Resting-state functional connectivity magnetic resonance imaging (rs-fMRI) is a sensitive tool for detecting early brain changes associated with Alzheimer’s disease, even in its preclinical stages. Amnestic mild cognitive impairment (aMCI) and late-life depression (LLD) are two prevalent conditions in older adults that significantly elevate the risk of cognitive decline and dementia. This study aimed to elucidate the underlying neurobiological substrates by longitudinally identifying and comparing distinct connectivity patterns in aMCI subjects and LLD patients, and by examining the associations between these patterns and clinical measures of cognitive and mood impairments.
MethodsThe study included three groups: 26 healthy controls (HCs), 15 individuals with aMCI, and 21 patients with LLD. All participants underwent rs-fMRI and neuropsychological assessments at baseline and at a 2-year follow-up. Functional connectivity was analyzed using a group Independent Component Analysis (ICA) model to investigate both group differences and longitudinal changes over time.
ResultsAt baseline, individuals with aMCI exhibited reduced functional connectivity in the precuneus, whereas LLD patients showed decreased connectivity in frontal, insular, and postcentral regions alongside increased connectivity in posterior parietal and cuneal cortices. Correlation analyses revealed that lower baseline insular connectivity predicted higher depressive symptoms at follow-up in aMCI subjects. In LLD, reduced baseline precuneus connectivity was associated with better two‐year outcomes in global cognition and long‐term memory.
ConclusionsThis study provides evidence of distinct alterations in resting-state functional connectivity in individuals with aMCI and LLD, underscoring region-specific vulnerabilities that may contribute to cognitive decline and depressive symptomatology in older adults.
The global burden of dementia continues to rise, with nearly 10 million new cases diagnosed annually (WHO, 2024). Alzheimer's disease (AD), the most common cause of dementia, is associated with neurodegenerative changes that may begin 20–30 years before clinical symptoms manifest (Jansen et al., 2015). Despite global efforts, effective interventions for AD prevention remain elusive, largely due to challenges in identifying high-risk individuals early enough for therapeutic interventions. Amnestic mild cognitive impairment (aMCI) and late-life depression (LLD) are highly prevalent conditions that often coexist and significantly elevate the risk of cognitive decline and dementia in older adults (Brailean et al., 2017; Rajji et al., 2024; Zacková et al., 2021). Although aMCI and LLD are considered distinct clinical entities, it remains unclear whether they share common pathogenic mechanisms or involve distinct pathways (Xie et al., 2013). Given that cognitive impairment is a common feature of LLD, and that depression may increase the risk of cognitive decline in otherwise cognitively intact older adults compared to those without depressive symptoms, further research is needed to elucidate the neural correlates underlying the connection between depressive symptoms and cognitive dysfunction.
Aiming to elucidate the neurobiological mechanisms underlying the risk for AD, several neuroimaging studies have employed resting-state functional magnetic resonance imaging (rs-fMRI) (Albert et al., 2011; Teipel et al., 2018) to identify localized abnormalities in brain function and connectivity. These studies reveal that cognitive impairments—particularly in executive function and processing speed within the frontal‐executive circuit, and in episodic memory within the corticolimbic circuit—are common across LLD, aMCI, and AD (Rashidi-Ranjbar et al., 2020). Although these studies have advanced our understanding of the neural correlates of LLD (Trapp et al., 2023; Zhao et al., 2019) and aMCI (Matsuoka et al., 2021; Munro et al., 2015), challenges remain in characterizing and comparing resting-state connectivity patterns across these at-risk populations. First, many investigations have employed a region-of-interest (ROI) approach to examine functional connectivity (FC) changes in specific brain areas, revealing both increased and decreased connectivity in regions such as the superior and inferior frontal gyri, precuneus, precentral gyrus, cingulate gyrus, parahippocampal cortex, cerebellum, and putamen (Berron et al., 2020; Ibrahim et al., 2021; Jellinger, 2023; Liang et al., 2020). However, the reliance on predefined ROIs may introduce biases that overlook key areas associated with the core pathological changes in these conditions. Second, cross-sectional studies suggest that LLD and aMCI share common impairments in brain regions, potentially reflecting a continuum of disease progression toward AD (Invernizzi et al., 2021); however, longitudinal studies are essential to validate this hypothesis. To date, only a limited number of rs-fMRI studies (Jovicich et al., 2019; Khodadadi Arpanahi et al., 2025; Schultz et al., 2020; Wang et al., 2015) have examined longitudinal connectivity changes in individuals at risk for AD. Third, most studies investigating LLD and aMCI have examined these disorders separately, often using heterogeneous cohorts, thereby failing to elucidate the similarities and differences between them.
To address these limitations, this study aimed to identify distinct connectivity patterns in individuals with aMCI, patients with LLD, and a comparable group of healthy controls (HCs) by comparing baseline and two-year follow-up findings using independent component analysis (ICA). ICA extracts functional connectivity information by identifying spatially independent and temporally synchronous brain regions without relying on a priori assumptions. We hypothesized that participants with aMCI and patients with LLD would exhibit specific functional connectivity disruptions on resting-state fMRI that will correlate with clinical measures of cognitive and mood impairment. Furthermore, we anticipated that these connectivity patterns would differ among the three groups.
MethodsParticipantsThe study comprised three groups: 26 HCs, 15 individuals with aMCI, and 21 patients with LLD. Participants from the aMCI and LLD groups were consecutively recruited from the Neurology and Psychiatry Departments of Bellvitge University Hospital (Barcelona, Spain), while HCs were recruited via advertisements within the same hospital’s catchment area. To assess the presence of psychiatric disorders, all participants underwent the Spanish version of the Mini-International Neuropsychiatric Interview (MINI) (Ferrando et al., 1998). Diagnoses of LLD were confirmed by two experienced psychiatrists (MU and VS) according to DSM-IV-TR (American Psychiatric Association., 2000) criteria (which do not substantially differ from DSM-5-TR criteria and are aligned with the diagnostic criteria of the interview used to identify comorbid symptoms), and aMCI diagnoses were determined by two experienced neurologists (JG and RR) based on Petersen’s criteria (Petersen, 2004), utilizing a syndromic categorical cognitive staging approach.
Participants were subject to several exclusion criteria: (1) ages <60 or >75 years, to minimize effects of altered neurovascular coupling, (2) a past or current diagnosis of other major psychiatric disorders, including substance abuse or dependence (excluding nicotine), (3) intellectual disability or neurodevelopmental disorders, (4) neurological disorders, (5) a Hachinski Ischemic Score (Hachinski et al., 1975) greater than 5, to exclude those at high risk for vascular cognitive impairment, (6) dementia diagnosis according to DSM-IV-TR (American Psychiatric Association., 2000) criteria and/or a Clinical Dementia Rating (CDR) (Hughes et al., 1982) score greater than 1, (7) severe medical conditions, (8) electroconvulsive therapy within the past year, (9) conditions impeding neuropsychological assessment or magnetic resonance imaging (MRI) examinations, and (10) anatomical abnormalities detected in the MRI scan.
Written informed consent was obtained from all participants prior to their involvement in the study. All study procedures adhered to the Declaration of Helsinki and were approved by the Clinical Research Ethics Committee of Bellvitge University Hospital (reference PR156/15, February 17th, 2016).
Clinical assessmentAt both baseline and the 2-year follow-up, all participants completed the Spanish version of the Mini-Mental State Examination (MMSE) (Folstein et al., 1975). Depressive symptom severity was assessed, though not for diagnostic purposes, using the validated Spanish versions of the Geriatric Depression Scale (GDS) (Yesavage et al., 1982) and the 17-item Hamilton Depression Rating Scale (HDRS17) (Hamilton, 1960). Additionally, objective long-term memory impairments were identified as scores 1.5 standard deviations below age- and education-adjusted normative values on the Delayed Recall Test from the Wechsler Memory Scale – Third Edition (WMS-III) (Wechsler D., 2004).
Imaging data acquisitionBaseline and 2-year follow-up MRI data were acquired using the same 3.0 T clinical MRI scanner (Ingenia, Philips Healthcare, Best, The Netherlands) equipped with a 32-channel head coil at the Imaging Diagnostic Institute (IDI) in Barcelona, Spain. Participants underwent a resting-state fMRI sequence while fixating on a cross and being instructed to “clear their mind” without focusing on specific thoughts. Data were collected using an echo planar imaging (EPI) sequence, sensitive to fluctuations in the Blood Oxygenation Dependent Level (BOLD) contrast, with the following parameters: 240 vol (excluding the four initial dummy volumes), 40 axial interleaved slices perpendicular to the floor of the fourth ventricle, over a matrix of 80 mm × 80 mm, repetition time (TR) = 2000 ms, echo time (TE) = 25 ms, flip angle (FA) = 90°, 3 mm isometric voxel size, field of view (FOV) = 24 cm. The duration of this acquisition was 8 min and 12 s. The scanning also included a whole-brain T1 weighted three-dimensional inversion-recovery prepared spoiled gradient echo sequence (TR = 10.46 ms, TE = 4.79 ms, FA = 8°, 0.75 mm isometric voxel size, FOV = 24 cm, 233 axial slices over a matrix of 320 mm × 318 mm), lasting 5 minutes 4 seconds.
rs-fMRI preprocessingBoth functional and anatomical images underwent preprocessing using the CONN functional connectivity toolbox (release 21.a). A comprehensive description of the preprocessing pipeline is provided in the Supplementary Material.
rs-fMRI connectivity analysisFirst-level analysisGroup-level independent component analysis (group-ICA (Calhoun et al., 2001) was performed to estimate 20 temporally coherent resting-state networks (RSNs) from the rs-fMRI data combined across all participants (HCs, aMCI, and LLD). The BOLD signal from every timepoint and voxel in the brain was concatenated across subjects along the temporal dimension. A singular value decomposition of the z-score normalized BOLD signal (subject-level SVD) with 64 components separately for each subject was used as a subject-specific dimensionality reduction step. The dimensionality of the concatenated data was further reduced using a singular value decomposition (group-level SVD) with 20 components, and a fast-ICA fixed-point algorithm (Hyvarinen, 1999) with hyperbolic tangent (G1) contrast function was used to identify spatially independent group-level networks from the resulting components. Last, GICA3 back-projection (Erhardt et al., 2011) was used to compute ICA maps associated with these same networks separately for each individual subject.
Group-level analysesGroup-level analyses were performed using a General Linear Model (GLM) (Nieto-Castanon, 2020). For each individual voxel a separate GLM was estimated, with first-level connectivity measures at this voxel as dependent variables (one independent sample per subject and one measurement per experimental condition), and groups as independent variables. Voxel-level hypotheses were evaluated using multivariate parametric statistics with random-effects across subjects and sample covariance estimation across multiple measurements. Inferences were performed at the level of individual clusters (groups of contiguous voxels). Cluster-level inferences were based on parametric statistics from Gaussian Random Field theory (Nieto-Castanon, 2020; Worsley et al., 1996). Results were thresholded using a combination of a cluster-forming p < 0.001 voxel-level threshold, and a familywise corrected p-FDR < 0.05 cluster-size threshold (Chumbley et al., 2010). To explore the association between significant brain-derived between group differences from the RSNs and clinical measures, peak activation eigenvalues from regions displaying significant differences were extracted.
Statistical analyses of non-imaging dataNon-imaging data were analyzed using IBM SPSS Statistics version 23. Statistical significance was set at p < 0.05. A one-way ANOVA was employed to compare study variables across groups (including demographic and clinical data). To identify group × time interactions we assessed repeated measures ANOVA and further post-hoc Scheffé test. Furthermore, the linear associations between brain-derived eigenvalues and clinical data, controlling for age, were assessed using first-order partial correlations.
ResultsDemographic and clinical characterizationTable 1 summarizes the demographic and clinical characteristics of the three groups at baseline and the 2-year follow-up. The most relevant findings reported in the table are that HCs consistently scored higher on global cognition compared to participants in the aMCI and LLD groups, and that the rate of cognitive decline over time differed significantly between the groups, as indicated by a significant group-by-time interaction (F(2,59) = 9.66, p < 0.001, η² = 0.058). A significant group-by-time interaction was also observed for depression symptoms (HDRS17: F(2, 59) = 12.44, p < 0.001, η² = 0.100), suggesting that the group differences in depressive symptom severity changed over time. Specifically, while patients with LLD showed higher depression severity at baseline compared to HCs and aMCI subjects (GDS: F(2,59) = 10.53, p < 0.001, η² = 0.263; HDRS17: F(2,59) = 23.51, p < 0.001, η² = 0.444), these differences were not maintained at follow-up. Additionally, significant age differences were observed between groups and were controlled for in subsequent analyses, as previously noted.
Sample description.
| Baseline | 2-year follow-up | Longitudinal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HCs; n = 26 | aMCI; n = 15 | LLD; n = 21 | Statistica (p-value) | HCs; n = 26 | aMCI; n = 15 | LLD; n = 21 | Statistica (p-value) | Statisticb (p-value) | |
| Agemean (SD) [range] | 67.04 (3.96) [60–75] | 70.80 (3.03) [64–75] | 68.29 (3.80) [62–74] | 4.91* (<0.01) | 69.00 (3.98)[62–77] | 72.67 (3.11) [66–77] | 70.29 (3.80) [64–76] | 4.60* (<0.01) | 1.00(0.37) |
| Sexmale; n ( %) | 9 (34.6 %) | 8 (53.3 %) | 4 (19.0 %) | 4.60(0.10) | |||||
| MMSEmean (SD) [range] | 28.85 (1.46) [24–30] | 26.00 (2.00) [22–29] | 26.90 (2.28) [21–30] | 12.22* (<0.01) | 28.81 (1.30)[25–30] | 22.00 (4.09) [16–27] | 25.48 (2.75) [19–29] | 31.11* (<0.01) | 9.66*(<0.01) |
| GDSmean (SD) [range] | 1.31 (2.45)[0–12] | 2.53 (2.47)[0–9] | 5.57 (4.27)[0–12] | 10.53* (<0.01) | 1.81 (2.70)[0–9] | 2.67 (3.72)[0–15] | 5.43 (3.88)[0–13] | 6.93* (<0.01) | 0.44(0.65) |
| HDRS17mean (SD) [range] | 1.35 (3.03)[0–15] | 4.87 (3.87)[0–14] | 10.29 (6.03)[1–19] | 23.51* (<0.01) | 1.88 (2.00)[0–7] | 5.20 (7.67)[0–30] | 3.57 (4.13)[0–16] | 2.51(0.09) | 12.44*(<0.01) |
| Long Term Memorycmean (SD) [range] | 8.23 (1.63)[6–12] | 2.27 (1.28)[0–4] | 6.43 (1.69)[4–10] | 68.45*(<0.01) | 8.04 (1.73)[3–11] | 2.07 (2.58)[0–7] | 6.05 (2.65)[0–11] | 32.45*(<0.01) | 0.07(0.93) |
Abbreviations: aMCI, amnestic type mild cognitive impairment subjects; GDS, Geriatric Depression Scale; HCs, healthy controls; HDRS17, Hamilton Depression Rating Scale; LLD, late-life depression patients; MMSE, Mini-Mental State Examination.
A total of 20 components were derived from probabilistic ICA, with 7 components representing the most typical RSNs (see Figures S1 and S2 for detailed spatial correlation of independent components).
At baseline, individuals with aMCI demonstrated significantly reduced connectivity in the precuneus cortex within component 1 (representing the dorsal attention network) compared to HCs (p-FDR= 0.04; Table 2; Fig. 1A). The repeated-measures ANOVA revealed a significant group-by-time interaction in the precuneus cortex (F(2, 59) = 4.40, p = 0.016, η² = 0.076; Fig. 1B). Scheffé‐adjusted pairwise comparisons revealed that baseline precuneus connectivity was lower in the aMCI group than in HCs (t(59) = 4.27, p Scheffé = 0.006). No other between-group or within-group contrasts survived Scheffé correction (all p Scheffé ≥ 0.166).
Group comparisons of local correlation differences at baseline.
Abbreviations: aMCI, amnestic type mild cognitive impairment subjects; FDR, false discovery rate; HCs, healthy controls; L, left; LLD, late-life depression patients; R, right.
Group differences in functional connectivity in the precuneus cortex. (A) Baseline analysis reveals significantly decreased connectivity in the precuneus cortex in subjects with amnestic mild cognitive impairment (aMCI) compared to healthy controls (HCs); the color bar represents t-values. (B) Longitudinal changes in functional connectivity among HCs, individuals with aMCI, and patients with late-life depression (LLD) are presented at baseline and at the 2-year follow-up.
*p < 0.05, **p Scheffé < 0.05.
At baseline, patients with LLD exhibited significantly decreased connectivity compared to HCs in the right middle frontal gyrus (p-FDR< 0.01; Table 2; Fig. 2A) and right angular gyrus (p-FDR= 0.01; Table 2; Fig. 2A) within the component 5 (representing the salience network). A repeated-measures ANOVA revealed a significant group-by-time interaction in both the right middle frontal gyrus (F(2, 59) = 6.84, p = 0.002, η² = 0.081; Fig. 2B) and the right angular gyrus (F(2, 59) = 7.44, p = 0.001, η² = 0.109; Fig. 2C). Scheffé‐adjusted pairwise comparisons at baseline showed that LLD patients had lower right middle frontal gyrus connectivity than HCs (t(59) = 5.24, p Scheffé < 0.001), whereas the LLD vs aMCI contrast was nonsignificant (t(59)= 1.91, p Scheffé = 0.604). Over time, only HCs showed a significant decrease in middle frontal gyrus connectivity (t(59) = 3.90, p Scheffé = 0.016); neither the aMCI nor LLD groups changed significantly, and no between-group differences at 2-year follow-up survived Scheffé correction (all p Scheffé > 0.93). For the right angular gyrus connectivity, baseline LLD vs HCs connectivity differences were likewise significant (t(59) = 4.80, p Scheffé = 0.001), with a nonsignificant LLD-aMCI contrast after correction (t(59)= 2.56, p = 0.013, p Scheffé = 0.604). Only HCs showed a longitudinal decrease in angular gyrus connectivity (t(59) = 4.82, p Scheffé = 0.001), and no group contrasts at 2-year follow-up remained significant (all p Scheffé > 0.95).
Group differences in functional connectivity in the middle frontal and angular gyri. (A) At baseline, patients with late-life depression (LLD) exhibited reduced connectivity in the right middle frontal and right angular gyri relative to healthy controls (HCs); the color bar indicates t-values. (B) Longitudinal changes in right middle frontal gyrus connectivity are shown for HCs, individuals with amnestic mild cognitive impairment (aMCI), and patients with LLD, measured at baseline and at a 2-year follow-up. (C) Longitudinal changes in right angular gyrus connectivity are depicted for HCs, individuals with aMCI, and patients with LLD, measured at baseline and at a 2-year follow-up.
*p < 0.05, **p Scheffé <0.05.
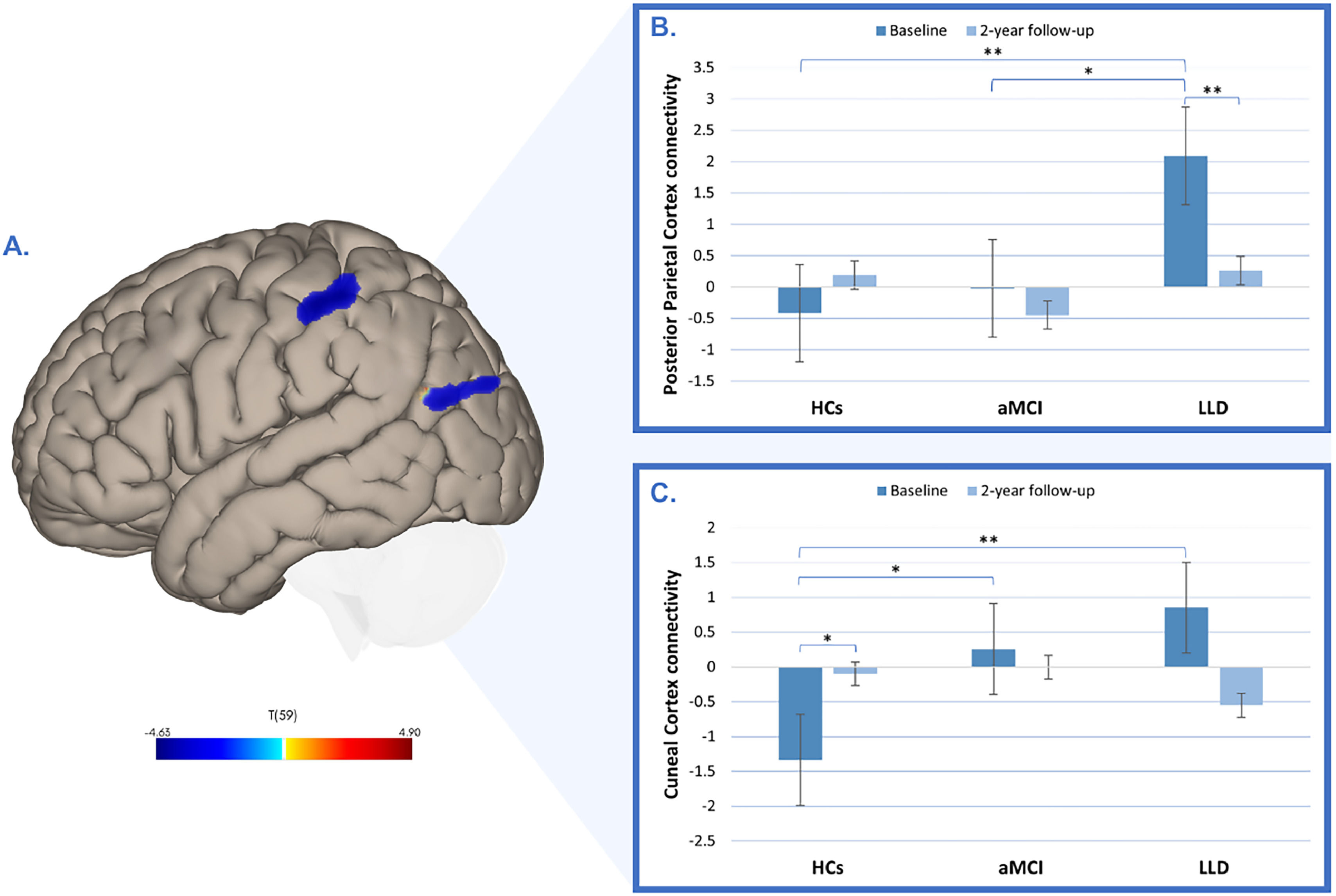
Interestingly, compared to HCs, patients with LLD also showed significantly increased connectivity in the left posterior parietal cortex (p-FDR< 0.01; Table 2; Fig. 3A) and left cuneal cortex (p-FDR = 0.02; Table 2; Fig. 3A) within the component 5 (representing the salience network). A repeated-measures ANOVA revealed a significant group-by-time interactions for both the left posterior parietal cortex (F(2, 59) = 9.35, p <0.001, η² = 0.083; Fig.3B) and the left cuneal cortex (F(2, 59) = 8.96, p = <0.001, η² = 0.124; Fig.3C). Scheffé‐adjusted pairwise comparisons at baseline showed higher connectivity in LLD patients than HCs in the posterior parietal cortex connectivity (t(59) = −4.57, p Scheffé = 0.003), with the aMCI-LLD contrast not significant after correction (t(59)= −3.34, p = 0.001, p Scheffé = 0.063). Over time, only LLD patients exhibited a significant decrease in posterior parietal cortex connectivity (t(59) = 4.36, p Scheffé = 0.005); no between-group differences at 2-year follow-up survived correction (all p Scheffé > 0.82). For the cuneal cortex, only the baseline HCs-LLD contrast was significant (t(59) = −4.70, p Scheffé = 0.002), with a nonsignificant aMCI-LLD contrast (t(59)= −1.11, p Scheffé = 0.941). No other contrasts survived correction (all p Scheffé ≥ 0.105).
Group differences in functional connectivity in the posterior parietal and cuneal cortices. (A) Baseline increases in functional connectivity in the left posterior parietal cortex and left cuneal cortex in patients with late-life depression (LLD) compared to healthy controls (HCs). The color bar indicates t-values. (B) Longitudinal changes in functional connectivity in the left posterior parietal cortex across HCs, individuals with amnestic mild cognitive impairment (aMCI), and patients with LLD, measured at baseline and at the 2-year follow-up. (C) Longitudinal changes in functional connectivity in the left cuneal cortex across HCs, individuals with aMCI, and patients with LLD, measured at baseline and at the 2-year follow-up.
*p < 0.05, **p Scheffé <0.05.
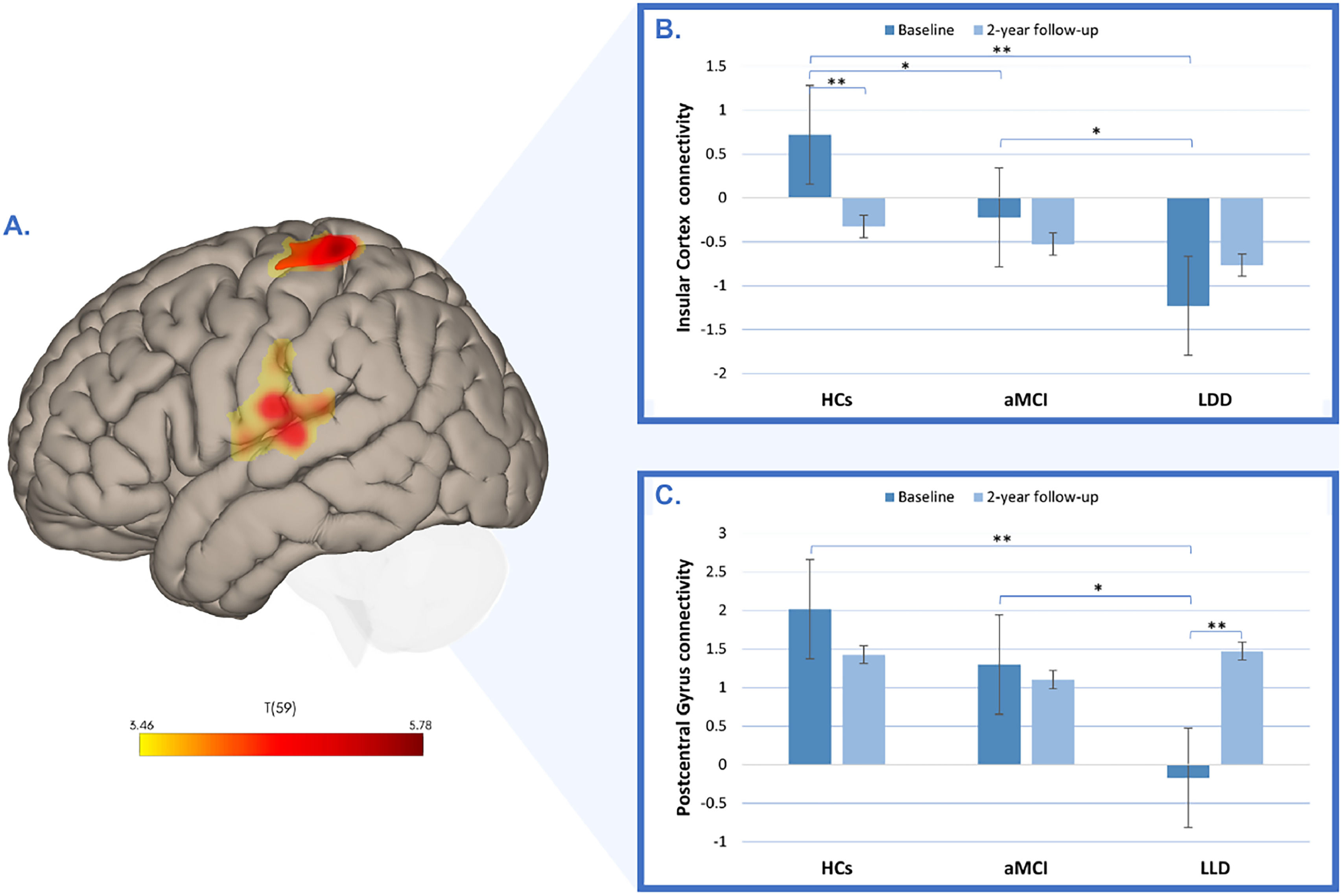
Additionally, patients with LLD showed decreased connectivity in the right insular cortex (p-FDR< 0.01; Table 2; Fig. 4A) and left postcentral gyrus (p-FDR< 0.01; Table 2; Fig. 4A) within component 20 (representing the sensorimotor network). The repeated-measures ANOVA revealed a significant group-by-time interactions for both the right insular cortex (F(2, 59) = 6.17, p = 0.004, η² = 0.065; Fig.4B) and the left postcentral gyrus (F(2, 59) = 12.70, p = <0.001, η² = 0.121; Fig.4C). Scheffé‐adjusted pairwise comparisons at baseline showed lower connectivity in LLD patients than HCs in the right insular cortex connectivity (t(59) = 6.01, p Scheffé < 0.001), with the HCs-aMCI contrast (t(59)= 2.62, p = 0.011, p Scheffé = 0.245) and the aMCI-LLD contrast (t(59)= 2.69, p = 0.009, p Scheffé = 0.219) not significant after correction. Over time, only HCs exhibited a significant decrease in insular cortex connectivity (t(59) = 3.64, p Scheffé = 0.032); no between-group differences at 2-year follow-up survived correction (all p Scheffé > 0.67). For the left postcentral gyrus, only the baseline HCs-LLD contrast was significant (t(59) = 5.43, p Scheffé <0.001), with a nonsignificant aMCI-LLD contrast after correction (t(59)= 3.15, p = 0.003, p Scheffé = 0.094). Longitudinally, only LLD patients exhibited a significant increase in postcentral gyrus connectivity (t(59) = −4.81, p Scheffé = 0.001), and no other contrasts survived correction (all p Scheffé ≥ 0.094).
Group differences in functional connectivity in the insular cortex and postcentral gyrus. (A) Baseline decreased connectivity in the right insular cortex and left postcentral gyrus in patients with late-life depression (LLD) compared to healthy controls (HCs). The color bar represents t-values. (B) Longitudinal changes in functional connectivity in the right insular cortex across HCs, individuals with amnestic mild cognitive impairment (aMCI), and patients with LLD, measured at baseline and at the 2-year follow-up. (C) Longitudinal changes in functional connectivity in the left postcentral gyrus across HCs, individuals with aMCI, and patients with LLD, measured at baseline and at the 2-year follow-up.
*p < 0.05, **p Scheffé <0.05.
Detailed post hoc Scheffé test results are provided in Supplementary Table S1 and Supplementary Table S2.
Correlations between group-ICA results and clinical dataCorrelation analysis revealed that lower baseline connectivity in the left posterior parietal cortex among HCs was significantly correlated with higher baseline Long-Term Memory scores (r=−0.42; p = 0.03; Fig. 5A). Additionally, in the aMCI group, baseline connectivity in the left cuneal cortex positively correlated with higher baseline GDS scores (r = 0.61; p = 0.02; Fig. 5B).
Correlations between functional connectivity and clinical variables at baseline. (A) Depicts the correlation between left posterior parietal cortex connectivity and Long-Term Memory (age-adjusted scores). (B) Depicts the correlation between left cuneal cortex connectivity and GDS (age-adjusted scores).
Also in the aMCI group, lower baseline connectivity in the right insular cortex was significantly associated with higher depression scores at follow-up (GDS: r=−0.56; p = 0.04; Figure 6A; HDRS17: r=−0.65; p = 0.02; Fig. 6B). Conversely, baseline connectivity in the left postcentral gyrus showed a significant positive correlation with follow-up GDS scores (r = 0.56; p = 0.04; Fig. 6C), Additionally, baseline connectivity in the left cuneal cortex demonstrated a significant positive correlation with follow-up adjusted HDRS17 scores (r = 0.56; p = 0.04; Fig. 6D).
Correlations between baseline functional connectivity and follow-up depression scores. (A) Depicts the correlation between baseline connectivity in the insular cortex and follow-up and baseline GDS age-adjusted scores. (B) Depicts the correlation between baseline connectivity in the insular cortex and follow-up and baseline HDRS17 age-adjusted scores. (C) Depicts the correlation between baseline connectivity in the left postcentral gyrus and follow-up baseline GDS age-adjusted scores. (D) Depicts the correlation between baseline connectivity in the left cuneal cortex and follow-up and baseline HDRS17 age-adjusted scores.
In the LLD group, lower baseline connectivity in the precuneus cortex significantly correlated with higher follow-up MMSE scores (r=−0.46; p = 0.04; Fig. 7A) and higher follow-up Long Term Memory scores (r=−0.65; p ≤ 0.01; Fig. 7B).
Correlations between baseline functional connectivity and follow-up cognitive performance. (A) Depicts the correlation between baseline connectivity in the precuneus cortex and follow-up and baseline MMSE age-adjusted scores. (B) Depicts the correlation between baseline connectivity in the precuneus cortex and follow-up and baseline age-adjusted Long-Term Memory scores.
Partial correlation analyses revealed no significant associations between changes in clinical and neuroimaging measures.
DiscussionOur study employed ICA to longitudinally compare resting-state functional connectivity patterns in HCs and two groups at heightened risk for AD. At baseline, both aMCI and LLD groups exhibited distinct functional connectivity profiles relative to HCs. The aMCI group showed markedly reduced connectivity in the precuneus cortex compared to HCs, whereas LLD patients exhibited decreased connectivity in the right middle frontal gyrus, right angular gyrus, right insular cortex, and left postcentral gyrus. In contrast, LLD patients also displayed increased connectivity in the left posterior parietal cortex and left cuneal cortex. Notably, baseline connectivity patterns in these regions correlated with follow-up clinical measures of depressive symptoms and cognitive performance, highlighting that individual differences in these connectivity patterns may account for important differences in cognitive and behavioral functions. Understanding these patterns can play an important role in predicting the early onset of neurodegenerative diseases and in monitoring disease care and treatment.
At baseline, individuals with aMCI showed reduced precuneus functional connectivity compared to HCs, which is consistent with previous findings (Li et al., 2020; Qi et al., 2010). This alteration underscores the precuneus’s altered resting-state activity as a meaningful functional hallmark distinguishing aMCI from HCs (Yan et al., 2013). The precuneus, a key node of the posteromedial cortex, participates in distinct functional networks and supports a wide range of cognitive processes, including self-referential thinking, episodic memory, and visuospatial functions, making it particularly susceptible to both aging and cognitive-related alterations (Cavanna & Trimble, 2006). Furthermore, emerging evidence suggests that aberrant precuneus connectivity is linked to early amyloid pathology in AD and is associated with cholinergic dysfunction, contributing to cognitive decline (Ikonomovic et al., 2011; Mi et al., 2017). Thus, it is consistent that before any manifestations of cognitive or behavioral changes, early manifestation of Aβ toxicity could be detected with resting state fMRI (Sheline & Raichle, 2013). Interestingly, in our study, in patients with LLD, lower precuneus functional connectivity at baseline was significantly associated with better cognitive performance at the two-year follow-up, as reflected by higher MMSE and Long-Term Memory scores. This finding concurs with previous findings indicating that the precuneus may play an important role in depression-related cognitive dysfunctions (Szymkowicz et al., 2017). Further studies are required to elucidate how precuneus functional alterations contribute to psychological and cognitive processes in older adults.
Patients with LLD exhibit distinctive patterns of decreased functional connectivity, particularly in the right middle frontal gyrus and right angular gyrus, at baseline compared to HCs. The right middle frontal gyrus, a component of the prefrontal cortex, is involved in executive functions, including decision-making, attention, and working memory (Kolk & Rakic, 2022), as well as cognitive control and emotional regulation (Kaiser et al., 2015; Zhu et al., 2022). Reduced connectivity in this region may impair cognitive control and emotional regulation, manifesting as difficulties in attention and emotion management—symptoms frequently observed in LLD (Liu et al., 2021; Zhu et al., 2022). Meanwhile, the right angular gyrus is associated with language, numerical processing, memory retrieval, and attention (Wang et al., 2016; Zhu et al., 2021). Diminished connectivity in the right angular gyrus can disrupt sensory integration and complex cognitive functions, potentially contributing to the memory and attention deficits commonly seen in LLD (Wang et al., 2013; Zhu et al., 2021) Moreover, altered connectivity in these regions may promote self-referential thinking and rumination, hallmark features of MDD that intensify depressive symptoms by reinforcing negative thought patterns and reducing engagement with the external environment (Hao et al., 2020). Finally, imbalances between these regions and other networks involved in emotion and salience processing may hinder mood regulation and the perception of emotional stimuli, thereby exacerbating the emotional symptoms of LLD (Pan et al., 2022; Yang et al., 2017)
At baseline, patients with LLD also exhibit decreased insular cortex functional connectivity—a region critical for detecting and integrating internal and external signals to guide goal-directed activity—thereby impacting emotional experiences, cognitive processes, and overt behaviors (Lamm & Singer, 2010; Pimontel et al., 2021). This aligns with previous evidence linking insular dysfunction to reduced network switching between the default mode and central executive networks, potentially contributing to symptom severity in LLD and other neuropsychiatric disorders (Diener et al., 2012; Manoliu et al., 2014). Moreover, we observed that in subjects with aMCI, lower baseline insular connectivity was associated with the presence of more depressive symptoms at follow-up, echoing prior research suggesting depression in individuals with aMCI may reflect underlying neuropathological processes, including early AD (Lee et al., 2012). Indeed, depression in aMCI has been identified as a possible clinical marker for progression to AD (Gabryelewicz et al., 2007; Lee et al., 2012), although the precise neurobiological mechanisms remain incompletely understood. Collectively, these findings suggest that depression may denote more advanced structural and functional changes in neurocognitive pathways, underscoring the need for additional longitudinal and multimodal imaging studies (e.g., amyloid PET) to elucidate the relationship between insular dysfunction, depression, and the transition to AD.
At baseline, patients diagnosed with LLD exhibited diminished functional connectivity in the left postcentral gyrus within the sensorimotor network compared to HCs, consistent with evidence implicating the primary somatosensory cortex in emotion regulation (Kropf et al., 2019; Xia et al., 2024). Although traditionally considered spared in early AD, emerging data suggest that this region may undergo subtle changes in the prodromal stages, potentially serving as an early marker for disease progression (Wiesman et al., 2021). Notably, in individuals with aMCI, higher baseline functional connectivity of the left postcentral gyrus correlated with greater severity of depressive symptoms at the two-year follow-up, underscoring the sensorimotor system’s broader role in both mood regulation and cognitive function. Furthermore, the structural and functional interactions between the primary somatosensory cortex and the thalamus—crucial for cognitive processes—offer a possible link to the cognitive deficits observed in LLD (Kang et al., 2018). Future research is warranted to clarify the mechanisms driving these associations, particularly with respect to depressive symptoms in aMCI subjects.
Functional connectivity in the cuneal cortex was increased at baseline in patients with LLD compared to HCs, extending previous research suggesting that the cuneus—traditionally recognized for its role in visual processing—also contributes to emotional regulation and self-referential processes (Dotson et al., 2022). Through its structural connections with frontolimbic regions, such as the orbitofrontal cortex (Burks et al., 2018), the cuneus may influence depressive symptoms by modulating interactions within networks implicated in emotion and cognition. Prior work has revealed depression-related structural changes in the cuneus (Dotson et al., 2022; Szymkowicz et al., 2016), as well as connectivity deficits in both aMCI (Li et al., 2018) and AD (Zhou et al., 2010). Notably, we found that aMCI participants with higher cuneal cortex connectivity reported more depressive symptoms both at baseline and follow-up, complementing evidence linking cuneal structural alterations to depressive severity (Dotson et al., 2022). Although frontolimbic regions have historically been the primary focus of depression research, our results underscore the importance of investigating the cuneus’s role in depressive symptomatology and highlight the need for further examination of occipital contributions to the pathophysiology of depression.
Although no longitudinal differences emerged in our neuroimaging data, baseline connectivity measures were predictive of subsequent clinical outcomes. Our results reveal a complex pattern of cross-domain associations between specific functional brain networks and long-term clinical outcomes in two distinct clinical populations. Thus, while patients with aMCI showed decreased connectivity within the precuneus network, in LLD, as discussed above, decreased connectivity within this same network was associated with better long-term cognitive performance. On the other hand, decreased connectivity in the right insular network—hypoconnected in LLD—is similarly associated with greater depression severity at follow-up in aMCI, mirroring the functional profile observed in LLD. Conversely, increased connectivity within the left postcentral gyrus network, also reduced in LLD, correlates with higher depression scores in aMCI at follow-up, implicating somatosensory integration in the evolution of mood symptoms. Finally, the cuneus network, which shows hyperconnectivity in LLD, is positively associated with both baseline and two-year depressive symptoms in aMCI. Together, these findings support the idea of a domain-specific and cross-group influence of intrinsic connectivity patterns, whereby the preserved domain in each group (cognition in LLD; mood in aMCI) remains susceptible to modulation by functional network alterations. This is study is not without limitations. Thus, it is important to note that while all participants underwent thorough clinical and neuropsychological assessments, the inherent nature of our naturalistic and sequential recruitment approach resulted in the age-related aMCI group being both older and smaller in size compared to the other groups. This discrepancy may have compromised our ability to identify significant variances. Future research with larger and more demographically balanced samples is needed to validate our findings and ensure their generalizability. Furthermore, our functional connectivity analyses relied on Pearson’s correlation and covariance matrices as measures of statistical dependence, but this methodology can produce overly dense networks, as it fails to distinguish between direct and indirect effects between variables. Future research using multivariate functional connectivity methods such as partial correlations and multiple regression is advised.
ConclusionsIn summary, our results demonstrate distinct alterations in resting-state functional connectivity in individuals with aMCI and LLD, highlighting region-specific vulnerabilities that may underlie cognitive decline and depressive symptomatology in older adults. These findings advance our understanding of the neurobiological substrates of these disorders while acknowledging the challenges of differentiating overlapping clinical profiles. Although two-year longitudinal connectivity trajectories did not distinguish aMCI from LLD, baseline rs-fMRI metrics showed promise in predicting subsequent clinical outcomes. Future studies with larger cohorts and longer follow-up periods are warranted to validate these observations and to assess their implications for early diagnosis and the development of targeted therapeutic interventions.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Inés del Cerro reports a relationship with Worldwide Clinical Trials that includes: consulting or advisory. The author, Carles Soriano-Mas is an Advisory Board Member for International Journal of Clinical and Health Psychology and was not involved in the editorial review or the decision to publish this article. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.