Primary lateral sclerosis (PLS) is a variant of amyotrophic lateral sclerosis (ALS) in which the upper motor neuron and, secondarily, the corticospinal tract degenerate, with no clinical or neurophysiological involvement of the lower motor neuron.1 Clinically, the disease is characterised by progressive spasticity and poor limb coordination. Prognosis of PLS is significantly better than that of classic ALS, with slower progression and higher survival rates.2 Treating spasticity can have a meaningful impact on patients’ quality of life, although few studies have addressed this topic. We present the case of a patient with PLS presenting with severe spasticity from onset, whose symptoms improved significantly following treatment with botulinum toxin (BTX).
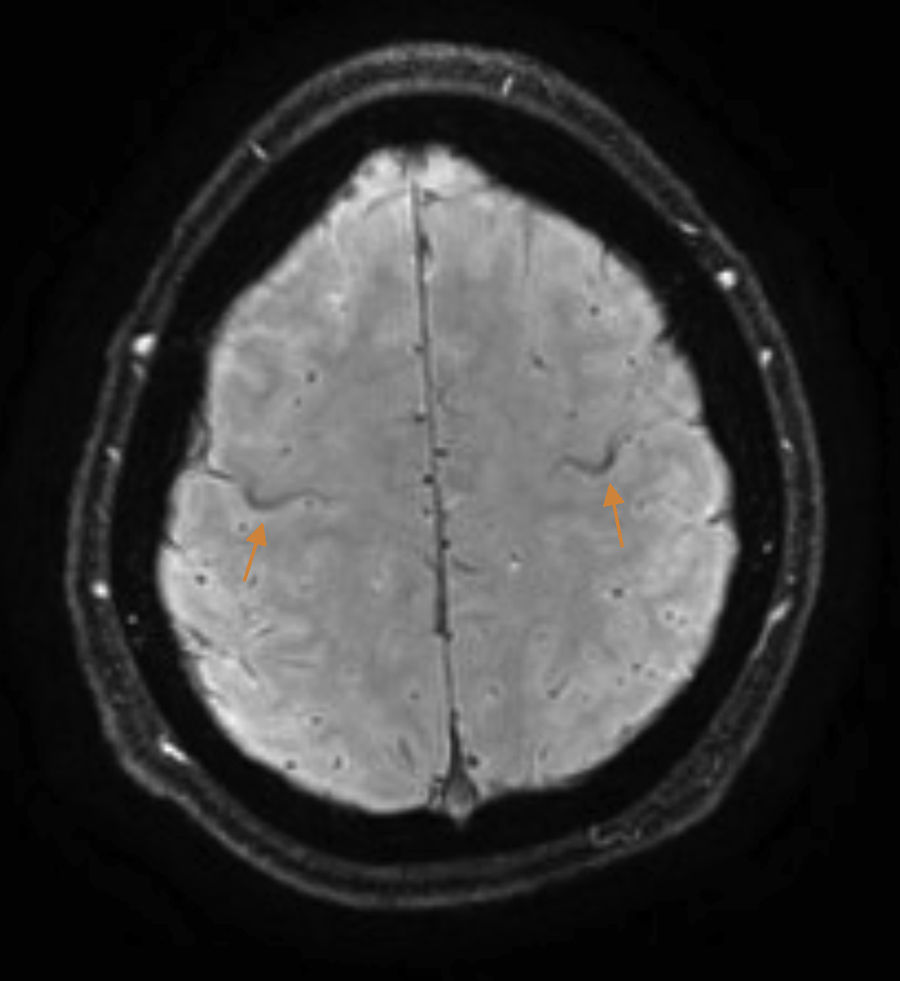
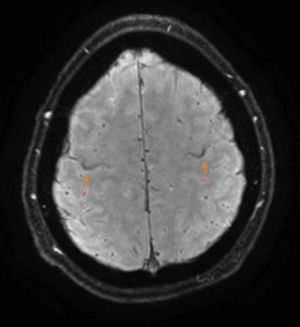
The patient is a 37-year-old man who began experiencing gait alterations in 2010. Examination revealed hyperreflexia, spasticity, and poor coordination of the lower limbs, with no sensory involvement. Blood testing (including an autoimmune study and monoclonal component detection) and a lumbar puncture yielded normal results. The neurophysiological study ruled out lower motor neuron involvement. A brain magnetic resonance imaging (MRI) scan revealed hyperintensity in the corticospinal tracts on the FLAIR sequence, with an SWI sequence revealing a hypointense rim in the precentral gyrus (Fig. 1). Based on these findings, we diagnosed the patient with suspected PLS and initiated treatment with riluzole. As the disease progressed, the patient presented pyramidal signs in the upper limbs, and spastic dysarthria. An additional electromyography performed 4 years after symptom onset ruled out lower motor neuron involvement; we were therefore able to establish a definitive diagnosis of PLS.1 Initial treatment with tetrazepam reduced spasticity, but caused generalised weakness which prevented the patient from standing. In September 2012, the patient displayed spastic gait and was only able to walk short distances. He required 2 canes to walk indoors, with frequent falls, and a wheelchair to move outdoors. He also had poor coordination in the right hand and had a tendency to drop objects. The patient scored 32 on the ALS Functional Rating Scale (ALSFRS-R) (Table 1) and 3-4/5. Deep tendon reflexes were hyperactive in the lower limbs, with persistent clonus and extensor cutaneous plantar reflexes. Spasticity scored 2 on the Ashworth Scale and increased when he began walking, with the patient experiencing freezing. The 10-Metre Walk Test could not be performed, as he was unable to walk 10 metres.
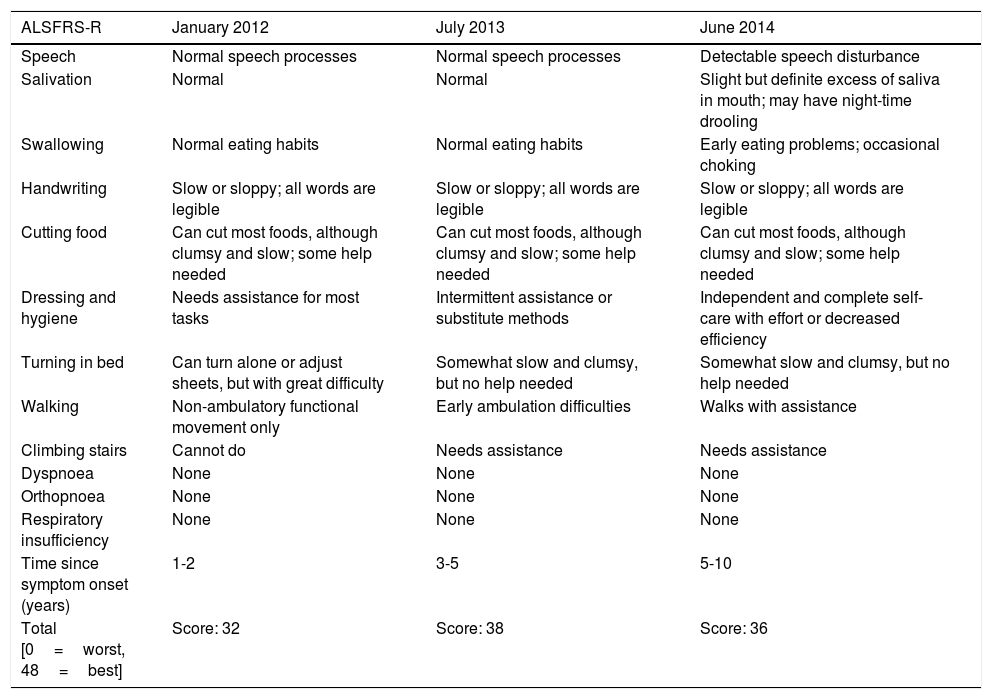
Changes in the patient's ALS Functional Rating Scale (ALSFRS-R) score.
| ALSFRS-R | January 2012 | July 2013 | June 2014 |
|---|---|---|---|
| Speech | Normal speech processes | Normal speech processes | Detectable speech disturbance |
| Salivation | Normal | Normal | Slight but definite excess of saliva in mouth; may have night-time drooling |
| Swallowing | Normal eating habits | Normal eating habits | Early eating problems; occasional choking |
| Handwriting | Slow or sloppy; all words are legible | Slow or sloppy; all words are legible | Slow or sloppy; all words are legible |
| Cutting food | Can cut most foods, although clumsy and slow; some help needed | Can cut most foods, although clumsy and slow; some help needed | Can cut most foods, although clumsy and slow; some help needed |
| Dressing and hygiene | Needs assistance for most tasks | Intermittent assistance or substitute methods | Independent and complete self-care with effort or decreased efficiency |
| Turning in bed | Can turn alone or adjust sheets, but with great difficulty | Somewhat slow and clumsy, but no help needed | Somewhat slow and clumsy, but no help needed |
| Walking | Non-ambulatory functional movement only | Early ambulation difficulties | Walks with assistance |
| Climbing stairs | Cannot do | Needs assistance | Needs assistance |
| Dyspnoea | None | None | None |
| Orthopnoea | None | None | None |
| Respiratory insufficiency | None | None | None |
| Time since symptom onset (years) | 1-2 | 3-5 | 5-10 |
| Total [0=worst, 48=best] | Score: 32 | Score: 38 | Score: 36 |
We began treatment with Lioresal® in combination with stretches in the therapeutic gymnasium and hydrotherapy. Lioresal® was not tolerated, and gait function did not change with the physiotherapy. We therefore proposed attempting to control spasticity with local BTX injections. In February 2013, 50 units of BTX were injected into the left and right solei. The patient continued with the physiotherapy programme, progressively performing stretches, muscle toning, balance control exercises, and gait re-education. One month later, there was a significant improvement in the patient's spasticity, resulting also in a functional improvement. The patient was able to walk indoors with one cane and short distances outdoors with 2. Falls had become less frequent. This made the patient feel safer and enabled him to improve his physical condition by performing his pool and toning exercises, according to the instruction he had received, outside the setting of the therapeutic gymnasium (ALSFRS-R, Table 1). Examination revealed improved strength and spasticity; clonus had resolved. The patient completed the 10-Metre Walk Test in 20seconds, taking 21 steps.
Four months later (June 2013) and at subsequent sessions, the patient received injections of 50 units of BTX, distributed between the left and right solei and gastrocnemii. The patient's current ALSFRS-R score is 36 due to the appearance of bulbar symptoms (Table 1). During this time, the patient has continued walking, although he currently requires 2 canes to walk both indoors and outdoors.
Numerous studies have demonstrated the effectiveness and safety of BTX in treating sialorrhoea in patients with ALS, with no cases describing a global worsening of the disease.3–5 We found only 2 references to BTX use for the treatment of spasticity associated with ALS, and none for PLS. Kent et al.6 present the case of a patient who responded poorly to the treatment, with remote secondary effects (dysphagia and dysarthria). Deffontaines-Rufin et al.7 describe a series of 39 patients with ALS, over half of whom displayed improved spasticity following an initial injection of BTX; however, this was reported in a communication delivered at a congress, and clinical data are unavailable. One of these patients developed transient respiratory symptoms; no other secondary effects were reported. There is no physiological basis to contraindicate the use of BTX in patients with PLS; there should therefore be no worsening of the disease if the proper dose is administered.8–10
In the light of the above, and given our patient's poor response to medical treatment and physiotherapy, we decided to begin treatment with local injections of BTX, aiming to achieve functional results. Infiltrations were administered periodically for over 2 years, achieving the desired functional objective and with no secondary effects.
The improvement in such an important function as gait, and being able to continue partaking in social activities, have afforded our patient a significantly higher quality of life, despite the progression of his condition, which is following the expected course for PLS.
FundingThis study has received no funding of any kind.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Alabajos Cea A, Máñez Añón I, Roda Alcayde C, Vázquez Costa JF, Guevara Salazar M. Mejoría de la espasticidad en esclerosis lateral primaria tras la inyección de toxina botulínica. A propósito de un caso. Neurología. 2018;33:131–133.