Leucine-rich glioma inactivated 1 (LGI1) antibody-related autoimmune encephalitis is easily misdiagnosed clinically because of its complex and diverse clinical manifestations. We present two cases of LGI1 antibody-related encephalitis with negative imaging findings and perform a literature review on this disease entity.
Case descriptionThe first case was that of a 60-year-old man who presented with involuntary movement of the paroxysmal right limb. The second case was that of a 66-year-old man who presented with hearing hallucinations, involuntary shaking of the right limb, and progressive cognitive impairment. Both patients in this study showed negative magnetic resonance imaging (MRI) results. Routine cerebrospinal fluid (CSF) and biochemical examinations showed no significant abnormalities, and positive LGI1 antibodies were detected in both the CSF and serum.
ConclusionBased on our experience and the literature review, we recommend that LGI1 antibody-related encephalitis should be considered when faciobrachial dystonic seizures, acute and subacute-onset seizures, low serum sodium (possibly with low CSF chloride), and cognitive-psychiatric disorders are encountered, even in the absence of specific radiographic and altered CSF findings.
La encefalitis autoinmunitaria relacionada con anticuerpos LGI1 puede ser fácilmente mal diagnosticada clínicamente debido a sus manifestaciones clínicas complejas y diversas. Presentamos dos casos de encefalitis relacionada con anticuerpos LGI1 con hallazgos de imágenes negativas y realizamos una revisión de la literatura sobre esta entidad patológica.
Descripción de casosEl primer caso fue el de un hombre de 60años que presentó movimientos involuntarios del miembro derecho paroxístico. El segundo caso fue el de un hombre de 66años que presentó alucinaciones auditivas, temblores involuntarios del miembro derecho y un deterioro cognitivo progresivo. Ambos pacientes en este estudio mostraron resultados negativos de imágenes por resonancia magnética (RM). Los exámenes rutinarios de líquido cefalorraquídeo (LCR) y bioquímicos no mostraron anomalías significativas, pero se detectaron anticuerpos LGI1 positivos en ambos: LCR y suero.
ConclusiónBasándonos en nuestra experiencia y en la revisión de la literatura, recomendamos considerar la posibilidad de encefalitis relacionada con anticuerpos LGI1 cuando aparezcan crisis distónicas faciobraquiales, convulsiones de inicio agudo o subagudo, hiponatremia (posiblemente con hipoclorhidria del LCR) y trastornos cognitivo-psiquiátricos, incluso en ausencia de hallazgos radiográficos específicos o modificaciones en el LCR.
The leucine-rich glioma inactivated 1 (LGI1) protein, first described in 2010, is a secretory synaptic protein, which is a part of the voltage-gated potassium channel (VGKC) complex, and mainly exists in the hippocampus, amygdala, and temporal lobe cortex.1 LGI1 antibody-related autoimmune encephalitis is a condition in which LGI1 antibodies participate in the pathogenesis. LGI1 antibodies can improve nerve tissue's excitability by interfering with synaptic protein interactions. This results in a series of clinical manifestations, most of which are seen in middle-aged and elderly people, mostly men, have acute or subacute-onset, and mainly manifest as borderline encephalitis syndromes, such as transient memory impairment, seizures, and mental disorders.2 Seizures or cognitive impairment are often the first symptoms of LGI1 antibody-related autoimmune encephalitis and can manifest as psychiatric abnormalities and personality changes.3 In 2013, the first confirmed case of anti-LGI1 antibody detection was reported in China. It is easily misdiagnosed clinically because of its complex, diverse clinical manifestations and lack of specificity inspections. We report two cases of LGI1 antibody-related autoimmune encephalitis with negative imaging which will improve our understanding of LGI1 antibody-related autoimmune encephalitis and provide evidence for its clinical diagnosis and treatment.
Case presentationThis case report was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (Approval No.: 2023YX016), and informed consent was obtained from the patients.
Case 1A 60-year-old man with a history of type 2 diabetes and hypertension was admitted to the hospital on March 1, 2021, because of paroxysmal right limb involuntary movement for 6h. His vital signs on admission included temperature (36.5°C), pulse (78beats/min), respiration (20breaths/min), and blood pressure (141/78mmHg). No obvious positive signs were found upon medical or nervous system examination. On preliminary admission examination, electrocardiography was normal, cranial computed tomography (CT) revealed lacunar infarctions in the bilateral basal ganglia, CT angiography (CTA) of the head and neck showed multiple atherosclerosis of the internal and external cranial arteries with local stenosis. No obvious abnormalities were found in routine blood tests, liver and kidney function, thyroid function, blood lipid, and tumor marker screening. Thus, involuntary movement may be caused by vascular disease—transient ischemic attack, hemichorea associated with nonketotic diabetes, and epileptic seizures. We first provided comprehensive treatment, such as antiplatelet therapy, lipid regulation, and plaque stabilization, improving circulation, and controlling blood sugar and pressure. On days 1 (admission) and 2, intermittent attacks reoccurred in the mouth and face, and the right limb twitched involuntarily twice, with a lower frequency than before. On day 3, the attack reoccurred in the mouth and face at night, the right limb twitched involuntarily once and developed into a full-blown tonic–clonic attack, which lasted for approximately 2min before he recovered consciousness. Thus, epileptic seizures were confirmed and the patient was treated with phenobarbital and sodium valproate. Recurrent attacks were closely observed, to clarify the cause. The patient had no history of epilepsy, secondary epilepsy was confirmed, and the cause was investigated.
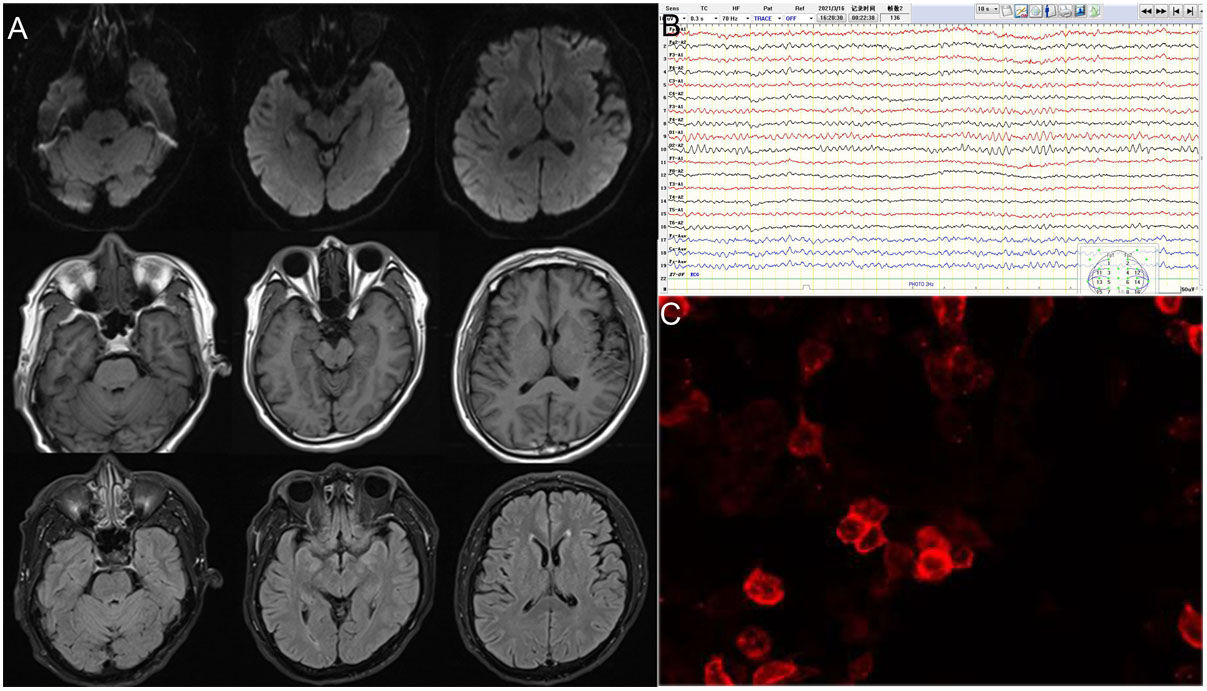
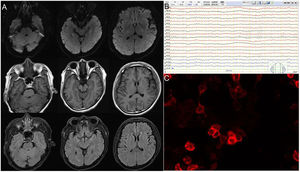
Brain magnetic resonance imaging (MRI), long-term electroencephalography (EEG), blood and cerebrospinal fluid (CSF) immunity, and tumor-related screening were performed. Brain MRI results revealed no specific changes. EEG revealed a short, frequent, episodic right upper limb involuntary twitch (Video 1). CSF contained normal leukocytes, protein and glucose, decreased chloride (116.0mmol/L, reference 120–130mmol/L), and increased IgG (62.06mg/L, reference 10–40mg/L). Autoimmune brain biomarkers (NMDAR-ab, AMPAR2-Ab, GABABR-Ab, and Caspr2-Ab), tumor markers (CEA, AFP, CA125, CA19-9, CA15-3, CA724, SCCAG, NSE, T-PSA, and CYFRA21-1), and paraneoplastic nerve antibodies (anti-Hu, -Ri, -Yo, Ma/Ta, Amphiphysin, -CV2, -SOX1, -Tr) (Table 1 and Fig. 1) had no obvious abnormalities. Anti-LGI1 antibodies in the blood and CSF were 1:10 (Fig. 1). Thus, the patient was diagnosed with LGI1 antibody-related autoimmune encephalitis and treated with 0.2g/kg immunoglobulin for 5 days and antiepileptics. His condition was stable, and convulsions did not recur.
Laboratory, imaging, electrophysiological examination and results of two cases with LGI1 antibody-related autoimmune encephalitis.
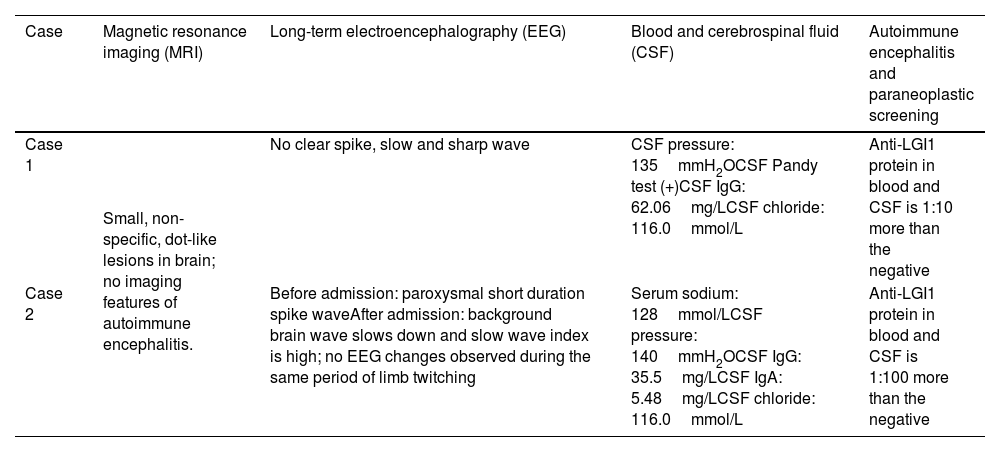
| Case | Magnetic resonance imaging (MRI) | Long-term electroencephalography (EEG) | Blood and cerebrospinal fluid (CSF) | Autoimmune encephalitis and paraneoplastic screening |
|---|---|---|---|---|
| Case 1 | Small, non-specific, dot-like lesions in brain; no imaging features of autoimmune encephalitis. | No clear spike, slow and sharp wave | CSF pressure: 135mmH2OCSF Pandy test (+)CSF IgG: 62.06mg/LCSF chloride: 116.0mmol/L | Anti-LGI1 protein in blood and CSF is 1:10 more than the negative |
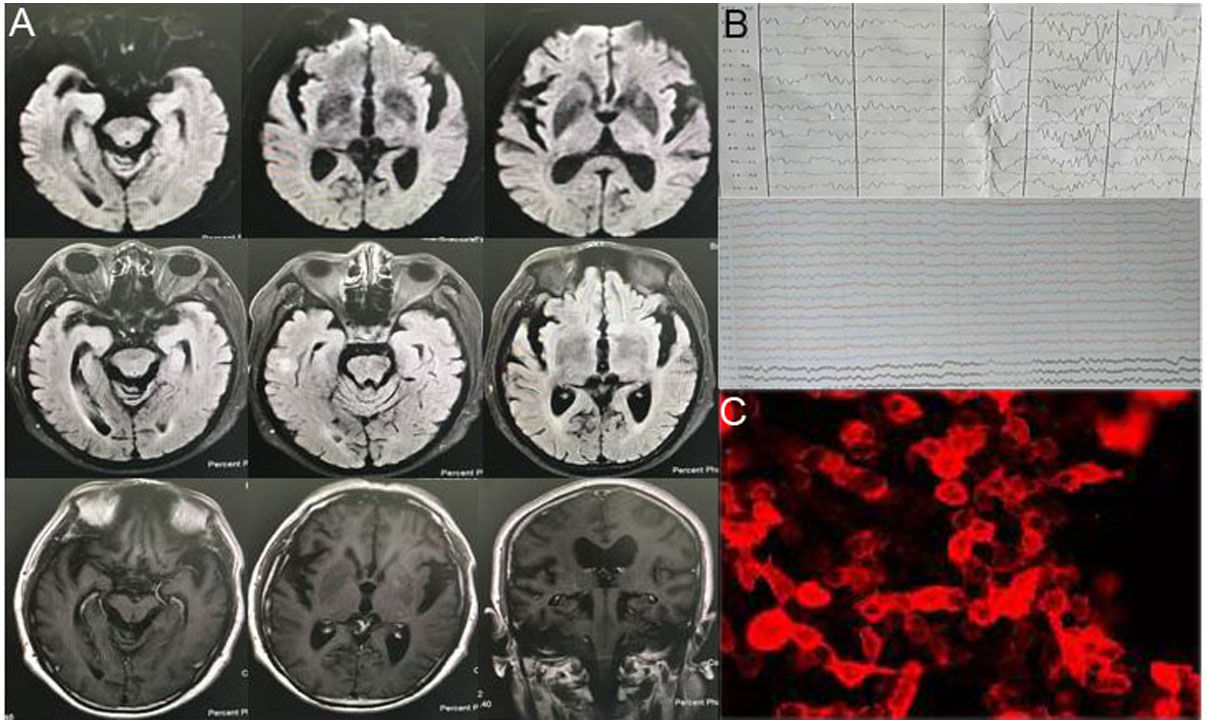
| Case 2 | Before admission: paroxysmal short duration spike waveAfter admission: background brain wave slows down and slow wave index is high; no EEG changes observed during the same period of limb twitching | Serum sodium: 128mmol/LCSF pressure: 140mmH2OCSF IgG: 35.5mg/LCSF IgA: 5.48mg/LCSF chloride: 116.0mmol/L | Anti-LGI1 protein in blood and CSF is 1:100 more than the negative |
A 66-year-old man with a history of type 2 diabetes was admitted to the hospital on September 6, 2021, because of auditory hallucinations, involuntary right limb shaking for 6 months, and aggravation with progressive cognitive impairment for 2 months. He experienced a pontine hemorrhage with numbness on the right side of the face in 2017. His vital signs on admission included temperature (36.7°C), pulse (75beats/min), respiration (20breaths/min), and blood pressure (140/70mmHg).
No obvious abnormalities were found in the cardiopulmonary complex after medical examination. Physical examination of the nervous system revealed that his mind was clear; however, he was in low spirits, indifferent. He experienced predominantly auditory hallucinations. His cognitive function decreased significantly [Mini-Mental State Examination (MMSE) score: 10; Montreal Cognitive Assessment (MoCA) score: 2]. His right side and right limbs violently twitched. Neurological examination shows no obvious abnormalities. Spike waves were found on a previous EEG, supporting epileptic seizures. Because he had no history of epilepsy and pre-disease cognitive function was normal, secondary epilepsy was confirmed.
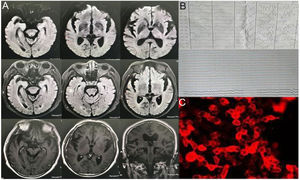
Brain MRI, long-term EEG, blood and CSF immunity, and tumor-related screening were performed. No specific changes were found on the brain MRI. EEG revealed a short, frequent, episodic right upper limb involuntary twitch (Fig. 2, Video 2). Laboratory test results show serum sodium: 128mmol/L. CSF showed normal leukocytes, glucose, and protein; decreased chloride (116.0mmol/L, reference 120–130mmol/L); increased IgG, and decreased IgA (5.48mg/L, reference 10–40mg/L). Autoimmune brain biomarkers (NMDAR-ab, AMPAR2-Ab, GABABR-Ab, Caspr2-Ab), tumor markers (CEA, AFP, CA125, CA19-9, CA15-3, CA724, SCCAG, NSE, T-PSA, CYFRA21-1), or paraneoplastic nerve antibodies (anti-Hu, -Ri, -Yo, Ma/Ta, Amphiphysin, -CV2, -SOX1, -Tr) showed no obvious abnormalities. Anti-LGI1 antibodies in the blood and CSF were 1:100 (Table 1). Thus, the patient was diagnosed with LGI1 antibody-related autoimmune encephalitis. He was treated with intravenous gamma globulins, hormones, anti-epilepsy drugs, cognitive improvement techniques, electrolyte imbalance correction, etc. When he left the hospital, his cognition improved, his auditory hallucinations decreased, and the involuntary right limb and face twitch improved (reduced frequency). The MMSE score was 10, and the MoCA score was 18.
DiscussionThe most common clinical manifestation feature of the above two cases is faciobrachial dystonic seizure (FBDS), characterized as one side of the face, upper limb, and shoulder, and can be accompanied by short (usually less than 3s), frequent (6–360times/day) dystonic lower limb twitches, or bilateral dystonic seizures, presage of abnormal sensation, stupidity, change of consciousness, etc.4 FBDS is a characteristic symptom of autoimmune encephalitis with anti-LGI1 antibodies; although it is considered a specific type of epileptic seizure, the EEG is non-specific and may show epileptiform discharges in one or both temporal lobes, with focal or diffuse slow-wave activity. A study showed that this manifestation is a movement disorder rather than an epileptic seizure, and whether it is cortical or extrapyramidal in location is controversial.5 Case 1 was also considered an extrapyramidal lesion by the supervising physician at the time of admission; therefore, the possibility of autoimmune encephalitis with anti-LGI1 antibodies should be further excluded by considering a specific episode of FBDS together with a similar clinical presentation.
Our patients also had cognitive dysfunction, seen in 95% of patients with anti-LGI1 antibody-related encephalitis, which is characterized by a decline in short-term memory. Cognitive dysfunction is the most common and sometimes only clinical manifestation of anti-LGI1 antibody-related encephalitis, which is often accompanied by mental and behavioral abnormalities, such as hallucinations, anxiety, depression, restlessness, hyperactivity, or obsessive–compulsive disorder.6 Some patients with anti-LGI1 antibody-related encephalitis also have language disorders, sleep disorders, cerebellar ataxia, etc. Hyponatremia may be one of the characteristic manifestations of anti-LGI1 antibody-related encephalitis and is associated with the simultaneous expression of LGI1 in the hypothalamus and kidneys, leading to abnormal secretion of antidiuretic hormones.7 The two cases in question have presented with different levels of serum sodium, one exhibiting a decrease and the other recording normal levels. Both subjects, however, have demonstrated a decline in cerebrospinal fluid chloride levels. While it is plausible that the lowered cerebrospinal fluid chloride levels may be correlated to the decreased serum sodium, no known evidence currently confirms whether the disease itself has any impact on the metabolism of chloride ions in neural cells. Further comprehensive investigations and ongoing observations are required to explore this area in depth.
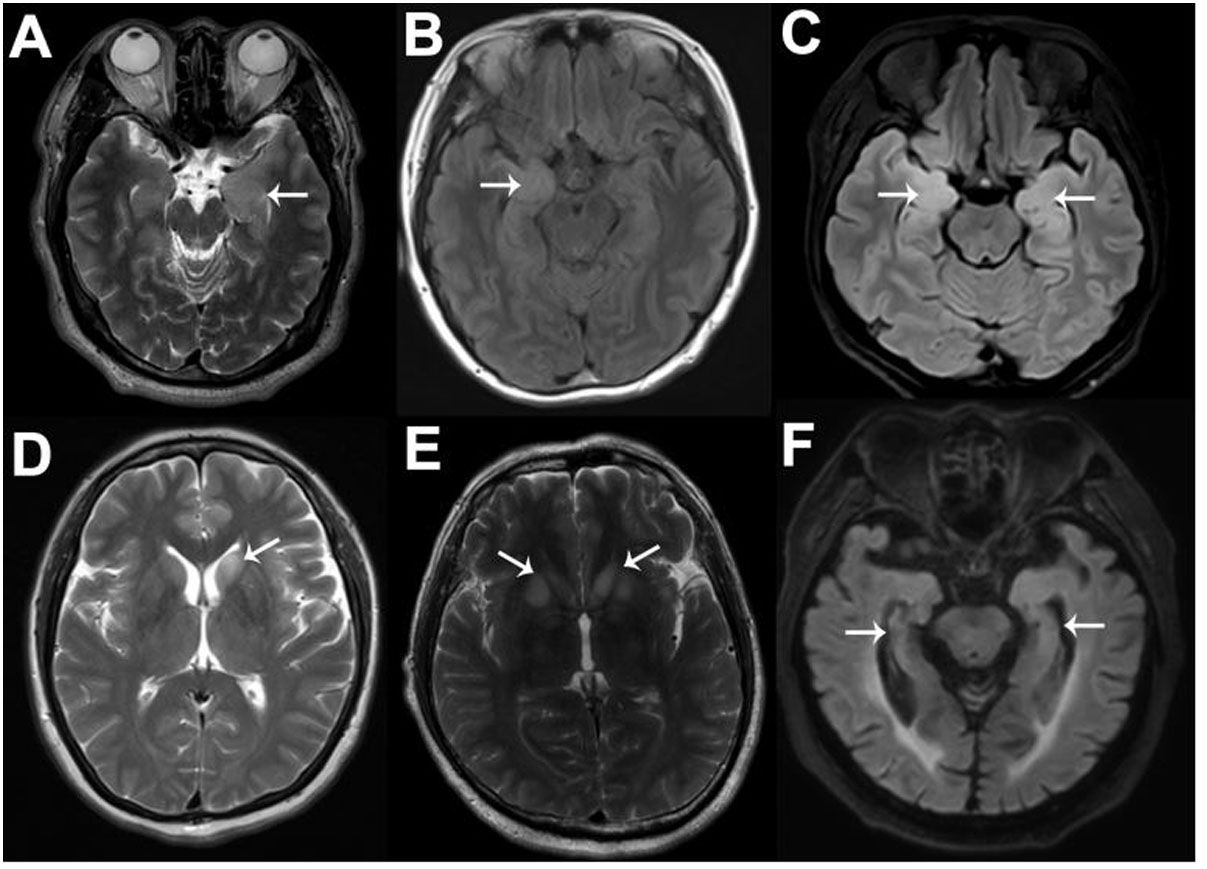
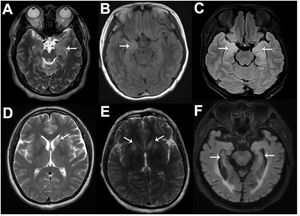
Sixty percent of patients with anti-LGI1-related encephalitis showed high signal intensity in the bilateral hippocampus of T2 and Flair sequences on MRI. In addition to the medial temporal lobe, the basal ganglia can also be affected (Fig. 3), and abnormal signals are only present on one side of the hippocampus or their MRI results appear normal in some patients.8 Negative MRI often makes early screening of anti-LGI1 antibody-related encephalitis difficult, and both patients in this study had negative MRI examinations. Functional imaging (e.g., Positron Emission Tomography [PET]) often shows hypermetabolic changes in the hippocampus, basal ganglia, or both and abnormal changes in low metabolism.9 PET is often more sensitive than MRI in showing anti-LGI1 antibody-related encephalitis lesions, especially in the early stages of the disease. A study showed that 18-fluoro-deoxyglucose (18F-FDG) PET in the parietal and occipital cortices of patients with anti-LGI1 antibody-related encephalitis can show low metabolism and high metabolism in the basal ganglia.10 The results of routine CSF and biochemical examinations in patients with LGI1 antibody-related encephalitis were non-specific and the positive results of serum and CSF for LGI1 antibodies were characteristic changes, which were consistent with the CSF findings in our two cases.
Typical magnetic resonance imaging (MRI) images of six anti-LGI1-related encephalitis patients. As shown by the white arrows, increased signals on MRI fluid-attenuated inversion recovery or T2 sequences can be seen in the left MTL (A), right MTL (B), bilateral MTL (C), left BA (D), and bilateral BA (E), and hippocampal atrophy can be seen (F).
Although LGI1 antibody-related encephalitis has distinctive characteristics, case reports prove that the clinical manifestations are diverse and its onset forms vary. In some instances, the disease may present with mild initial symptoms despite a severe and ominous course in the long-term. The negative imaging results may lead to misdiagnosis as another condition, ultimately resulting in a worse prognosis. Although most patients with anti-LGI1 antibody-related encephalitis had post-treatment seizure control, 28.0–66.7% maintained moderate to severe cognitive impairment, 21% reported persistent insomnia, and only 24–43% were able to return to work or all premorbid activities.6 In addition, up to 77.8–88.9% of patients with anti-LGI1 antibody-related encephalitis develop visually observable hippocampal atrophy in the long-term. A long-term follow-up cohort study suggested that the long-term prognosis of patients with poor initial treatment and relapse was poor. Old age and the incidence of abnormal CSF may be risk factors for poor prognosis. However, further confirmation is still needed. We will also continue long-term follow-up on the two cases to further observe their changes and outcomes.
ConclusionAlthough LGI1 antibody-related encephalitis has the typical manifestations of FBDS, it is easily confused with extravertebral symptoms and often cannot be recognized at first sight if the patient has a complex underlying disease and negative imaging. In addition, the long-term prognosis of anti-LGI1 antibody-related encephalitis is not ideal as one might think, and early recognition, diagnosis, and treatment are important.
Therefore, further improvement in clinical awareness of the disease is required. Features such as faciobrachial dystonic seizures (FBDS), acute and subacute-onset seizures, hyponatremia, and cognitive-psychiatric disorders are characteristic of LGI1 antibody-related encephalitis. Although it remains to be seen whether low cerebrospinal fluid chloride levels represent an additional defining feature, further observation with a larger number of cases is warranted. Thus, LGI1 antibody-related encephalitis should be considered in the presence of these clinical features, even without specific radiographic evidence.
Ethical considerationsThis study was conducted in accordance with the principles outlined in the Helsinki Declaration and was supervised by the Ethics Committee of the Second Clinical Medical College at Shanxi Medical University. The committee approved an exemption from obtaining informed consent from patients for this study, with approval no: 2023YX016.
FundingThis work was supported by National Natural Science Foundation of China (No. 82160237), Key Research and Development Program in Hainan Province (No. ZDYF2023SHFZ104), Natural Science Foundation of Hainan Province (No. 822MS210).
Conflict of interestGaiqing Wang receives research support from the National Natural Science Foundation of China, Key Research and Development Program in Hainan Province and Natural Science Foundation of Hainan Province. The other authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.