56.21% increase is mainly due to the increase in rivastigmine and memantine consumption (85.0% and 88.77%, respectively). Costs associated with galantamine use increased by 47.50% and those linked to donepezil consumption increased by 16.20%.
DiscussionDrug consumption studies constitute a useful tool for evaluating drug prescription tendencies and consumption trends, and analysing variability in clinical practice. This type of study is especially relevant for conditions such as AD, which will constitute one of the greatest public health problems in the coming years, as mentioned previously.14–16
Our results reveal a considerable increase in the consumption of all 4 drugs analysed here between 2006 and 2011; this increase has been accompanied by a change in prescription patterns. Although donepezil consumption increased moderately during the study period, the consumption of ACEs overall remained stable due to increased prescription of alternatives such as galantamine and rivastigmine. Memantine use was also observed to increase during the study period.
Several hypotheses may explain the increases in consumption of these drugs. On the one hand, several studies show that the prevalence of AD is increasing1,2,17–20 and a rising number of patients will therefore need treatment. This is partly due to population ageing, since age is directly correlated with development of AD.21–23 On the other hand, studies carried out in the past few years have placed particular emphasis on early diagnosis, resulting in greater numbers of identified and treated patients.
Regarding changes in consumption for each active ingredient, donepezil is the oldest of the drugs analysed here and its patent has expired, a fact that may have lessened its commercial appeal. In addition, galantamine is priced lower than donepezil (this is not the case for rivastigmine), which may have led to increased galantamine prescription. The increase in revastigmine use is linked to the introduction of a transdermal patch format in 2008. Transdermal patches are associated with greater tolerability and fewer adverse effects compared to other formats,24–27 and with greater treatment adherence compared to other ACEs.28–30
Regarding drug consumption by province, we found differences both in total consumption and in consumption by each drug separately. The case of Álava is particularly noteworthy: total drug consumption during the study period increased a mere 16.34%, compared to 50.49% and 57.37% in Bizkaia and Gipuzkoa, respectively. Similarly, when comparing our data to the results published in a similar study conducted in Castille-La Mancha in 2008,31 we found differences in drug consumption between these 2 autonomous communities. Donepezil was the most frequently prescribed ACE in both autonomous communities, although there were marked differences: 11 DDI in Castille-La Mancha and 6.65 DDI in the Basque Country. Rivastigmine and galantamine consumption was also slightly higher in Castille-La Mancha, but memantine consumption was higher in the Basque Country (4.64 vs. 1.76 DDI).
These differences may be attributed to the different commercial strategies employed by pharmaceutical companies in each region. Likewise, the influence of specialists may be decisive when it comes to prescribing one drug or another. According to the literature, although primary care doctors are the ones to prescribe drugs on a regular basis, their prescriptions are influenced by specialists in many cases.32–34 This phenomenon of ‘recommended prescription’ is especially visible in the case of new drugs,35 which may partially explain the increase in rivastigmine use since the patch formulation was introduced.
Regarding the economic impact of these changes, our data indicate a significant increase in costs associated with these drugs: from €11.5 million in 2006 to €18.1 million in 2011. This increase is due to greater consumption of these drugs, whose prices have remained largely unchanged throughout the study period. As for the ‘recommended prescription’ phenomenon mentioned previously, some studies suggest that it may have an impact on pharmaceutical costs, since the mean cost of prescriptions issued by specialists is higher than that of prescriptions in primary care.32,33
Although anticipating future trends is difficult, our data suggest that pharmaceutical costs will increase in the coming years, mainly due to population ageing, increasingly early diagnosis (accompanied by early treatment), and the potential development of new active ingredients, which would normally be marketed at higher prices than current drugs. However, we should also be mindful that a number of cost containment measures have been implemented in recent years, including copayments, generic drug prescriptions, and price reductions.36 It should be stressed that pharmaceutical costs are, quite simply, an investment in health. A recent assessment report by the National Institute for Health Research37,38 concludes that these 4 drugs are more cost-effective than was previously believed and could become even more cost-effective in the event of a hypothetical reduction in prices. In addition, some studies indicate that treatment with ACEs and/or memantine in patients with AD is not only cost-effective but may also help reduce costs, since it yields additional health-related benefits.39,40 However, as the organisation Alzheimer Europe points out,41 the existing cost-effectiveness models cannot be applied to AD since they do not assess such indirect benefits as caregiver burden and quality of life.
Although all 4 of these drugs are authorised for prescription in Spain and covered by the Spanish Health System, there are major concerns about equal access to treatment.41 According to a recent study,42 in 2004 only 40% of the patients diagnosed with AD in Spain were receiving treatment with any one of these drugs. Similar percentages were found elsewhere in Europe, although situations varied greatly from country to country. While additional studies should be conducted to update these results, current data expose barriers to accessing proper treatment.41 This is due in part to differences in treatment access and availability between countries and the fact that many patients with AD have not been correctly diagnosed.2,41
Our study has a number of limitations that should be considered for proper interpretation of results. Firstly, we only included the prescriptions invoiced to the Basque Health System, overlooking data for in-hospital drug dispensation, prescriptions in the private sector by other entities (for example, MUFACE or ISFAS, 2 special Social Security regimens for civil servants and military workers, respectively), and drugs dispensed without prescription (in theory, this should be zero since these drugs require a prescription). We must also be mindful that DDI, the unit used to analyse consumption, is calculated based on the DDD defined by the WHO.12 Although DDI is useful for measuring and comparing drug consumption and analysing consumption trends, it should be used with caution since it is based on DDDs,43 which have been established by consensus and may not coincide with the actual doses prescribed in clinical practice. Lastly, we should point out that our study analyses the costs of drugs specifically used in dementia and does not include other drugs that are frequently prescribed to these patients, such as antidepressants and antipsychotics.
The increased use of drugs used specifically in AD and other dementias, along with the foreseeable increase in AD prevalence in the coming years underscore the need for a consensus on rational drug use. Studies similar to our own should be conducted to analyse consumption trends in different regions with a view to reducing variability and improving management of patients with AD or other types of dementia.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Villanueva G, López de Argumedo M, Elizondo I. Consumo de medicamentos para el tratamiento de la demencia en la Comunidad Autónoma Vasca durante el periodo 2006-2011. Neurología. 2016;31:613–619.