Edited by: Assoc. Professor Joaquim Reis
(Piaget Institute, Lisbon, Portugal)
Dr. Luzia Travado
(Champalimaud Foundation, Lisboa, Portugal)
Dr. Michael Antoni
(University of Miami, Coral Gables, Florida, United States of America)
Last update: November 2025
More infoCancer-related fatigue (CRF) is a prevalent and debilitating symptom experienced by many patients, affecting both their physical and psychological well-being. This study aims to explore the network organization of three dimensions of CRF (i.e., general, physical, and psychological) and to examine how patient’s resources such as coping strategies interact with these distinct aspects of fatigue.
MethodThis study involves data from a previous observational study on patients with metastatic colorectal cancer undergoing chemotherapy. Participants (N = 169) completed several questionnaires at baseline. Partial correlation network analysis was used to model the relationships between patients’ symptoms (i.e., CRF, emotional distress) and resources (i.e., perceived control of the illness, coping strategies, perceived social support), in three distinct networks, each of them including one dimension of fatigue (i.e., general, physical, or psychological). In each network, a core variable (i.e., a symptom or a resource) was identified based on the highest centrality indices.
ResultsCoping strategies emerged as the core variable in the three networks, while depression was the symptom with the strongest association with CRF. These findings underline the interconnection between emotional state and fatigue, but most of all suggest the centrality of the patients’ resources, specifically coping strategies used to manage their symptoms, and their potential role in influencing the symptoms.
ConclusionOur findings are expected to provide insights into targeted therapeutic approaches and enhance patient care. Understanding the complex interplay between the dimensions of fatigue and the coping strategies employed by patients is crucial for developing effective interventions.
Cancer-related fatigue (CRF) is defined as “a distressing persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (Berger et al., 2010). CRF is almost universal in patients undergoing chemotherapy (Iop et al., 2004; Wagner & Cella, 2004) and its prevalence is approximately 75 % in patients with metastatic disease (Curtis et al., 1991; Ventafridda et al., 1990; Wang et al., 2014). Patients with metastatic colorectal cancer (mCRC) may undergo multiple cycles of chemotherapy, and their functional status tends to deteriorate with each line of treatment (Mayrbäurl et al., 2016; Wagland et al., 2015, 2016).
Fatigue can be conceptualized as a unidimensional construct—referred to as general fatigue—and assessed using a single-item Visual Analog Scale (e.g., 'I feel tired') or graded using clinical scales for asthenia (ranging from 0 for no fatigue to 3 for severe fatigue). However, fatigue is more commonly considered a multidimensional symptom. A recent review explored how CRF has been defined and assessed in adult patients with cancer worldwide (Keane et al., 2024) and underlined that across all CRF assessment tools, some dimensions are used more frequently (i.e., “Physical”, “Mental”, “Cognitive”) than others. Physical and cognitive fatigue are the most frequently assessed dimensions, particularly in clinical trials, as they are closely related to disease and treatment side effects—for example, chemo-induced anemia contributing to physical fatigue (Saligan & Kim, 2012) or chemotherapy-related cognitive impairment (chemo-fog) (Hardy et al., 2018). The physical dimension of CRF reflects the physical sensation linked to the feeling of tiredness (e.g. energy loss or muscular fatigability), whereas the cognitive or mental dimensions refer to a lack of concentration and/or memory, or a drop in motivation. These two dimensions reflect major complaints in patients with cancer, and are, at least partly, linked to the intensity and duration of cancer treatments (Grusdat et al., 2022; National Comprehensive Cancer Network (NCCN), 2022). Psychological fatigue is also a relevant dimension but remains under-investigated (respectively 21,58 % and 40,29 % of quantitative articles (N = 139) included in Keane et al., 2024review)). This paper, summarizing the most commonly used clinical assessment tools, some of which include items related to emotional or affective fatigue (e.g., the Cancer Fatigue Scale, the EORTC QLQ, and the Multidimensional Fatigue Symptom Inventory; seeTable 2 in Keane et al., 2024), helps us to define psychological fatigue as feelings of demoralization, frustration, or emotional exhaustion—reflecting the profound emotional impact of cancer from diagnosis through post-treatment. During structured interviews, when cancer patients are asked about their symptoms of fatigue, the term ‘weary’ appears in the majority of verbatim, reflecting the important place this dimension has in patients' daily lives (Baussard et al., 2017). CRF is associated with many other symptoms endured by patients with cancer, one of them being emotional distress (Baussard et al., 2024; Götze et al., 2020; Lin et al., 2022; Ma et al., 2020; Mehnert et al., 2018; Schmidt et al., 2018). Emotional distress, is “a multifactorial, unpleasant experience of a psychologic (i.e., cognitive, behavioral, emotional), social, spiritual, and/or physical nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment” (Mitchell et al., 2011; Riba et al., 2019). Emotional distress not only affects psychological well-being but also triggers physiological changes. Substantial evidence shows that emotional distress is linked to lower quality of life in cancer patients (Riba et al., 2019) and contributes to biobehavioral dysregulation, particularly via alterations in the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system activation (Cohen et al., 2007; Spiegel et al., 2006). The HPA axis, a core component of the stress response system, may become chronically dysregulated—manifested by abnormal cortisol rhythms—and has been associated with fatigue, sleep disturbances, cognitive dysfunction, and emotional distress in cancer populations (Bower, 2005; Ryan et al., 2007). Recent findings (Kanter et al., 2024) support HPA dysfunction as a widespread phenomenon across cancer types, and suggest that fatigue is part of a symptom cluster linked to this dysregulation. These results underscore the potential role of hormonal imbalances in the development and maintenance of CRF, reinforcing the need to integrate emotional and biological factors in clinical care.
To manage these difficulties, patients can rely on various resources that promote better adaptation to the illness, such as coping strategies, perceived control over the illness, and perceived social support. Firstly, coping strategies can be defined as the thoughts and behaviors used to deal with stressful situations (Taylor & Stanton, 2007). Maladaptive coping strategies (e.g., avoidance, denial, rumination, self-blame) are linked with higher emotional distress, higher fatigue, and lower quality of life (Dahal & Meheta, 2018; Dev et al., 2024; Fasano et al., 2020; Nipp et al., 2016; Schaab et al., 2023; Wright et al., 2020). Secondly, perceived control over the course of the disease involves believing that we have personal resources enabling us to confront and manage one or more events linked to the disease (Bruchon-Schweitzer & Boujut, 2014). Several studies highlight the beneficial role of perceived control and patients’ quality of life (Brown et al., 2017; Cousson-Gélie, 2014), as well as in mitigating emotional distress (Henselmans et al., 2009; Ranchor et al., 2010). However, while perceived control over CRF has been insufficiently studied, it is noteworthy that patients perceive it as "completely uncontrollable" (Corbett et al., 2016). When fatigued patients are surveyed, nearly half believe they must live with their fatigue, and only 22 % of them feel they can control it (Stone et al., 2000). This suggests that the link between fatigue and quality of life may be potentially moderated by the level of perceived control patients have over their fatigue. Finally, perceived social support is defined as "the subjective impact of the help provided by an individual’s social network" but also as the perception that "their needs and expectations (in terms of support) are being satisfactorily met" (Procidano, 1992). It has been widely observed that satisfactory social support is associated with better quality of life, lower anxiety, and reduced depression (Mehnert et al., 2010; Pinar et al., 2012; Zhou et al., 2010). However, the effects of social support on fatigue, like those on perceived control, remain underexplored. Nonetheless, it has been noted that emotional or informational social support could lead to a reduction in general and physical fatigue (Soares et al., 2013), whereas insufficient perceived social support is associated with higher fatigue levels in patients (Peters et al., 2014).
As described above, many symptoms reported by patients with cancer are linked to each other, even reinforcing each other (Chirico et al., 2024; Kwekkeboom, 2016; Miaskowski et al., 2017; Schellekens et al., 2020; Zhu et al., 2022). Studying multiple symptoms concurrently as well as their interactions is thus particularly relevant and important. To do so, network analyses can be used. They allow to study symptoms in their full complexity, as dynamic systems of interacting symptoms (Borsboom & Cramer, 2013; de Rooij et al., 2021; Lin et al., 2022; Rha & Lee, 2021). Network analyses are often used to study symptoms, but other variables such as coping strategies can also be added in the networks (Baussard et al., 2024; Chirico et al., 2024; Grégoire et al., 2024; Schellekens et al., 2020). This would allow a better understanding of the relationships between patients’ symptoms and ressources. Core symptoms/variables within a network are the ones with the strongest associations with the other symptoms/variables. They could play a role in activating them (Hevey, 2018). Thus, it is important to identifying them, as they may represent a relevant target to impact the other symptoms through innovative interventions (Baussard et al., 2024; de Rooij et al., 2021; Grégoire et al., 2024; Kwekkeboom, 2016; Rha & Lee, 2021; Schellekens et al., 2020; Shim et al., 2021; Zhu et al., 2022).
This research aims to investigate the network organization of CRF in CRCm patients undergoing chemotherapy. We will consider symptoms and patients’ resources at the same level, to underline how patients cope with the disease. We assume that 1) the network structures of general, physical, and psychological fatigue will be distinct (i.e., the relationships between the CRF dimension and the other variables will be different between the three networks), confirming the multidimensional aspects of CRF, 2) emotional distress, especially depressive symptoms, will be the core symptom in the three networks, based on several similar studies on various cancer patients populations (Baussard et al., 2024; Chirico et al., 2024; Lin et al., 2022; Schellekens et al., 2020) and, 3) patients' resources are expected to show strong associations with CRF and to serve as central variables within the network structure., as shown in recent studies (Baussard et al., 2024; Chirico et al., 2024).
MethodsDesignThis study is a secondary analysis of data from a longitudinal study conducted by our team. The longitudinal study was approved by the French Data Protection Authority (CNIL; no. DR-2015–730) and by the Sud-Méditerranée II IRB and the trial was then registered (Baussard, 2018) on isrctn.org (no. ISRCTN18044948). Written informed consent was provided by all participants prior to study participation. This longitudinal study (Baussard et al., 2022) followed patients with mCRC undergoing chemotherapy and aimed to identify distinct trajectories of fatigue and to explain the trajectory belonging of each patient by psychosocial variables. In this study, participants had to complete several questionnaires at the inclusion and after completing their chemotherapy cycle (6 months), plus an assessment of fatigue every two weeks. Only their baseline data will be considered in the present paper.
ParticipantsPatients with mCRC undergoing chemotherapy were recruited in four hospitals in France between 2015 and 2019, in the context of another study (Baussard et al., 2022). Patients younger than 18 years, those not able to understand French and those with brain metastasis, cognitive impairment, or psychiatric disorder were excluded. As the present cohort came from a previous study of our team, no sample size has been calculated specifically for the present study. In addition, it seems that no standard procedure to determine the ideal sample size for network analysis is commonly used (de Rooij et al., 2021; Shim et al., 2021; Zhu et al., 2022).
MeasuresDemographic and clinical variables were obtained from self-reports and from medical records.
Demographics: information regarding age, sex, relationship status, education level, time since diagnosis, location of the metastases and treatment received was collected.
Patients’ symptoms- -
Fatigue (primary outcome): assessed using The Daily Fatigue Cancer Scale (DFCS), a standardized visual analog scale developed and validated among French patients with cancer (Baussard et al., 2017). It consists of three questions measuring general (“I feel tired”), physical (“I lack energy”), and psychological fatigue (“I feel weary”). For each question, patients answered by moving a cursor along a scale ranging from 0 (Not at all) to 10 (Extremely). In the present study, we will estimate three networks based on each fatigue dimension.
- -
Depression and Anxiety: assessed using the Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, 1983). This scale is often used in clinical or oncology research (Bjelland et al., 2002). It includes 14 items (seven for each dimension). Items are scored on a 4-point scale ranging from 0 to 3. For both subscales, the maximum score is 21, and a cut-off score ≥ 8 indicates a clinical level of anxiety or depression (Castelli et al., 2010).
- -
Perceived control of the illness: assessed using the Cancer Locus of Control Scale (Pruyn et al., 1988). We used the 14-item French version (Cousson-Gelie et al., 2005), were patients are requested to indicate agreement on a 4-point scale ranging from 1 (Not at all in agreement) to 4 (Full agreement). The scale has three dimensions: perceived internal control over the course of the illness (5 items, scores between 6–24), internal causal attribution (i.e., perceived causal attribution of the illness; 6 items, scores between 5–20), and perceived religious control over the cause and course of the illness (3 items, scores between 3–12).
- -
Coping strategies: the French version of the Ways of Coping Checklist (WCC) (Folkman & Lazarus, 1980), validated among patient with cancer (Cousson-Gélie et al., 2010) was used to assess coping strategies. The 21 items are scored on a 4-point Likert-type scale ranging from No to Yes and asses 3 coping strategies: problem-focused strategy (i.e., directed at solving the impact of the stressful event, scores between 8–32), emotion-focused strategy (i.e., direct at affect regulation, scores between 7–28), and social support seeking (scores between 6–24). Patients indicate their agreement with 21 items on a 4-point Likert-type scale ranging from No to Yes.
- -
Perceived social support: assessed using the Cancer-Specific Social Support Questionnaire (Segrestan et al., 2007; Segrestan-Crouzet, 2010). This is a self-administered questionnaire measuring perceived social support of cancer patients in 20 items, according to a five-point Likert scale, ranging from 1 (Never) to 5 (Very often). It assesses four dimensions: emotional support (9 items, scores between 9–45), material/distractive support (5 items, scores between 5–25), informative support (2 items, scores between 2–10) and negative support (4 items, scores between 4–20).
All statistical analyses were conducted using R software (version 4.1.1), specifically the R packages qgraph and bootnet for network analysis. Descriptive statistics were employed to describe the sample (means, standard deviations, and percentages). Categorical variables are presented as counts ( %) and quantitative variables as means with standard deviations (SD). Partial Spearman correlation networks were used to model the conditional independence relationships between variables (network nodes). To introduce sparsity in the networks, a regularized Lasso estimation was applied, with the tuning parameter selected based on the Bayesian Information Criterion (BIC). Sparse networks, rather than complete ones, are presented. The centrality indices used were strength (i.e., the sum of absolute edge weights, indicating how strongly a node is directly connected to other nodes), closeness (i.e., a node’s relationship to all other nodes), betweenness (i.e., the role of a node in the shortest paths between pairs of other nodes), and expected influence (i.e., the sum of a node’s connections, reflecting its relative importance in the network (Robinaugh et al., 2016)). The core symptom in each network was identified based on the highest centrality indices. Bootstrapping (nBoots = 1000) was used to assess the accuracy of the networks, providing estimates and confidence intervals (CI) for node strength and edge weights.
ResultsDescription of the sampleOverall sample characteristics are provided in Table 1. The 169 patients had a mean age of 64.36 years (range = 36–90) and were mostly male (58.6 %). The majority had a partner (75.7 %), 51.5 % had a low educational level, and 85.8 % were unemployed (retired or for medical reasons). The majority had received their diagnosis within the previous 2 years (71.7 %) and were diagnosed with a colon carcinoma (53.8 %). Other localization is rectum (32,5 %) or not defined (13,7 %). Most had Stage III cancer (69.3 %), and metastases were located in the liver (39.6 %), lungs (11.8 %), or both liver and lungs (17.2 %). The sample reported few symptoms. Indeed, the mean scores for CRF ranged from 2.34 to 2.96/10, while anxiety and depression scores were subclinical (<8/21). Regarding the resources used by the patients, the internal control and the problem-focused coping were particularly used, while emotional support was the type of social support mostly reported by the patients.
Patient Characteristics (N = 169).
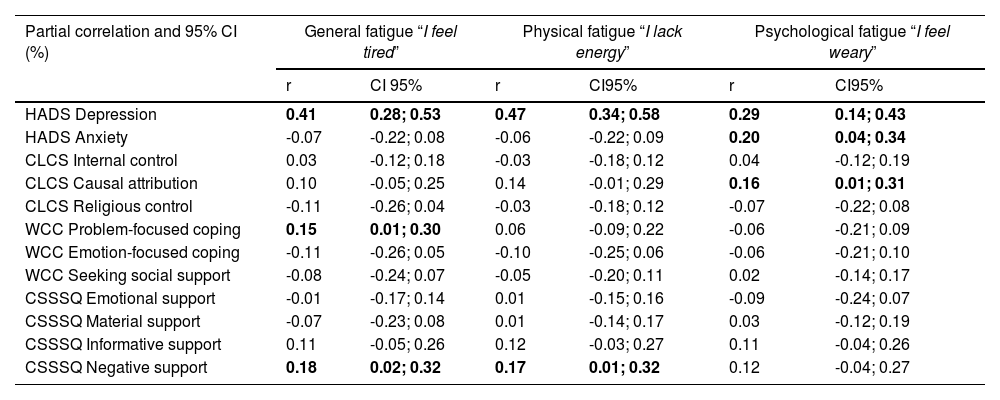
Table 3 details the partial correlations and 95 % CI between each fatigue dimension and the other variables. Fig. 1 illustrates the three networks (see detailed weights in Table 1 from supplementary material) each of which includes one specific fatigue dimension. Depression is significantly associated with each dimension of fatigue (r ranging from 0.29 to 0.47). Anxiety is only significantly associated with psychological fatigue (i.e., “I feel weary”; r = 0.20). Considering patients’ resources, general fatigue (i.e., “I feel tired”) is significantly associated with problem-focused coping (r = 0.15) and negative support (r = 0.18). Physical fatigue (i.e., “I lack energy”) is only significantly associated with negative support (r = 0.17). Finally, psychological fatigue is only significantly associated with causal attribution. Depression and negative social support are the variables with the strongest associations with fatigue nodes in all three networks. These observations were confirmed by bootstrap procedures, confirming the network accuracy (see Fig. 1 in Supplementary Material).
Fig. 2 illustrates the centrality indices of the three networks (also see Table 2 in the Supplementary Material). Based on these indices, especially “expected influence” (Robinaugh et al., 2016), emotion-focused coping strategies and problem-focused coping strategies showed the highest influence in all networks (rexpected influence = 1.03 – 1.10). Regarding the other indices, these coping strategies also revealed the highest strength (i.e., highest number and strength of direct connections; rstrength = 1.00 – 1.16), betweenness (rbetweeness = 21 – 34), and closeness (rcloseness =8.10 – 9.01). Depression is the symptom showing the highest strength after taking into account the importance of coping strategies (rstrength = 0.66 – 0.88). The centrality of each symptom is similar in the three networks. These results were confirmed by bootstrap procedures (see Fig. 2 in the Supplementary Material).
Clinical symptoms and adjustment strategies reported in the sample (N = 169).
Partial correlations and 95% confidence intervals (CI) between each VAS of fatigue and the other symptoms. Bold values indicate significant associations.
This study aimed to explore, through network analyses, the relationships between, on the one hand, three dimensions of CRF (i.e., general, physical, and psychological fatigue) and, on the other hand, emotional distress and patients’ resources (i.e., perceived control of illness, coping strategies, and perceived social support), on a population of 169 patients with metastatic colorectal cancer. Our first hypothesis was that the network structure of each dimension of CRF would be different. Our second hypothesis was that depressive symptoms would be the core symptom in the three networks. Finally, our last hypothesis was that patients’ resources would have strong relationships with CRF. Our first hypothesis is verified. Indeed, we can observe some similarities between the three networks (e.g., depression is strongly associated with each CRF dimension), but also many differences. Each CRF dimension had specific relationships with other variables, as shown in section 3.2. Indeed, anxiety and causal attribution are only significantly correlated with psychological fatigue, while problem-focused coping is only correlated with general fatigue. Negative support is also significantly correlated with general and physical fatigue only. These first results also allow us to confirm our third hypothesis regarding the existence of strong connections between patients’ resources and CRF. Concerning the importance of depression in the networks, it is in line with other studies showing the very strong connections between fatigue and depression and emotional distress in other populations of patients with cancer (Baussard et al., 2024; Götze et al., 2020; Lin et al., 2022; Ma et al., 2020; Mehnert et al., 2018; Schmidt et al., 2018). We could hypothesize that being tired or weary represents the "admissible" aspect of the symptom, whereas depressive thoughts might still be too taboo to express. Regarding the differences between the three networks, the association between anxiety and psychological fatigue is in line with another study showing the association between affective fatigue and psycho-social factors such as mental difficulties and worries about the future (Schmidt et al., 2018). The associations between some resources and CRF is also in line with recent studies showing the link between maladaptive coping strategies and higher fatigue (Dev et al., 2024; Wright et al., 2020). However, these studies considered fatigue as a unique symptom, and not its distinct dimensions. To our knowledge, no study has explored the relationships between the dimensions of CRF and different coping strategies. Our results suggest that general fatigue is particularly linked with problem-focused coping, and negative social support, that physical fatigue is particularly linked with negative social support, and that psychological fatigue is particularly linked with causal attribution. The positive association between general fatigue and problem-focused coping suggests that greater use of this strategy may result in increased fatigue, indicating that it could function in a potentially dysfunctional way. Even if the literature on the effectiveness of coping strategies does not universally favor one approach over another (Faye et al., 2006), a possible explanation is that the perceived controllability of the situation plays a critical role (Carver et al., 1989; Folkman & Lazarus, 1988) and that depending on the level of perceived control, an emotion regulation strategy may yield different outcomes (Fang et al., 2006). As previously mentioned, patients may perceive their fatigue as uncontrollable (Corbett et al., 2016). Consequently, attempting to cope actively with it could exacerbate their exhaustion. It emphasizes the importance of flexibility in selecting strategies based on the demands of the situation. This aligns with the ARC model (Kangas & Gross, 2020), which highlights the role of flexibility in emotion regulation strategies. Indeed, in cancer patients, such flexibility is associated with better psychological adjustment (Cheng, 2003; Cheng et al., 2012; Eto et al., 2022). In conclusion, these results showed the differences in the configuration of the three networks, and confirm the results of recent studies which emphasized the multidimensional aspect of CRF and the need to address each dimension of CRF separately (Person et al., 2020; Schmidt et al., 2018).
On the contrary, our second hypothesis is not verified. Even if depression is a very important variable in all networks, as discussed above, it is not considered as the core variable. Indeed, emotion-focused and problem-focused coping strategies had the highest centrality indices in all networks. This is not surprising as it is known that coping strategies and other resources such as social support influence many symptoms such as emotional distress and fatigue, as well as quality of life, in patients with cancer but also in the general population (Dahal & Meheta, 2018; Dev et al., 2024; Fasano et al., 2020; Freire et al., 2016; Mayordomo-Rodríguez et al., 2016; Nipp et al., 2016; Schaab et al., 2023; Tomás et al., 2012; Wright et al., 2020; Zamanian et al., 2021). It is thus logical that these variables have such central and important positions in our networks. In addition, a recent study conducted a network analysis on 992 patients with mixed cancer diagnoses, combining distress symptoms (i.e., depression and anxiety) and resources factors (Chirico et al., 2024). It showed the importance (i.e., high centrality indices) of resources factors (e.g., coping efficacy, seeking and receiving support) in the network. Altogether, our results suggest that variations in patients’ depressive symptoms and resources, such as coping, social support and causal attribution, could lead to variations in the three CRF dimensions. More specifically, variation of negative social support could be linked to variation of general and physical fatigue, variation of problem-focused coping could lead to variation of general fatigue, and variation of causal attribution could lead to variation of psychological fatigue. This highlights the mediating effect of resources as defined in transactional approaches.
Our results underline the importance of investigating interacting symptoms and resources as a complex and global system. They also suggest relevant therapeutic targets to design interventions to improve the well-being of patients with cancer, mostly their CRF and emotional distress, following recommendations from previous studies (Baussard et al., 2024; de Rooij et al., 2021; Grégoire et al., 2024; Kwekkeboom, 2016; Rha & Lee, 2021; Schellekens et al., 2020; Shim et al., 2021; Zhu et al., 2022).
LimitationsSome limitations to this study must be acknowledged. First, this study is a secondary analysis of data collected in another project (Baussard, 2018; Baussard et al., 2022), not originally intended to be used in network analyses. We do not underestimate the importance of pre-registered studies to limit potential biases emerging from second analyses (Baldwin et al., 2022). In fact, the extensive exploration of numerous variable relationships within a dataset, where the protocol was not designed for this purpose, pose methodological challenges and limit the generalization of the results. Second, the sample size is small. There is no standard procedure to determine the sample size needed to perform network analyses, and the number of patients included in this study is close to those used in other similar studies (e.g., 172 in Lin et al. (2022), 190 patients in de Rooij et al. (2021), 159 in Baussard et al. (2024)). However, it could be possible that a larger sample size would have allowed more significant results to appear. Third, covariates (e.g., age, time since diagnosis) were not controlled in the present analysis. Lastly, the baseline symptoms of the participants were low (i.e., subclinical anxiety and depression, and all CRF scores < 3/10). This was quite unexpected, as emotional distress and CRF are very often reported as major difficulties by patients with cancer, notably with metastatic colorectal cancer. Regarding fatigue, this could be due to the measurement tool used (i.e., a VAS). Indeed, numerical rating scales are usually recommended to investigate such symptoms, due to higher compliance rates, responsiveness, ease of use and applicability (Hjermstad et al., 2011). It is possible that these lower mean scores for both fatigue and distress influenced our results and may limit the generalizability of the findings. This underscores the need for future studies focusing on populations in which CRF is more prominent.
Implications for clinical practice and future researchOur results nevertheless underline the multidimensionality of CRF and the importance of the patients’ resources. They also suggest some therapeutic targets that could be used to design more cost-effective and personalized interventions to improve CRF and emotional distress of patients with cancer. By integrating HPA axis dysregulation into our conceptual framework, we adopt a more comprehensive biopsychosocial view of cancer-related fatigue and support this idea. If emotional distress contributes to both psychological suffering and physiological dysregulation, addressing it becomes essential not only for mental health but also for the management of physical symptoms such as fatigue. Psychological interventions—such as cognitive-behavioral therapy, mindfulness-based stress reduction, and structured stress management programs—have demonstrated effectiveness in reducing both distress and fatigue, possibly by normalizing cortisol patterns and enhancing coping strategies (Bower et al., 2011; Lengacher et al., 2012). In metastatic colorectal cancer, where symptom burden is particularly high, early and systematic management of emotional distress may mitigate fatigue and improve overall quality of life.
To do so, fostering the development and use of personal resources and more adaptive coping strategies (e.g., positive reappraisal, acceptance, optimism) appears to be important, as well as enhancing positive social support. Future research could also extend these findings to other populations of patients with cancer, to better understand their specific reality, and suggest relevant targets for personalized intervention programs. Following this idea, an ongoing study is using network analyses to explore the relationships between several common symptoms (e.g., pain, CRF, sleep difficulties, emotional distress, cognitive difficulties) and resources (i.e., coping strategies, self-compassion) on two distinct populations of cancer survivors (i.e., breast and digestive cancer) (Grégoire et al., 2024). In a second phase of this study, the researchers aim to develop a more personalized mind-body intervention to target the core symptom of the network for each cohort. Hopefully, this cost-effective intervention will allow an improvement not only of the core symptoms, but also of several other symptoms of the networks, through a “domino effect”. Another strategy could be to apply existing interventions to target the most central symptom, rather than developing new ones. This approach may be more feasible in terms of time and resources. However, it requires identifying interventions that specifically target a single symptom, which is challenging, as most psychosocial interventions in oncology are designed to address multiple symptoms simultaneously. Moreover, the added value of a network-based intervention approach lies in examining how changes in a central symptom influence the broader symptom network. This dynamic cannot be adequately assessed when using broad-spectrum interventions.
Another perspective would be to explore the evolution of such networks over time. Temporal network analysis allows for the comparison of distinct networks (e.g., across populations or time points), which may be particularly relevant in metastatic cancer. Given the progressive deterioration of functional status with successive treatment lines, such analyses could help identify phase-specific therapeutic targets, according to the treatment stage or time since diagnosis.
We are convinced that network analysis is a powerful tool which allows researchers to align with the clinical reality of the patient and develop appropriate management strategies. Some recent studies even used such analyses at an individual level with patients in oncology, to better understand the relationships between their personal difficulties and improve their management (Bickel et al., 2022; Bootsma et al., 2022).
ConclusionOur study confirmed the multidimensional aspect of CRF and showed the specific connections of each of its dimensions with other variables. Among them, depressive symptoms were associated with all CRF dimensions, while anxiety was correlated with psychological fatigue only. Some patients’ resources also had specific connections with CRF dimensions, and problem- and emotion-oriented coping strategies were the core variables of the three networks. Our results are in line with many other studies in the field. However, these studies have mainly be conducted on women with breast cancer. Our study thus provides new insights on the condition of patients with metastatic colorectal cancer, as well as regarding the links between coping strategies and fatigue. Management of depressive symptoms, as well as development of more adaptive coping strategies and positive social support should then be considered when designing interventions to improve CRF, and more generally the well-being, of patients with metastatic colorectal cancer.
CRediT authorship contribution statementCharlotte Grégoire: Writing – original draft, Writing – review & editing. Florence Cousson-Gélie: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing. Louise Baussard: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Funding acquisition, Methodology, Project administration, Supervision.
The authors declare no conflicts of interest
Funding sources: SHSESP 2015, French National Cancer Institute (no. INCA_9565), 2023 postdoctoral contract FRS-FNRS (Télévie, Belgium).