Coronary artery disease (CAD) is the leading cause of myocardial ischemia, contributing significantly to global cardiovascular mortality. Coronary artery bypass grafting (CABG) remains a cornerstone in the management of multivessel CAD.
Our objective was to evaluate the effects of complete coronary artery surgical revascularization on left ventricular function in patients with ischemic heart disease.
This prospective cross-sectional study was conducted at the Iraqi Center for Cardiac Diseases, Baghdad, from July to December 2019. Fifty patients who underwent CABG were included. Preoperative, early postoperative (2 weeks), and late postoperative (1 year) echocardiographic evaluations were performed. Echocardiographic data were obtained using the biplane modified Simpson’s method. Variables analyzed included left ventricular ejection fraction (LVEF), left ventricular internal diameters, and wall thicknesses.
No significant changes in LVEF or ventricular dimensions were observed within two weeks post-surgery. However, at one-year follow-up, patients with preoperative EF <50% (n=15) demonstrated a 10% increase in LVEF. Mild changes in left atrial diameter, septal wall thickness, and ventricular dimensions were also observed.
In conclusion, Patients with impaired preoperative systolic function showed significant improvement in LVEF following complete surgical revascularization. The absence of preoperative myocardial viability assessment and incomplete adherence to guideline-directed therapy were noted limitations.
La enfermedad arterial coronaria (EAC) es la principal causa de isquemia miocárdica y contribuye significativamente a la mortalidad cardiovascular global. La cirugía de revascularización coronaria (CABG) sigue siendo fundamental en el tratamiento de la EAC multivaso.
Evaluar los efectos de la revascularización quirúrgica coronaria completa sobre la función ventricular izquierda en pacientes con cardiopatía isquémica.
Este estudio transversal prospectivo se realizó en el Centro Iraquí de Enfermedades Cardíacas, Bagdad, de julio a diciembre de 2019. Se incluyeron cincuenta pacientes sometidos a CABG. Se realizaron evaluaciones ecocardiográficas preoperatorias, postoperatorias tempranas (2 semanas) y postoperatorias tardías (1 año). Los datos ecocardiográficos se obtuvieron mediante el método de Simpson modificado biplanar. Las variables analizadas incluyeron la fracción de eyección del ventrículo izquierdo (FEVI), los diámetros internos del ventrículo izquierdo y el grosor de la pared.
No se observaron cambios significativos en la FEVI ni en las dimensiones ventriculares en las dos semanas posteriores a la cirugía. Sin embargo, tras un año de seguimiento, los pacientes con una FE preoperatoria <50% (n=15) mostraron un aumento del 10% en la FEVI. También se observaron cambios leves en el diámetro auricular izquierdo, el grosor de la pared septal y las dimensiones ventriculares.
Los pacientes con deterioro de la función sistólica preoperatoria mostraron una mejoría significativa de la FEVI tras la revascularización quirúrgica completa. La ausencia de una evaluación preoperatoria de la viabilidad miocárdica y la adherencia incompleta al tratamiento recomendado por las guías clínicas fueron limitaciones observadas.
Coronary artery disease (CAD) remain the predominant cause of myocardial ischemia, primarily due to subintimal atheromatous plaque deposition leading to luminal narrowing or occlusion. CAD continues to be a major contributor to morbidity and mortality worldwide.1
CABG and percutaneous coronary intervention (PCI) are primary revascularization strategies. Elective CABG carries a mortality rate of approximately 1.7%. Common complications include bleeding, renal impairment, heart failure, and cerebrovascular events. Despite these risks, CABG provides substantial benefits in symptom relief, functional improvement, and survival, particularly in patients with multivessel disease.1
Indications for CABGCABG is recommended based on clinical symptoms, angiographic findings, and left ventricular function. Indications include:
- •
Significant left main coronary artery disease.
- •
Severe proximal LAD disease with multivessel involvement.
- •
Symptomatic two- or three-vessel disease.
- •
Angina refractory to optimal medical therapy.
- •
Ischemic cardiomyopathy with viable myocardium.
- •
Refractory ischemia following myocardial infarction or failed PCI.2–3
Complete revascularization lacks a universal definition. It generally implies the grafting of all significant coronary stenosis (>70%) supplying viable myocardial territories. Functional completeness considers both anatomical severity and perfusion status. In this study, complete revascularization refers to bypassing all significant lesions with good distal runoff, excluding non-dominant RCA or small-caliber branches.4
ObjectivesTo assess the effect of complete coronary artery surgical revascularization on left ventricular function in patients with ischemic heart disease and varying degrees of preoperative ventricular function.
MethodsStudy design and settingA prospective cross-sectional study was conducted at the Iraqi Center for Cardiac Diseases from July to December 2019.
ParticipantsFifty patients undergoing CABG during the study period were included. Patients with prior cardiac surgery, valve replacement, or permanent pacemaker implantation were excluded.
Data collectionData were collected preoperatively, at two weeks, and at one year postoperatively. Clinical data included comorbidities, medications, and surgical details. Echocardiographic data were collected using GE Vivid E9. LVEF was assessed via the biplane modified Simpson’s method using apical four- and two-chamber views. Left ventricular diameters and wall thicknesses were measured using 2D imaging. Left atrial volume was calculated via the biplane method of disks.
Doppler ultrasound assessed carotid and peripheral arterial territories for vascular disease. CAD was defined based on angiographic stenosis >70% and clinical ischemia.
Surgical techniqueAll patients underwent median sternotomy and cardiopulmonary bypass. Grafts included LIMA and saphenous veins. Grafts were placed distal to significant lesions to ensure myocardial perfusion.
Statistical analysisSPSS v25 was used. Normality was assessed using the Shapiro-Wilk test. Continuous variables were expressed as mean ± standard deviation. Paired t-tests were used for intra-group comparisons. A p-value <0.05 was considered statistically significant.
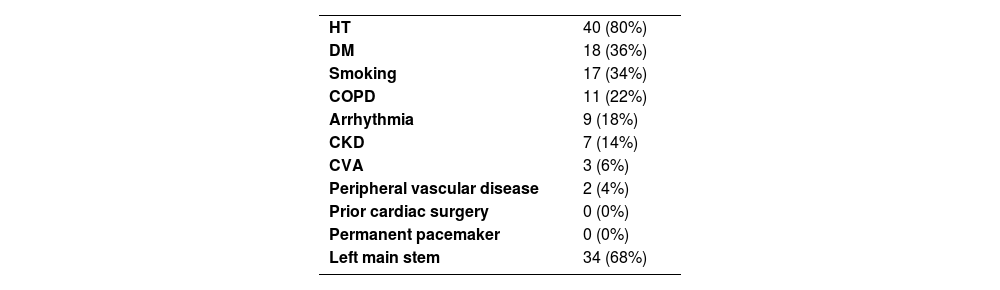
ResultsDemographics and risk factorsThe mean age was 57.1±7.7 years; 78% were male. Mean BMI was 29.3±3.7 kg/m2. Hypertension was present in 80%, diabetes in 36%, and 34% were smokers.
Patients with a history of smoking were seventeen (34%), while patients with a history of chronic obstructive pulmonary disease (COPD) were eleven (22%).
By history and laboratory findings, seven patients had chronic kidney disease (CKD) (14%) while forty-three (86%) had a normal renal function test and had no history of kidney disease.
By electrocardiography, nine patients had arrhythmia (18%) while forty-one (82%) patients had sinus rhythm and no history of arrhythmia.
Three patients with a history of cerebrovascular accident (CVA) (6%) and two patients with a history of Peripheral vascular disease (4%).
By angiography, patients with left main stem diagnosed preoperatively by angiography were thirty-four (68%) as shown in Table 1.
Regarding the timing of surgery, twenty-seven patients (54%) were elective cases and twenty-three patients (46%) were emergency cases.
In pre-operative data collection, the left ventricular ejection fraction of the patients was (20% - 70%) and the mean was 53.37%; the left ventricular diameter during diastole was (3.7 cm – 7.4 cm) and the mean was 5.2894; the left ventricular diameter during systole was (2.3 cm – 5.9 cm) and the mean was 3.7542.
The left atrial diameter was (2cm – 4.9cm) and the mean is 3.306; the posterior wall thickness was (0.9cm – 1.5cm) and the mean was 1.068; the septal wall thickness was (0.9cm – 1.3 cm) and the mean was 1.008.
Operative and echocardiographic dataComplete revascularization involved 1-4 grafts. Grafting distribution: 10% had 1 graft, 30% had 2, 46% had 3, and 14% had 4. Average bypass and cross-clamp times were 100±28 and 54±17 minutes, respectively.
Preoperative LVEF ranged from 20 to 72% (mean 53.4%). At one-year follow-up, LVEF increased to 55.5% ± 7.5. Improvements were significant in patients with baseline EF <50%, while those with EF >50% showed minimal change.
Medical therapyPostoperatively, 92% received beta-blockers, 46% received ACEIs, and 4% received digoxin. All patients received diuretics. No patients were treated with aldosterone antagonists or SGLT2 inhibitors, representing a deviation from current heart failure guidelines.
Functional outcomesPatients with EF<50% demonstrated a significant LVEF increase (mean 37.5% to 47.1%, p=0.001). Those with EF>50% had a slight reduction (60.4% to 59.2%, p<0.001). LAD and SWT increased postoperatively, while LVIDd, LVIDs, and PWT slightly decreased.
In the early post-operative period (the 1st two weeks), the left ventricular ejection fraction, left ventricular internal diameter, left atrial diameter, septal wall thickness and posterior wall thickness of patients showed no significant changes in comparison with the data preoperatively.
In the late follow up which was done during the first year post-operatively, the left ventricular ejection fraction of the patients was (35% - 65%) and the mean was 55.47%; the left ventricular diameter during diastole was (4.1 cm – 7.5 cm) and the mean was 5.133; the left ventricular diameter during systole was (2.8 cm – 5.9 cm) and the mean was 3.763 as shown in Table 2.
The left atrial diameter was (2.7cm – 4.9cm) and the mean was 3.637; the posterior wall thickness was (0.9cm – 1.2cm) and the mean was 1.02; the septal wall thickness was (0.8cm – 1.2 cm) and the mean was 1.018.
Patients with EF>50% (34 patients) showed a slight decrease of 1% in LVEF postoperatively along with PWT, while a slight increase in LVIDs, LVIDd, LAD and SWT were noticed as shown in Table 3.
Echocardiographic data before and after coronary artery bypass grafting in the study population.
| Variables | All patients n=49 | ||
|---|---|---|---|
| Pre-CABG | Post-CABG | P value | |
| LVEF% | 53.37+12.323 | 55.47+7.459 | 0.001 |
| LVIDd cm | 5.2894+0.91769 | 5.133+0.6917 | < 0.001 |
| LVIDs cm | 3.7542+0.98521 | 3.763+0.7328 | < 0.001 |
| LAD cm | 3.306+0.7026 | 3.637+0.5345 | < 0.001 |
| PWT cm | 1.068+0.15 | 1.02+0.0735 | < 0.001 |
| SWT cm | 1.008+0.1085 | 1.018+0.0635 | < 0.001 |
Patients with EF<50% (15 patients) displayed a 10% increase in LVEF postoperatively along with LAD and SWT, while a slight reduction in LVIDd, LVIDs and PWT was observed as shown in Table 4.
Echocardiographic data before and after coronary artery bypass grafting in the study population in subgroups of patients with normal and decreased pre-operative left ventricular ejection fraction.
| Variables | LVEF>50%, n=34 | LVEF<50%, n=15 | ||||
|---|---|---|---|---|---|---|
| Pre-CABG | Post-CABG | P value | Pre-CABG | Post-CABG | P value | |
| LVEF% | 60.38+ 5.779 | 59.15+ 4.201 | <0.001 | 37.47+ 7.18 | 47.13+ 6.468 | 0.001 |
| LVIDd cm | 4.833+ 0.591 | 4.874+ 0.426 | <0.001 | 6.25+ 0.761 | 5.72+ 0.825 | <0.001 |
| LVIDs cm | 3.228+ 0.506 | 3.424+ 0.381 | <0.001 | 4.853+ 0.872 | 4.533+ 0.763 | <0.001 |
| LAD cm | 3.076+ 0.58 | 3.524+ 0.457 | 0.009 | 3.72+ 0.769 | 3.893+ 0.622 | 0.174 |
| PWT cm | 1.103+ 0.159 | 1.032+ 0.073 | <0.001 | 1+ 0.100 | 0.993+ 0.070 | 0.016 |
| SWT cm | 1.021+ 0.123 | 1.024+ 0.061 | 0.001 | 0.987+ 0.064 | 1.007+ 0.070 | 0.217 |
Patients with one, two and four grafts demonstrated an increase in the mean of LVEF while patients with three grafts had no change in the mean of LVEF.
One male patient had died in the surgical ward on the 8th post-operative day. He had a chronic kidney disease and was on dialysis pre- and postoperatively.
His pre-operative left ventricular ejection fraction was 40%. He had a CABG procedure with a single vessel grafted (left internal mammary artery to left anterior descending coronary artery).
DiscussionThe research reveals that the mean age of patients was 57.12 years, aligning closely with the findings of Saravia et al., who reported a mean age of 61.8 years among patients undergoing CABG.5 This similarity may be attributed to the prevalence of multiple risk factors within our community, as observed in our cohort, where 80% of patients had hypertension, 38% had diabetes mellitus, 34% were smokers, and the mean body mass index (BMI) was 29.28. These factors, combined with lifestyle influences, likely contribute to the observed trends.
The predominant risk factor was found to be mainly hypertension (80%), followed by diabetes mellitus (38%) and smoking (34%). These findings are consistent with the results of Saravia et al., who identified hypertension (49.5%) as the most prevalent cardiovascular risk factor, followed by smoking (38%) and diabetes (29.9%). Notably, smoking has been widely recognized as a significant contributor to coronary artery disease, particularly among younger populations, as highlighted in numerous studies.5
Despite the high mean BMI of 29.28 observed in the study, it did not significantly influence outcomes following CABG. This finding aligns with the study by Reeves et al., which concluded that there is no substantial difference in morbidity and mortality between obese and normal-weight patients undergoing CABG, provided that imbalances in key prognostic factors are accounted for.6
A percentage of 46 patients underwent emergency CABG. According to the current guidelines from the American College of Surgeons, emergency CABG is indicated for patients presenting with “evolving myocardial ischemia refractory to optimal medical therapy, left main artery stenosis and/or three-vessel disease, ongoing ischemia despite successful or failed percutaneous coronary intervention (PCI), complications arising from PCI, or cardiogenic shock associated with complex coronary anatomy”.7
The improvement of left ventricular systolic function following myocardial revascularization is influenced not only by the surgical factors such as the surgeon's expertise, completeness of revascularization, cardiopulmonary bypass management, ischemia duration, and myocardial protection techniques but also by postoperative complications, the condition of the coronary arteries, and myocardial viability. Studies utilizing advanced diagnostic modalities, including cardiac catheterization, echocardiography, equilibrium radionuclide ventriculography, single-photon emission computed tomography (SPECT), positron emission tomography (PET), and magnetic resonance imaging (MRI), have identified several predictors of improved left ventricular function. Key predictors include the presence of viable myocardium, evidence of myocardial ischemia, and timely coronary revascularization.8
The results indicated a slight decrease in left ventricular ejection fraction (LVEF) among patients with a pre-operative LVEF of over 50%, while a significant improvement was observed in those with a pre-operative LVEF of less than 50%. These findings align with the study by Koene RJ et al., which reported a decline in left ventricular systolic function following CABG in patients with normal pre-operative LVEF, while patients with reduced pre-operative LVEF demonstrated notable improvement.9
The decline in left ventricular systolic function after CABG may be attributed to factors such as intra-operative global ischemia, myocardial stunning, or early post-operative graft failure. These results highlight the need for further validation through prospective studies involving unselected patient populations.9
Multiple factors influence outcomes in patients with baseline left ventricular systolic dysfunction undergoing CABG, including preoperative care, the severity of left ventricular dysfunction, surgical expertise, completeness of revascularization, myocardial protection techniques, cardiac anesthesia management, the availability of emergency cardiac facilities, and postoperative intensive care monitoring and management.9
Furthermore, a study by Khaled S et al. corroborated our findings, demonstrating significant improvement in left ventricular systolic function after CABG. This supports the hypothesis that surgical revascularization and restoration of blood flow to ischemic myocardium protect viable and functioning myocardial tissue from subsequent infarction, recruit hibernating myocardium, and reduce left ventricular remodeling and ischemic burden. Together, these mechanisms contribute to the recovery of left ventricular function.10
Improved LVEF following CABG is closely tied to the presence of viable myocardium and complete revascularization. Our findings align with prior studies indicating functional recovery in patients with impaired preoperative LVEF.
The lack of myocardial viability assessment is a key limitation. Viability assessment via stress echocardiography or cardiac MRI can predict post-revascularization improvement. Furthermore, the underuse of guideline-directed medical therapy (GDMT) such as aldosterone antagonists and SGLT2 inhibitors likely affected outcomes.
Incomplete revascularization in 10% of patients may have contributed to variability in outcomes. This should be considered when interpreting the results.
Limitation of the study- •
The era of COVID-19 epidemic affects our number of patients, timetable and the availability of patients for follow-up.
- •
Absence of myocardial viability testing.
- •
Lack of SGLT2 inhibitors and mineralocorticoid receptor antagonists.
- •
Incomplete revascularization in a subset of patients.
- •
Small sample size and single-center design.
In the early post-operative period (1st two weeks), patients showed no significant change in the left ventricular ejection fraction after complete coronary revascularization.
In the late follow-up which was done during the first year post-operatively, Patients with EF>50% demonstrated no significant change in the left ventricular ejection fraction after complete coronary revascularization.
Patients with left ventricular systolic dysfunction (EF <50%) displayed significant improvement in the left ventricular ejection fraction after complete coronary revascularization.
Complete coronary revascularization significantly improves LVEF in patients with reduced preoperative systolic function. Optimal outcomes require viability assessment and adherence to GDMT.
RecommendationComplete coronary surgical revascularization is the most important crucial measure in treating the coronary artery disease indicated for surgery, where the reperfusion of the different aspects of heart muscle will lead to improvement in the hibernated muscle function.
Future studies should include viability testing and optimize GDMT to assess the full impact of revascularization on ventricular recovery.
Ethical considerationsThis study was approved by the Institutional Ethics Committee of Health directory. Written informed consent was obtained from all individual participants included in the study. The research complied with the ethical guidelines of the Health directory.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.