Despite the advance in vaccination, the SARS-CoV-2 infection remains a challenge for the medical community. Outpatient and hospital therapy for COVID-19 are still improving. Our study aimed to report the results of a series of patients with COVID-19 who participated in an outpatient treatment protocol since the first clinical manifestation.
MethodsA case series report of individuals aged ≥18 years with clinical symptoms and a confirmed test for COVID-19 submitted to a treatment protocol. Patients were enrolled between May and September 2020 and followed for at least 15 days. The assessed clinical outcomes were the need for hospitalization, admission to the intensive care unit, orotracheal intubation, and death.
ResultsWe studied a 116 patients. The mean age was 48 ± 14 years. Females formed 53%. The main comorbidities wereobesity (15.5%), systemic arterial hypertension (10.3%) ,type II diabetes (6%), and lung diseases (6.0%). Temperature >37.7 °C (51.7%), cough (55.2%), myalgia (37.1%), headache (37.9%), and fatigue (34.5%) were the most frequent signs and symptoms. According to different disease staging, the most administered drugs were: azithromycin, ivermectin, corticosteroid, antibiotics, and anticoagulants. There was no death, and hospitalization accounted for only 8.6% of the patients (1 in ICU); none required orotracheal intubation. The mean length of hospital stay was 5.8 days.
a pesar del avance en la vacunación, la infección por SARS-CoV-2 sigue siendo un desafío para la comunidad médica. La terapia ambulatoria y hospitalaria para COVID-19 sigue mejorando. Nuestro estudio tuvo como objetivo informar los resultados de una serie de pacientes con COVID-19 que participaron en un protocolo de tratamiento ambulatorio desde la primera manifestación clínica.
Métodosestudio prospectivo con reportes de casos de personas ≥ 18 años con síntomas clínicos y pruebas confirmadas de COVID-19 en protocolo de tratamiento. Los pacientes se trataron consecutivamente entre mayo y septiembre de 2020 y se les dio seguimiento durante al menos 15 días. Los desenlaces clínicos evaluados fueron: la necesidad de hospitalización, la unidad de cuidados intensivos, la intubación orotraqueal y la muerte.
Resultadosse estudiaron 116 pacientes. La edad media fue de 48 ± 14 años. Las hembras formaron el 53%. Las principales comorbilidades fueron obesidad (15,5%), hipertensión arterial sistémica (10,3%), hipertensión arterial sistémica (10,3%), diabetes tipo II (6%), y enfermedades pulmonares (6,0%). Tos (55,2%), temperatura > 37,7 °C (51,7%), cefalea (37,9%), mialgia (37,1%) y fatiga (34,5%) fueron los signos y síntomas más frecuentes. Según los diferentes estadios de la enfermedad, los fármacos más administrados fueron: azitromicina, ivermectina, corticoides, antibióticos y anticoagulantes. La hospitalización representó solo el 8,6% de los pacientes (uno en UCI); ninguno requirió intubación orotraqueal y ningún fallecimiento. La estancia hospitalaria media fue de 5,8 días.
COVID-19 was the name chosen for a severe acute respiratory syndrome caused by a new coronavirus (SARS-COV-2) identified in Wuhan, China, in 2019 for the first time.1 Frequent symptoms and signs are fever, dry cough, headache, anosmia, ageusia, sore throat, myalgia, tremors, dyspnoea, and in more severe cases, pneumonia.2 Despite this, most patients remained asymptomatic; unfortunately, a small percentage with this highly contagious disease has a virulent curse, leading to mass deaths.3–5
Regrettably, despite disease knowledge advances and how to better avoid contamination by a coronavirus, we have not had vaccines or drugs scientifically approved to treat this disease in May 2020. There were already 514 200 cases of COVID-19, and 29 314 (5.7%) individuals died due to COVID-19 in Brazil at the end of May 2020.6 At that time, feelings of frustration, hope, and sadness led to anecdotal interventions.7 Yet, it is supposed that the infection by the new coronavirus interconnects several mechanisms, i.e., inflammation, coagulation disturbances, and thrombotic phenomena.1,8–12 These processes occur in different stages of the pathophysiology of COVID-19 as they initiate with the virus invasion or early infection (stage I), followed by the pulmonary phase (stage II), and the hyper inflammation phase (stage III).13,14 As Siddiqi and his co-author demonstrated in fig. 1 of their paper, these stages are potential therapeutic targets.14 In addition, other clinical complications due to these pathological mechanisms, such as opportunistic infections and the involvement of other organs, have been observed.4,15,16 Therefore, studies concerning early interventions with hydroxychloroquine and azithromycin were published.17,18 However, due to low efficacy and possible cardiac complications, the administration of hydroxychloroquine was almost abandoned.19 Instead, following the approval of FDA-USA, ivermectin was used to inhibit viral replication.20–24 Shortly, we faced several randomized clinical trials (RCTs), even systematic revisions with associations between medicines, including antivirals, "immunomodulators and corticosteroids".25 Nevertheless, despite the number of studies, we are still waiting for robust and well-designed RCTs or reliable and non-biased systematic revision with meta-analysis.17,26–30
ObjectiveWe aimed to report the results of a structured clinical protocol for early treatment of COVID-19 (CPET-COVID-19) in patients with symptoms of the disease and specific complementary exams that proved the disease.
RationaleWe expected to take action in the first 2 stages of the disease, combining the available repurposed drugs to reduce hospitalizations and deaths.
Patients and methodsStudy designA case series of patients treated based on a structured protocol.
Inclusion criteriaIndividuals aged ≥18 years, symptomatic (symptoms started up to 7 days before the first consultation), with a positive test for COVID-19 (RT-PCR or serology for COVID-19) between May and September 2020 and submitted to the CPET-COVID-19.
Symptomatic patientsFor the present study, symptomatic patients had one or more signs or symptoms: fever, dry cough, odynophagia, anosmia, ageusia, headache, myalgia, dyspnea, delirium, and diarrhoea.
Exclusion criteriaWe excluded patients who disagreed with joining the study, those with a dubitable or subsequent negative test for COVID-19, and those who lost follow-up.
Place of studyThe principal author and F.P. evaluated most patients in their office as an outpatient protocol. They also recruited all potential cases admitted to the Hospital da Cidade (H.C.) emergency rooms, Salvador-Brazil. Patients were admitted to this hospital when necessary.
Medical careThe first author of this study or F.P. examined and submitted every patient to the following laboratory exams and thorax imaging according to timing and symptoms of the disease process: hemogram, C-reactive protein, creatine phosphokinase, coagulation tests, liver transaminases, creatinine, urea, D-dimer, and thorax computed tomography or thorax X-ray. Based on the medical literature, we considered critical test values, for example, D-dimer 3 times the normal value; C-reactive protein >20; substantially reduced number of lymphocytes; Substantially elevated CK and CT scan of the chest with more than 50% ground-glass involvement, as well as acute renal dysfunction.
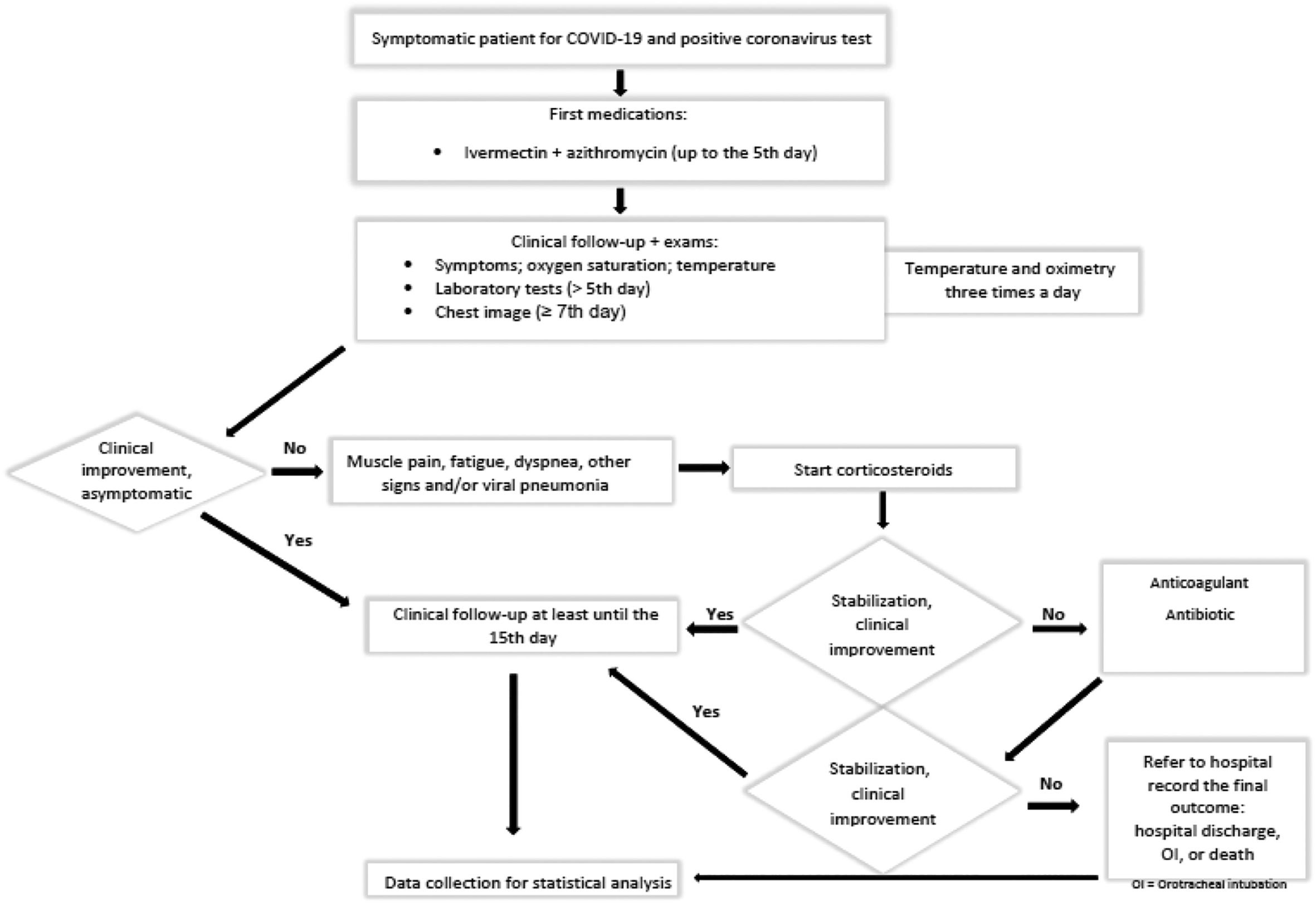
In addition, most cases were followed up daily through remote queries by the Zoom Platform or the WhatsApp application. Furthermore, we monitored oximetry and temperature at least 3 times a day. In addition, we have strictly monitored patients with comorbidities such as diabetes, obesity, and hypertension. Clinical complications received particular support, such as investigation, suitable treatment, and evaluations at the H.C. emergency department, as necessary (Fig. 1).
Early clinical treatment protocolCPET-COVID-19 uses the COVID-19 pathophysiology stages as therapeutic targets, as shown in Fig. 1.
ConsultationsThe authors obtained the opinions of G.M. (pulmonologist) regarding thorax image and AB (neurologist) concerning neurological complaints.
DrugsAccording to disease staging and patient characteristics, the principal author prescribed the following drugs: ivermectin, azithromycin, prednisolone, rivaroxaban, enoxaparin, antibiotics, and paracetamol or dipyrone.
OutcomesThe assessed clinical outcomes were the need for hospitalization, admission to the intensive care unit, orotracheal intubation, and death.
Statistical analysisWe ran Statistical Package for the Social Sciences (SPSS for Windows version 20.0) program; IBM, Armonk, NY, USA), 844052 Software: R Program, version 3.6.1 and Microsoft Excel, version 2016 (16.0.6769.2017). We used the mean and the standard deviation to describe quantitative variables with normal distribution. In addition, we expressed categorical variables in absolute numbers and percentages.
EthicsThe ethical committee for human research of Hospital Ana Nery has approved this protocol. Therefore, the author registered this study in the Brazilian Platform for Research. Furthermore, all included patients have signed the informed consent document. The drugs used were: azithromycin, ivermectin, enoxaparin, prednisolone, and symptomatic (dipyrone or paracetamol).
ResultsWe enrolled a 122 symptomatic patients. Six patients were negative for coronavirus, and 116 patients were eligible as a case and included in the study. Most cases are the author's patients; others came from other physicians' referrals or the emergency rooms of H.C. As COVID-19 is a contagious disease, some patients were relatives living in the same home. In addition, 7 patients joined the protocol with routine laboratory tests already done. The mean age observed was 48 years (SD ± 14), and females were 62 (53%). The most common comorbidities were obesity (15.5%), systemic arterial hypertension (10.3%), type II diabetes (6.0%), and lung diseases (6.0%). Temperature >37.7 °C (51.7%), cough (55.2%), myalgia (37.1%), headache (37.9%), and fatigue (34.5%) were the most frequent signs and symptoms. The following drugs and their percentage of uses were: azithromycin (100%), corticosteroids (75.9%), anticoagulants (34.5%), antibiotics (10.3%), and ivermectin (99.1%). Table 1 shows the laboratory tests concerning most inflammatory and coagulation disorders related to COVID-19 and their percentage of abnormal results. We did not mention renal function as there was no abnormal result. The significantly higher complementary test was C-reactive protein, followed by leukogram and D-dimer (see table below). One patient developed Rhabdomyolysis, and another significantly increased liver enzymes (transaminases).
The absolute number of patients and number of patients submitted to complementary exams and abnormal results in percentages.
| Exams | Patients | Abnormal results |
|---|---|---|
| n = 116 | ||
| White blood cell count | 109 | 19% |
| Lactate dehydrogenase (LDH) | 88 | 11% |
| C-reactive protein (CRP) | 109 | 42% |
| D- dimer | 56 | 18% |
| Creatine phosphokinase (CPK) | 83 | 18% |
| Prothrombin time (P.T.) | 80 | 18% |
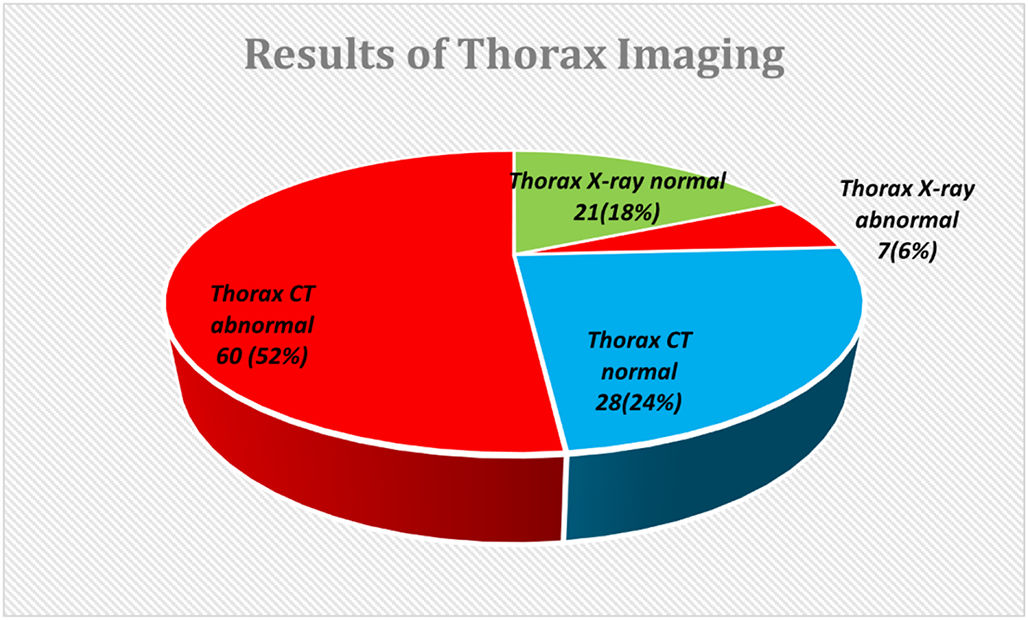
Both recovered very well in 2 weeks. Fig. 2 demonstrates the results of imaging tests (abnormal percentages of computed tomography and chest X-rays).
Hospitalization accounted for 10 (8.6%) patients, and 90% were male. Table 2 shows the characteristics of inpatients. Most non-hospitalized patients reached the status of cured up to 15 days.
Characteristics of patients with COVID-19 admitted to hospital units with viral pneumonia.
| Patient | Sex | Age (years) | Comorbidities | Hospital Unit | Oxygen supplement | LOS |
|---|---|---|---|---|---|---|
| 01-JRM | M | 40 | Obesity-1 | ICU | Mask | 06 days |
| 02-RJBS | M | 55 | Obesity-II/ AH/DM | ICU | Mask | 05 days |
| 03-NDMF | M | 61 | Overweight | ICU | Mask | 05 days |
| 04-DCAA | M | 33 | Obesity-II | ICU | Mask | 09 days |
| 05-DNG | M | 49 | No comorbidity | ICU | Mask | 09 days |
| 06-PCM | M | 56 | Overweight | ICU | Mask | 05 days |
| 07-GM | M | 55 | Obesity-I/A.H./Asthma | CW | NHF O2 | 02 days |
Patients with O2 saturation between 90% and 92% O2 were admitted to C.W., whereas those below 90% to ICU. No patient required oro-tracheal intubation. M = male sex, Obesity1 = class I obesity (BMI = 30.0–34.9 Kg/m2), Obesity-2 = class II obesity (BMI = 35.0–39.9 Kg/m2), BMI = Body Mass Index, AH = Arterial hypertension, DM = Diabetes Mellitus, ICU = intensive care unit, CW = COVID ward, NHF O2 = nasal high-flow O2, LOS = length of stay.
Seven patients (6%) entered the study with an average white blood cell count and normal C-reactive protein results. Most white cell disorders were lymphopenia.
DiscussionDespite the advance in vaccination, SARS-CoV-2 infection remains a challenge for the medical community. Therapy for COVID-19 has advanced, but it is already on the evolution curve. Our study reported a series of 116 cases of COVID-19 submitted to CPET-COVID-19. The theoretical protocol framework follows the disease's pathophysiological stages proposed by Siddiqi et al.14 According to this protocol by Siddiqi, our patients entered the study at "stage 1" of that protocol.
We developed this protocol from May to September 2020, when the medical community's therapeutic recommendations for COVID-19, especially those of the early and outpatient phases, were under intense discussion25 [31–34].
During this protocol period, COVID-19 devastated populations, so time and scientific rigour were competing [35]. Unfortunately, several reasons led to the scarcity of well-designed trials: the severity of COVID-19, different response to coronavirus infections among patients, physician concern of committing an unethical decision giving a placebo, and the use of compassionate treatment, i.e. the same has occurred with the Ebola pandemic [36]. Consequently, most studies were case series with repurposed medicines [31]. In addition, the FDA had approved ivermectin since in-vitro, and it inhibited SARS-COV 2 replication.23
The covid-19 scenario in Salvador in May 2020 was catastrophic since almost all hospitals had at least 90% of beds taken. Thus, health authorities recommended patients be isolated at home until respiratory symptoms occurred. Governments proclaimed partial lockdown and opened several new hospitals for COVID-19 [37]. The media showed a death scenario as May 2020 became the month with the highest number of deaths in Brazilian history [38]. So, we decided to initiate this protocol; however, several observational and interventional studies, even systematic reviews, most poorly designed, had shown debatable results.30 After balancing the pros and cons, we decided to keep the protocol without a control group pending patients' safety instead of scientific rigour. After all, we were testing a protocol, and we believe all drugs of this protocol are essential, even ivermectin.
Although we cannot find well-designed RCTs, 11 of the 18 RCTs had positive effects; nevertheless, the last guidelines of the European Society of Clinical Microbiology and Infectious Disease (ESCMID) do not recommend ivermectin to treat COVID-19 [39].
Yet, due to the low rate of side effects, some authors intend to repurpose ivermectin to treat COVID-19 [40]. It is worth mentioning that drugs for treating the early stage of COVID-19 are debatable [41]. Therefore, the prescription of ivermectin is still a concern [39]. In addition, several studies included azithromycin [39]; however, none found evidence to treat COVID-19 [42, 43]. Nonetheless, one study shows that a treatment kit for COVID-19 might reduce hospitalizations and deaths [44]. Overweight and obesity were our population's most critical risk factors for COVID-19 severity, and they were responsible for 80% of hospital admission. Several reasons led to obesity increased disease severity in COVID-19, such as virus entry enhancement, the immune system being unable to provide an adequate response, increased risk for thrombus formation, and hemorrhages; the adipose tissue now considered a significant reservoir for SARS-CoV-2 and an essential source of inflammatory mediators [45, 46]. Other frequent comorbidities such as diabetes mellitus, arterial hypertension, and lung disease are well-known risk factors for bad outcomes in COVID-19 [47–49].
Moreover, we faced a patient with Rhabdomyolysis and another with elevated liver enzymes, which are not rare complications [50, 51]. Both recovered after resting and hydration. Therefore, the hospital admission rate was low for our patients. Although 8.6% of our patients developed moderate to severe viral pneumonia, none required orotracheal intubation.
We decided to share this report as we observed highly favorable clinical outcomes in patients submitted to CPET-COVID-198. We still believe our treatment has changed the natural history of COVID-19. Additionally, it seems clear to us the relationship between the chronology of pathophysiological events of COVID-19, the therapeutic rationale tied to them, and the clinical manifestations observed in these patients. Moreover, we had no clinical complications attributable to the drugs we prescribed.
Weakness of the studyThe small sample size (n = 116) and the absence of a control group reduce the protocol's potential influence on the observed clinical outcomes.
Conclusions- 1.
Whereas in May 2020, the case fatality rate of COVID-19 in Brazil reached 5.7%, there was no death among symptomatic patients submitted to CPET-COVID-19.
- 2.
The study findings hypothesized that CPET-COVID-19 altered relevant clinical outcomes in patients with COVID-19; thus, randomized controlled trials should assess this hypothesis.
All patients agreed to sign the informed consent form.
Availability of data and materialsDatasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Author's contributionS.S. conceived the study idea, designed the methods, examined, and treated patients, collected data, and drafted the manuscript; F.P. collected data and cared for patients; G.M. performed the thorax imaging review end reviewed the manuscript. A.B. performed patient neurological reviews, drafted the manuscript, and executed English editing; All authors read, discussed, and approved the final manuscript.
Competitive interestsThere is no conflict of interest.
FinancingMinimum budget, funded by authors themselves.
We thank Silas Santana, Rutielen Souza, and Ricardo Fortes for their participation in collecting data, revising data, and studying design, respectively.