Given that stroke is currently a serious problem in the population, employing more reliable and objective techniques for determining diagnosis and prognosis is necessary in order to enable effective clinical decision-making. EEG is a simple, low-cost, non-invasive tool that can provide information about the changes occurring in the cerebral cortex during the recovery process after stroke. EEG provides data on the evolution of cortical activation patterns which can be used to establish a prognosis geared toward harnessing each patient's full potential. This strategy can be used to prevent compensation and maladaptive plasticity, redirect treatments, and develop new interventions that will let stroke patients reach their new maximum motor levels.
Actualmente, el ictus representa un problema grave en la población, por lo que es necesario emplear técnicas de evaluación de diagnóstico y pronóstico más fiables y objetivas que permitan una eficaz toma de decisiones clínicas. La electroencefalografía es una herramienta sencilla, de bajo coste y no invasiva, que puede proporcionar conocimiento acerca de los cambios que acontecen en el córtex cerebral en el proceso de recuperación tras un ictus. Proporciona datos de evolución de los patrones de activación corticales, que permiten establecer un pronóstico para aprovechar el máximo potencial de las personas evitando compensaciones y plasticidad maladaptativa, redireccionar los tratamientos y desarrollar nuevas intervenciones con el objetivo de alcanzar el nuevo máximo nivel motor de los pacientes que han sufrido un ictus.
Stroke is the second most frequent cause of death in the Spanish general population, and the leading cause of death in women.1 In 2002, the cumulative incidence rate per 100000 population in Catalonia among people older than 24 was 218 new cases in men and 127 new cases in women.2 It also represents the main cause of disability and generates a significant healthcare and social expenditure.1
Eighty per cent of the subjects who suffer a stroke survive the acute phase; 6 months later, 85% of them still present motor disorders as well as neurological deficits, communication disorders, cognitive disorders, and disorders in visuospatial perception.3,4
Rehabilitation is a therapeutic process aimed at maximising the patient's physical, psychological, and social potential.5 Although many subjects partially recover lower limb motor function, most of them cannot perform activities of daily living with the affected upper limb (UL) after months of rehabilitation.6 Functional recovery of the UL is observed in less than 15% of the subjects.7 Impairment of the UL significantly limits the patient's level of activity and both physical and social interactions.8
The different sequelae in the UL with an impact on activities of daily living have been clinically assessed using the available standardised scales. Considering the results on these scales, the greatest potential for recovery is reached between the second and fifth months after stroke. Prospects for functional recovery after 6 months are considered limited.9,10 However, these scales demonstrate a limited capacity for detecting less sensitive changes that are nonetheless necessary for measuring outcomes of stroke patients. In addition, they are subject to rater subjectivity.11 Therefore, we suggest combining imaging techniques with standard scales to obtain more accurate and objective assessments of recovery.12
New evaluation toolsRecovery after stroke constitutes one of the main enigmas of the neurosciences. Motor rehabilitation patterns are heterogeneous, and the only prognostic indicators to be used until recently were the degree of motor impairment13,14 and the cardioembolic stroke subtype. The latter represents 14% to 30% of ischaemic strokes, with cerebral infarct being the most severe due to its high recurrence and in-hospital mortality rates during the acute phase.15 However, a significant effort has been made to research changes to the central nervous system after stroke in order to establish more accurate prognoses and develop new strategies promoting motor rehabilitation.16
In this line, some neuroimaging techniques have undergone significant developments: functional magnetic resonance imaging (fMRI), transcranial magnetic stimulation (TMS), and positron emission tomography (PET).
Based on different studies which used these assessment techniques, researchers have managed to determine the changes induced by the application of new and groundbreaking rehabilitation approaches, such as robotics,17 music therapy,18 and TMS.19 Furthermore, neuroplastic changes have been proven to play an important role in the recovery after stroke. According to the scientific literature, changes contributing to motor rehabilitation can be found in both hemispheres. On the one hand, various authors have shown that the presence of motor evoked potentials (MEPs) triggered by TMS is a predictor of motor rehabilitation.20,21 On the other hand, some authors suggest that the plasticity associated with compensatory movements may contribute to maladaptive plasticity.22,23 The studies by Talelli et al.24 and Stinear et al.25 used TMS to analyse ipsilesional corticospinal tract projections. In both studies, TMS data was helpful in predicting motor rehabilitation and adapting rehabilitation strategies. Chelette et al.26 used TMS to analyse the changes caused by rehabilitation of the affected UL in chronic stroke patients. Between 7 and 20 months after stroke, researchers observed an increased area of activation in the contralesional hemisphere, followed by a decrease in the same after 21 months. However, ipsilesional motor maps remained stable between 7 and 13 months, and their areas started to expand between 13 and 31 months after stroke, especially at 20 to 21 months. At the end of the study, there was a 10% improvement on scores on standardised functional tests (Fugl-Meyer Assessment and Wolf Motor Function Test). This underscores the association between neuroplastic changes and motor rehabilitation. These findings suggest that a lack of MEPs during the earliest stages does not indicate a poor prognosis, that both hemispheres contribute to recovery during different stages, and that recovery after stroke is more dynamic than was once believed.26 Cramer et al.27 observed that decreased activity in fMRI studies of the ipsilesional cortex during movements of the paretic hand was actually associated with greater functional gains 6 weeks after rehabilitation in chronic-phase stroke patients. Ward et al.28 examined the degree of recovery in chronic stroke patients and the recruitment pattern of brain regions during a handgrip task as measured using fMRI. They observed that patients with poorer outcomes activated more areas in the contralesional hemisphere than did controls. Also, patients with better recovery showed relatively normal patterns. Therefore, there seems to be a negative association between functional outcomes and the degree of activity in the ipsilateral hemisphere during a motor task. Jang et al.29 used diffusion tensor tractography (DTT) and TMS to evaluate the corticospinal tract of a patient who showed motor apraxia at 30 months after stroke. After one month of rehabilitation and dopaminergic treatment, the patient's motor recovery increased by 22% (Motricity Index). However, no modifications were observed in the corticospinal tract fibres according to the motor evoked potential parameters measured using DDT and TMS. In this way, they demonstrated that the motor rehabilitation was mainly linked to improvement of motor apraxia and that the corticospinal tract was not impaired.29
The 2 studies published in 2008 and 2010 by Formaggio et al.30,31 combined fMRI and electroencephalography (EEG) to study the blood-oxygenation-level dependent signal and changes in brain oscillatory activity in motor areas associated with physiological and pathological events. These studies reported a significant correlation with both finger movements and motor imagery tasks as they observed a decreased event-related desynchronisation (ERD) in the α and β bands in the contralateral hemisphere during performance of the motor or motor imagery task. This decrease is associated with blood-oxygen-level dependent activation in the same areas. This combination provided useful information helping identify the regions contributing to changes in electrical responses more accurately.
In summary, neuroimaging techniques have important implications in terms of diagnosis and clinical decision-making.29 In general, evidence shows that the analysis of cortical activity using these tools expands our knowledge about motor rehabilitation mechanisms and may help us design new treatment strategies and sensitive outcome measures. However, these tools are not always accessible to everyone, since they are expensive and have contraindications and limited availability.32
EEG as a tool for diagnosing and assessing stroke patientsContrary to predictions issued by many authors, the introduction of neuroimaging techniques has not relegated EEG to a second plane; it is still widely used in neurosciences since it provides a model for real-time brain functioning.
EEG is commonly used to diagnose vascular epilepsy secondary to stroke in adults; it lets physicians study the characteristics and clinical outcomes of patients, as well as analyse the effectiveness of different antiepileptic treatments.33 Furthermore, EEG is typically used as a monitoring method during carotid endarterectomy to detect cerebral ischaemia, which causes an ipsilateral voltage attenuation.34 EEG is also performed to assess coma and pseudocoma states and differentiate them from other states of consciousness,35 as well as to confirm brain death.36
Characterising and studying electrophysiological signals of the central nervous system with EEG equipment is also well established because of different features which make it an optimal clinical evaluation tool. The technique is non-invasive and equipment is portable and inexpensive.37 Several authors have used it to assess cortical functional reorganisation after stroke.38–43 EEG studies have shown different patterns of movement-related electric brain activity and different neuronal mechanisms related to motor control.44 Furthermore, EEG has shown that activity-dependent brain plasticity takes place throughout an individual's lifetime. As such, analysing the development of plasticity may be a useful method for determining the functional sequelae of stroke and how they evolve.45 Considering this potential, an updated review should be conducted to analyse and compile available evidence on the use of EEG to study changes in the cerebral cortex in stroke patients. Additionally, we will attempt to answer the following unresolved questions: could EEG be a useful tool for increasing knowledge of the patterns of cortical changes after stroke? Could it constitute a diagnostic method in clinical practice? Could it be used as an objective tool for determining stroke patients’ outcomes? And lastly, can EEG shed light on the effectiveness of traditional rehabilitation interventions?
DevelopmentCharacteristics of movement-related cortical activity measured with EEG: slow potentials and cortical oscillatory activityBrain electrical activity is the consequence of ion currents generated by cellular biochemical processes; this theory was promulgated by Richard Caton in 1875.37,46 These currents manifest as oscillations or fluctuations of potentials in neuronal groups.
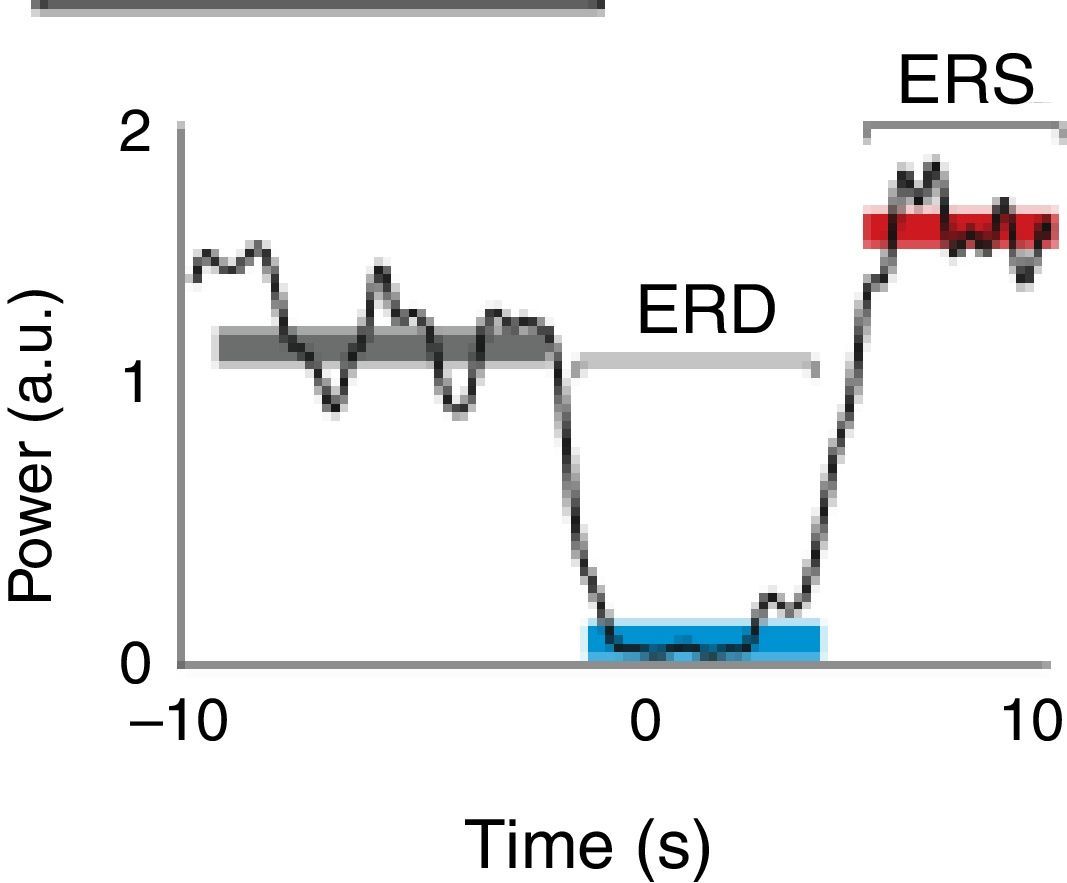
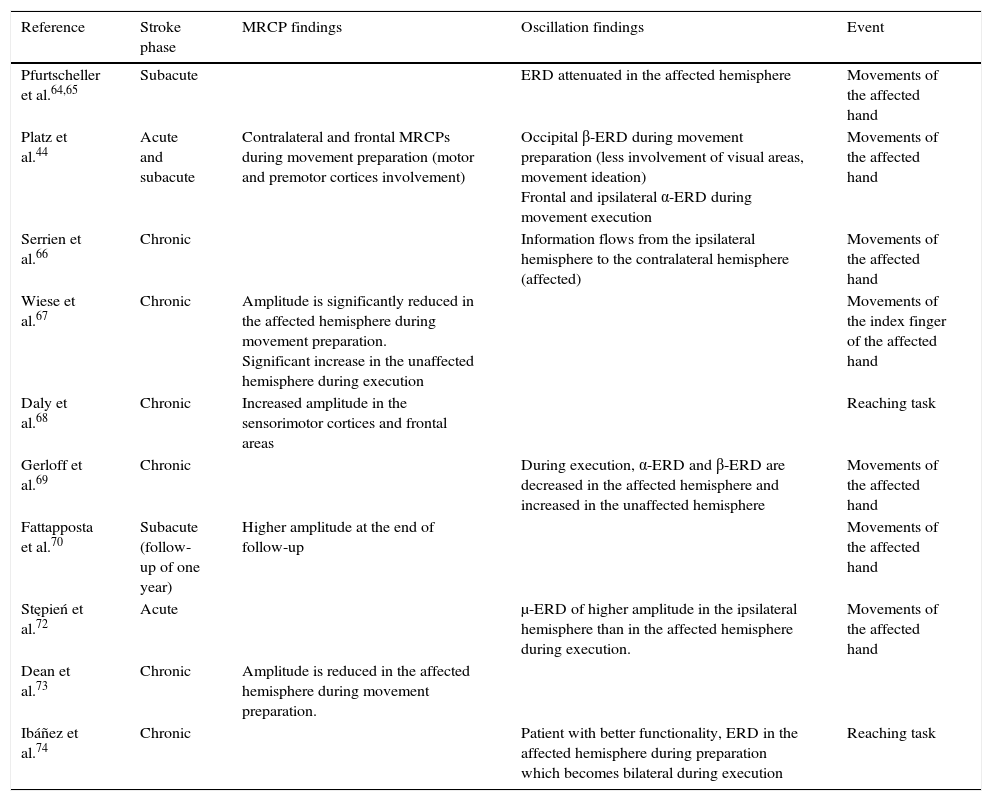
A motor event generates various responses within the neuronal structures of the brain. On the one hand, it provokes slow movement-related cortical potentials (MRCP) which represent the electroencephalographic evidence of the participation of motor cortex during movement and conscious preparation for the intentional movement.47 On the other hand, it causes changes in the amplitude of cortical oscillatory activity. In general terms, there are 2 types of cortical oscillations related to motor behaviour: the α and β rhythms.48–51 Motor-related changes in the α and β frequencies result in ERD or event-related synchronisation (ERS).52 The ERS represents an increase in the amplitude of α and β frequencies, whereas ERD represents a decrease (Fig. 1).47
Characteristic ERD/ERS pattern in a healthy subject. ERD/ERS pattern in a healthy subject before a voluntary and self-initiated reaching movement of the right arm. Movement onset is at t=0. The patterns were obtained using the techniques described by Ibáñez et al.74
The α frequency is the main background rhythm of normal EEGs in adults.46 It is measured with the patient awake and with eyes closed. This activity, whose maximum amplitude is recorded in the occipital regions, occurs during wakefulness and periods of relaxation and shows reactivity to eye closure, which is attenuated with opening of the eyes.37,46 By definition, rhythm frequency is 8 to 13Hz.37 Frequency amplitude is usually symmetrical, but it may often be higher in the non-dominant hemisphere. If the patient is not relaxed, α waves may not occur, and their absence in one hemisphere is a pathological sign.46
According to the cortical region in question, α frequency presents specific characteristics and has a set nomenclature. Mu (μ) rhythm is located around the central sulcus (primary sensorimotor cortices).37 Mu rhythms present a particular reactivity, since they occur at rest and resolve with contralateral limb movements. They are particularly pronounced around the area of the motor cortex associated with the hand and resolve when making a fist, or even during motor imagery tasks.50 They are regarded as intrinsic activity of the central region and are thus very useful in the functional evaluation of stroke patients.53
Low amplitude β activity presents frequencies of 14 to 30Hz and indicates mental activity and attention.37 Maximum β amplitude is observed in the fronto-central regions. The most significant finding in the analysis of β activity is interhemispheric asymmetry. A decrease in amplitude in one of the hemispheres tends to indicate presence of alterations.46
Amplitude of oscillatory activity decreases when frequency increases. Therefore, μ rhythm with a frequency of 8 to 13Hz has a higher amplitude than β activity. Furthermore, oscillation amplitude is proportional to the number of synchronised active neurons, so that lower oscillations involve more neurons than faster oscillations.52
In general, sensory stimulation and the actual movement or motor imagery task will result in ERD or ERS of μ and β rhythms. Movement is preceded by ERD in healthy subjects, and as the movement is carried out, variations occur in the synchronisation of cortical rhythms (ERS). Movement offset is followed by a β-ERS generated in the precentral region, which reflects a deactivation of motor cortex. This occurs in the first second after termination of voluntary movement, when the μ rhythm still presents a low-amplitude ERD. Bilateral movements cause bilateral activation.54
μ-ERD does not depend on movement duration or localisation, and it is the same for movements of the index, the thumb, or even the hand. This suggests that μ-ERD is due to a non-specific preactivation of neurons in the motor areas.52 In this line, studies with electrocorticography have shown that β-ERD is more discrete than μ-ERD. Likewise, it is located in the prerolandic cortex, a more anterior area (motor area), whereas μ-ERD is located in the postrolandic area (primary somatosensory cortex).55
ERD and ERS can take place simultaneously, both in α activity and β activity. In the first case, during perception of a visual stimulus, occipital α-ERD and μ-ERS of central rhythms can be observed in EEG recordings. However, occipital α-ERS and central μ-ERD occur during self-initiated voluntary movements.56 A possible explanation would be the involvement of thalamic structures which activate cortical structures engaged in some movements while deactivating others not involved in the task. At the β activity level, ERD and ERS take place simultaneously during a hand motor imagery task, which activates a contralateral ERD and ipsilateral ERS.52
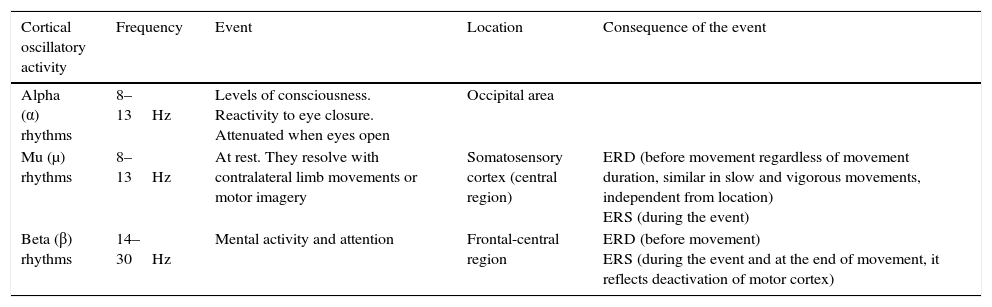
For ERD/ERS calculations using EEG at movement onset, variations of power in μ and β bands are expressed with reference to a value calculated in a reference time period, always taken at rest, long before the movement is to be performed, in order to remain as neutral as possible (Tables 1 and 2).57
Characteristics of cortical motor rhythms.
| Cortical oscillatory activity | Frequency | Event | Location | Consequence of the event |
|---|---|---|---|---|
| Alpha (α) rhythms | 8–13Hz | Levels of consciousness. Reactivity to eye closure. Attenuated when eyes open | Occipital area | |
| Mu (μ) rhythms | 8–13Hz | At rest. They resolve with contralateral limb movements or motor imagery | Somatosensory cortex (central region) | ERD (before movement regardless of movement duration, similar in slow and vigorous movements, independent from location) ERS (during the event) |
| Beta (β) rhythms | 14–30Hz | Mental activity and attention | Frontal-central region | ERD (before movement) ERS (during the event and at the end of movement, it reflects deactivation of motor cortex) |
ERD, event-related desynchronisation; ERS, event-related synchronisation.
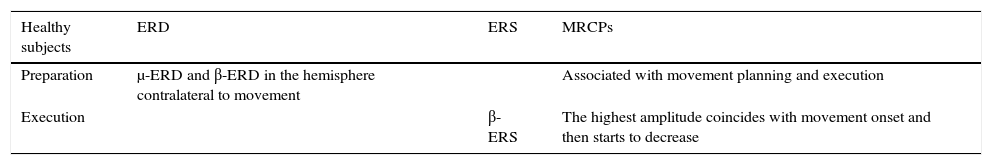
Characteristics of motor cortex oscillations in healthy subjects during movement planning and execution.
| Healthy subjects | ERD | ERS | MRCPs |
|---|---|---|---|
| Preparation | μ-ERD and β-ERD in the hemisphere contralateral to movement | Associated with movement planning and execution | |
| Execution | β-ERS | The highest amplitude coincides with movement onset and then starts to decrease |
μ-ERD, β-ERD, event-related desynchronisation in the μ and β frequency bands; β-ERS, event related synchronisation in the β frequency band.
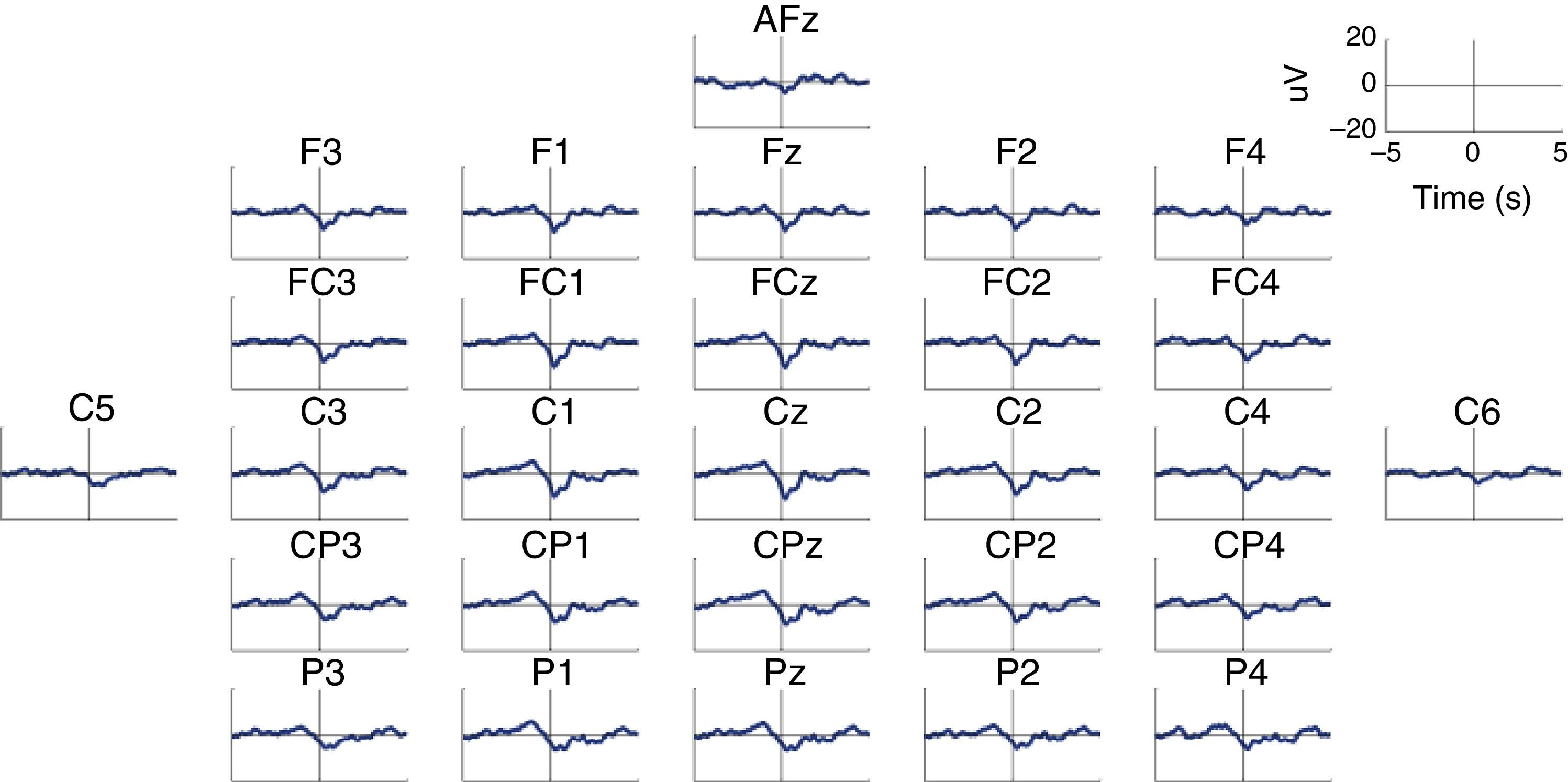
MRCP are especially relevant in the assessment of cortical activation patterns, since they are associated with movement planning and execution. This being the case, Bereitschaftspotential (BP) will require a special mention as they are considered the initial stage of MRCPs.41,47,58 This parameter, introduced by Kornhuber and Deecke in 1964, is an electrical signal corresponding to the supplementary motor area, which activates before the primary motor area.59 This is a slow and negative potential which reflects the preparatory activity and whose maximum peak coincides with voluntary movement.60 BP has 2 components: an early component initiating 2seconds before the movement starts, and a late component, which appears 400ms before the movement itself. Early BP is maximal at the midline of the central parietal area, and widely and symmetrically distributed across both hemispheres. Late BP is much larger over the right central region (contralateral to the movement) (Fig. 2). In general terms, when a movement pattern is present, the BP commonly starts much earlier, compared to movements executed in more natural conditions.47
Characteristic BP (Bereitschaftspotential) pattern in a healthy subject. The figure includes BP patterns obtained by 28 EEG channels in a healthy subject who performed a voluntary and self-initiated reaching movement of the right arm. Movement onset is at t=0. The patterns were obtained using the techniques described by Ibáñez et al.74
Although the mechanisms generating BP are yet to be understood, these potentials are modulated by the way the task is performed. Multiple factors influence the type of BP: the preparatory state, movement selection (free or repeated pattern), learning acquisition, movement repetition rhythm, practice, difficulty, speed, and pathological lesion in brain structures, among others.41,50 Neurological changes modify the time of appearance and amplitude of MRCPs; as such, they can be useful in diagnosis and follow-up.61
Characteristics of cortical activity in stroke patients measured with EEGStudies with EEG by Pfurtscheller62–65 in the 1980s revealed that the dynamic amplitude of neural oscillations represents an indicator of neuronal activation during movement preparation and execution. Pfurtscheller et al.64 found an attenuation in ERD in the affected hemisphere of stroke patients compared to the unaffected hemisphere during movements of the affected hand.
In the last 10 years, numerous studies have assessed cortical activity in stroke patients with EEG to analyse cortical reorganisation throughout the course of their recovery.
Platz et al.44 used EEG to study MRCPs and ERD in α and β frequencies in subjects with acute and subacute hemiparesis (3-20 weeks after stroke). They analysed the preparation and execution stages during self-initiated and repeated movements of the affected hand. The authors observed that in healthy controls, slow potentials extended from the central electrode throughout the cortex during movement preparation, to later become more obvious in the contralateral hemisphere during movement execution. They also verified that ERD started in the contralateral hemisphere and extended to the ipsilateral hemisphere, with bilateral distribution during execution. However, patients showed significant differences with regard to control subjects, especially during movement preparation. The authors observed a more contralateral and frontal distribution of MRCPs, which may suggest more extensive involvement of motor and premotor cortices in motor planning in stroke patients. In terms of cortical oscillatory activity, β-ERD was more localised in the occipital area in patients than in controls during movement preparation. This finding may suggest a higher involvement of visual areas (movement ideation). Furthermore, an increase in the α-ERD in the frontal and ipsilateral area of the cortex was observed during execution. This is associated with an increased need for concentration on the task and compensatory measures in the unaffected hemisphere.44
Serrien et al.66 described the ipsilateral hemisphere as playing a functional role during movement of the affected hand in chronic stroke patients. For the execution of the same task, somatosensory and motor cortical recruitment originates in the contralateral hemisphere in control subjects and well-recovered patients, and in the ipsilateral hemisphere in patients with a poorer recovery. They established that the more extensive the involvement of contralateral areas, the better the prognosis for functional recovery.
Wiese et al.67 compared MRCPs from chronic stroke patients and healthy control subjects as they moved the index finger on the affected hand. In the patient group, the component directly preceding movement onset was significantly reduced in the lesioned hemisphere. The researchers observed a significant increase in MRCP activity in the contralesional hemisphere during the movement itself. The presence of this early MRCP component, which is generated by the primary motor cortex, suggests that stroke is accompanied by damage to these structures in the affected hemisphere.
Daly et al.68 used EEG to study MRCPs in 10 patients with chronic stroke in order to determine motor planning time of a reaching task. They observed a high MRCP amplitude in the sensorimotor cortex as well as in the frontal areas, which reflects a lower degree of automatisation of the task and the need for compensatory strategies to perform the movement. Gerloff et al.69 showed that α-ERD and β-ERD were reduced in the injured hemisphere of stroke patients in the chronic stage, and increased in the unaffected hemisphere during movements of the affected hand. This suggests a shift in compensatory functional connectivity towards the contralesional hemisphere.
Fattapposta et al.70 followed up on 9 acute stroke patients during 1 year by taking a total of 4 measurements at 0, 3, 9, and 12 months. They conducted a study which assessed the pre-programming of movements taking place during execution of a simple task. Patients had a significantly lower BP amplitude than control subjects in the first recording, but amplitudes increased in the subsequent 3 recordings. This result may be associated with early neuronal plasticity and other processes involved in neurorepair, such as angiogenesis and neurogenesis, which occur in the early stages after the lesion.71 However, regaining automatisation of simple tasks would take longer. This provides an explanation for a habituation phenomenon in which the motor task becomes memorised. Mobility scales (Nine Hole Peg Test and Motricity Index Test) showed significant improvements in motor skills throughout the process. The authors stated that patients recovered a relatively good motor skill level, while lacking an adequate recovery of attention and control abilities in the skilled motor act, thereby requiring development of motor compensation strategies. These associative functions are crucial for automating skilled movements, considering that most of daily motor practice is automated.
Stępień et al.72 used EEG to assess ERD in stroke patients in the acute stage (less than 8 days of progression). They observed that movements of the affected hand caused a stronger α-ERD in the sensorimotor area (pericentral area) of the ipsilateral hemisphere than in the ipsilesional hemisphere. Furthermore, they reported that α-ERD amplitude was preserved in the affected hemisphere during movements of the unaffected hand; in contrast, activity of the affected hand caused a decrease in the frequency of α oscillations in the affected hemisphere. The ERD is sensitive to the recovery of networks after stroke. A relatively well-preserved ERS in the affected hemisphere during movements of the unaffected hand seems to indicate good potential for recovery, and therefore a positive prognosis. This also suggests that bilateral rehabilitation during at least the first stages of recovery is also important.72
Dean et al.73 studied motor planning in chronic stroke patients and observed reduced MRCP amplitude in the affected hemisphere, especially during preparation of two-handed movements. This shows altered anticipatory attention activity and motor processing in those patients with poorer functional recovery.
Ibáñez et al.74 used EEG to analyse the temporal precision of intention to perform a movement in 3 chronic stroke patients. They used ERD as a parameter to measure oscillatory activity. The authors observed that one subject presented bilateral ERD, while another presented a significant predominance of the central area; both situations were indicative of cortical reorganisation. The third subject presented activation of the regions contralateral to the affected limb that became bilateral once the patient began executing movement. This patient tested at the highest functional level on the UL (Table 3).
Summary of scientific evidence using EEG to assess motor cortex in stroke patients.
| Reference | Stroke phase | MRCP findings | Oscillation findings | Event |
|---|---|---|---|---|
| Pfurtscheller et al.64,65 | Subacute | ERD attenuated in the affected hemisphere | Movements of the affected hand | |
| Platz et al.44 | Acute and subacute | Contralateral and frontal MRCPs during movement preparation (motor and premotor cortices involvement) | Occipital β-ERD during movement preparation (less involvement of visual areas, movement ideation) Frontal and ipsilateral α-ERD during movement execution | Movements of the affected hand |
| Serrien et al.66 | Chronic | Information flows from the ipsilateral hemisphere to the contralateral hemisphere (affected) | Movements of the affected hand | |
| Wiese et al.67 | Chronic | Amplitude is significantly reduced in the affected hemisphere during movement preparation. Significant increase in the unaffected hemisphere during execution | Movements of the index finger of the affected hand | |
| Daly et al.68 | Chronic | Increased amplitude in the sensorimotor cortices and frontal areas | Reaching task | |
| Gerloff et al.69 | Chronic | During execution, α-ERD and β-ERD are decreased in the affected hemisphere and increased in the unaffected hemisphere | Movements of the affected hand | |
| Fattapposta et al.70 | Subacute (follow-up of one year) | Higher amplitude at the end of follow-up | Movements of the affected hand | |
| Stępień et al.72 | Acute | μ-ERD of higher amplitude in the ipsilateral hemisphere than in the affected hemisphere during execution. | Movements of the affected hand | |
| Dean et al.73 | Chronic | Amplitude is reduced in the affected hemisphere during movement preparation. | ||
| Ibáñez et al.74 | Chronic | Patient with better functionality, ERD in the affected hemisphere during preparation which becomes bilateral during execution | Reaching task |
ERD, event-related desynchronisation; ERS, event-related synchronisation; MRCPs, movement-related cortical potentials; α, β, and μ, frequency bands which show the oscillations.
There is little evidence on using EEGs for diagnostic purposes. Pfurtscheller et al.62 conducted a study with a large sample of patients (n=50) to assess cortical oscillatory activity during movements of the affected hand and predict the location of the lesion in the territory of the left middle cerebral artery. Results suggested that an increased rhythm in the ipsilateral hemisphere, together with a symmetric ERD, indicates a deep lesion with a probability of 95% (and a superficial lesion with a probability of 5%). Hemispheric μ amplitude symmetry and asymmetric ERD indicate superficial cortical ischaemia with a probability of 81%; an ipsilaterally attenuated μ rhythm accompanied by an asymmetric or abolished ERD indicates an infarct extending broadly over the entire arterial territory.
ConclusionsEEG-based analysis of movement preparation and execution by comparing stroke patients and healthy controls is useful for determining diagnoses and predicting outcomes. Near-normal cortical activation is associated with better functionality, and progression toward normal characteristics of cortical activation suggests that therapy has been effective. Beyond a certain progression time, patients are excluded from rehabilitation programmes after having been assessed using standardised scales. We need tools that are sensitive to functional changes and thus useful for assessing patient outcomes, effectiveness of interventions, and the real prognosis to facilitate decision-making in clinical practice.
EEG is an inexpensive, non-invasive, simple, portable, and potentially useful tool for diagnosis and prognosis. It provides insight into neuroplastic changes in terms of motor recovery, thereby making it possible to assess the effectiveness of interventions and in turn facilitating clinical decision-making. It also lets us maximise the recovery potential of patients with motor disability by preventing maladaptive plasticity, and developing new interventions intended to modulate cerebral function. An early prediction of the type of motor rehabilitation in question may help to determine the time, duration, and objectives of therapeutic protocols that will be tailored for each patient with neuroplasticity in mind.
A promising new line of research would entail studying motor cortex behaviour using surface EEG recordings, taking into account lesion type and time of progression, in a large sample of patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Monge-Pereira E, Molina-Rueda F, Rivas-Montero FM, Ibáñez J, Serrano JI, Alguacil-Diego IM, et al. Electroencefalografía como método de evaluación tras un ictus. Una revisión actualizada. Neurología. 2017;32:40–49.