The treatment of inflammatory bowel disease has undergone a significant transformation following the introduction of biologic drugs. Thanks to these drugs, treatment goals have evolved from clinical response and remission to more ambitious objectives, such as endoscopic or radiologic remission. However, even though biologics are highly effective, a significant percentage of patients will not achieve an initial response or may lose it over time. We know that there is a direct relationship between the trough concentrations of the biologic and its therapeutic efficacy, with more demanding therapeutic goals requiring higher drug levels, and inadequate exposure being common.
Therapeutic drug monitoring of biologic medications, along with pharmacokinetic models, provides us with the possibility of offering a personalized approach to treatment for patients with IBD. Over the past few years, relevant information has accumulated regarding its utility during or after induction, as well as in the maintenance of biologic treatment, in reactive or proactive strategies, and prior to withdrawal or treatment de-escalation.
The aim of this document is to establish recommendations regarding the utility of therapeutic drug monitoring of biologics in patients with inflammatory bowel disease, in different clinical practice scenarios, and to identify areas where its utility is evident, promising, or controversial.
El tratamiento de la enfermedad inflamatoria intestinal ha sufrido una gran transformación tras la introducción de los fármacos biológicos. Gracias a ellos, los objetivos del tratamiento han evolucionado desde la respuesta y remisión clínica a objetivos más ambiciosos, como la remisión endoscópica o radiológica. Sin embargo, aunque los biológicos son muy eficaces, un porcentaje importante de pacientes no obtendrá una respuesta inicial o la perderá a lo largo del tiempo. Sabemos que existe una relación directa entre las concentraciones valle del biológico y su eficacia terapéutica, que cuanto más exigente sea el objetivo terapéutico serán precisos niveles superiores del fármaco y que es frecuente la exposición insuficiente al mismo. La monitorización terapéutica de medicamentos biológicos, así como los modelos farmacocinéticos, nos brindan la posibilidad de ofrecer un enfoque personalizado del tratamiento en pacientes con enfermedad inflamatoria intestinal. Durante los últimos años se ha acumulado información relevante respecto a su utilidad durante o después de la inducción, así como en el mantenimiento del tratamiento biológico, en estrategias reactivas o proactivas y antes de la retirada o desintensificación del tratamiento.
El objetivo de este documento es establecer recomendaciones sobre la utilidad de la monitorización terapéutica de biológicos en pacientes con EII, en los diferentes escenarios de la práctica clínica e identificar las áreas donde su utilidad es evidente, prometedora o controvertida.
- 1
We recommend always monitoring with the same technique and in the same laboratory.
- 2
Reference levels in induction/post-induction:
Infliximab: we recommend measuring levels at week 14 in all patients.
The minimum trough concentration for clinical remission is >5 μg/ml and for endoscopic healing, 7–10 μg/ml.
In patients with a greater baseline inflammatory burden, such as in severe ulcerative colitis (UC) and perianal Crohn’s disease (CD), we recommend measuring levels early during induction with the aim of obtaining higher drug concentrations: 20–25 μg/ml at week 2; and 10–15 μg/ml at week 6.
Adalimumab: we recommend measuring adalimumab levels in all patients at week 4.
In those with CD, the suggested trough concentration is >5 μg/ml and in UC > 6.36 μg/ml.
Considering the objective of endoscopic healing, the proposed levels are >10–12 μg/ml.
Vedolizumab: there are insufficient data to recommend target levels.
Ustekinumab: there is insufficient information to make a formal recommendation. In the absence of better evidence, levels could be a measured at week 8.
In CD, the reference levels at week 8 are 3.9 μg/ml (range 2 to 7.3 μg/ml) for clinical remission and >11.1 μg/ml for endoscopic remission.
In patients with UC, the trough concentration associated with the clinical response at week 8 is ≥3.7 μg/ml.
- 3
Levels during maintenance:
Infliximab: we recommend levels >5–7 μg/ml to maintain clinical remission. If mucosal healing is not achieved, intensify with a target level of around 10 μg/ml.
Adalimumab: we recommend levels >10–12 μg/ml for clinical and endoscopic remission.
Vedolizumab: we believe that there is not enough evidence to recommend therapeutic monitoring of vedolizumab levels in maintenance.
Ustekinumab: we recommend levels of 1.5 to 3 μg/ml for clinical remission and >4.5 μg/ml for endoscopic response.
- 4
Carriers of the HLA-DQA1*05 allele.
Pending clinical trials, for patients carrying the HLA-DQA1*05 allele who require an anti-TNF, we recommend combined immunosuppression and to consider using strategies that improve drug persistence (proactive monitoring) or to use an alternative target.
- 5
Pharmacodynamic failure.
Inflammation must be confirmed and complications ruled out. After this, it is recommended to switch to a drug with a different mechanism of action.
- 6
Proactive strategy
A generalised proactive monitoring strategy is not recommended.
Pending additional information, its use should be considered during or at the end of the induction phase, especially in clinical scenarios that are difficult to manage (perianal disease, high inflammatory burden), during drug maintenance if there is a risk of secondary loss of response (monotherapy, second anti-TNF, carrier of HLA-DQA1*05) and in the case of de-escalation or reintroduction strategies after prolonged suspension.
- 7
De-escalation of anti-TNF drugs
We recommend adequate patient selection and close monitoring.
Therapeutic drug monitoring is mandatory after dose reduction of infliximab and adalimumab.
- 8
Withdrawal of an anti-TNF
Prior to withdrawing an anti-TNF, we recommend measuring drug concentrations and anti-drug antibodies.
- 9
After “drug holidays”
We recommend assessing anti-TNF antibody levels, especially before the second dose. If antibodies are detected before or after the first dose, a change of anti-TNF or target is necessary.
Biologicals have revolutionised the treatment of inflammatory bowel disease (IBD). However, up to a third of patients treated with anti-TNF do not show a primary response (primary non-response; PNR) and up to 50% of subjects develop a secondary loss of response (LOR) (or secondary non-response; SNR).1 In simplified terms, this may be a result of insufficient exposure to the drug due to rapid drug consumption (pharmacokinetic failure) caused by a high inflammatory burden, extensive intestinal involvement, the presence of hypoalbuminaemia or anti-drug antibodies (ADAs) (immunogenic failure); or because of inflammatory processes, probably related to an alternative inflammatory pathway (pharmacodynamic failure).
In recent years, there has been a change in therapeutic goals in IBD. Treatment is now recommended not only to achieve symptom remission but also endoscopic remission in patients with Crohn’s disease (CD) and ulcerative colitis (UC). This paradigm shift involves the use of biological and morphological data that will lead to drugs being selected on the basis of a personalised medicine model. There seems to be a clear correlation between the concentrations of biologicals and the clinical and endoscopic response obtained. This prompted us to explore the utility of different monitoring strategies for biological therapy, in order to improve its effectiveness and safety.
In short, there are two possible approaches. Reactive monitoring involves determining drug levels and ADAs in patients who develop secondary LOR, in order to guide the change in treatment (intensification or choice of a new drug). The proactive strategy proposes monitoring medication levels, regardless of clinical and/or biological activity, and make dose adjustments in order to reach a certain predefined level. The ultimate goal of the proactive strategy is to improve the response rate during induction, prevent clinical relapse and/or perform safer withdrawal of the immunosuppressant (IMS).
In this document, a large team of experts in therapeutic monitoring of biologicals, made up of specialists in gastroenterology and pharmacy, have participated in preparing these recommendations of the Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) [Spanish Working Group on Crohn’s Disease and Ulcerative Colitis]. The techniques currently available for monitoring biologicals and antibodies (Ab) are reviewed, as well as their possible applications in different clinical practice scenarios in IBD.
Monitoring techniquesWhat techniques are available for determining biological serum concentrations?Therapeutic monitoring of biological medicinal products in IBD is a challenge, largely due to the lack of standardisation of analytical methods for measuring drug concentrations and their ADAs.2,3 The development and validation of analytical techniques has been carried out mainly for infliximab and adalimumab2–4 and has subsequently been adapted for vedolizumab and ustekinumab.2,5,6
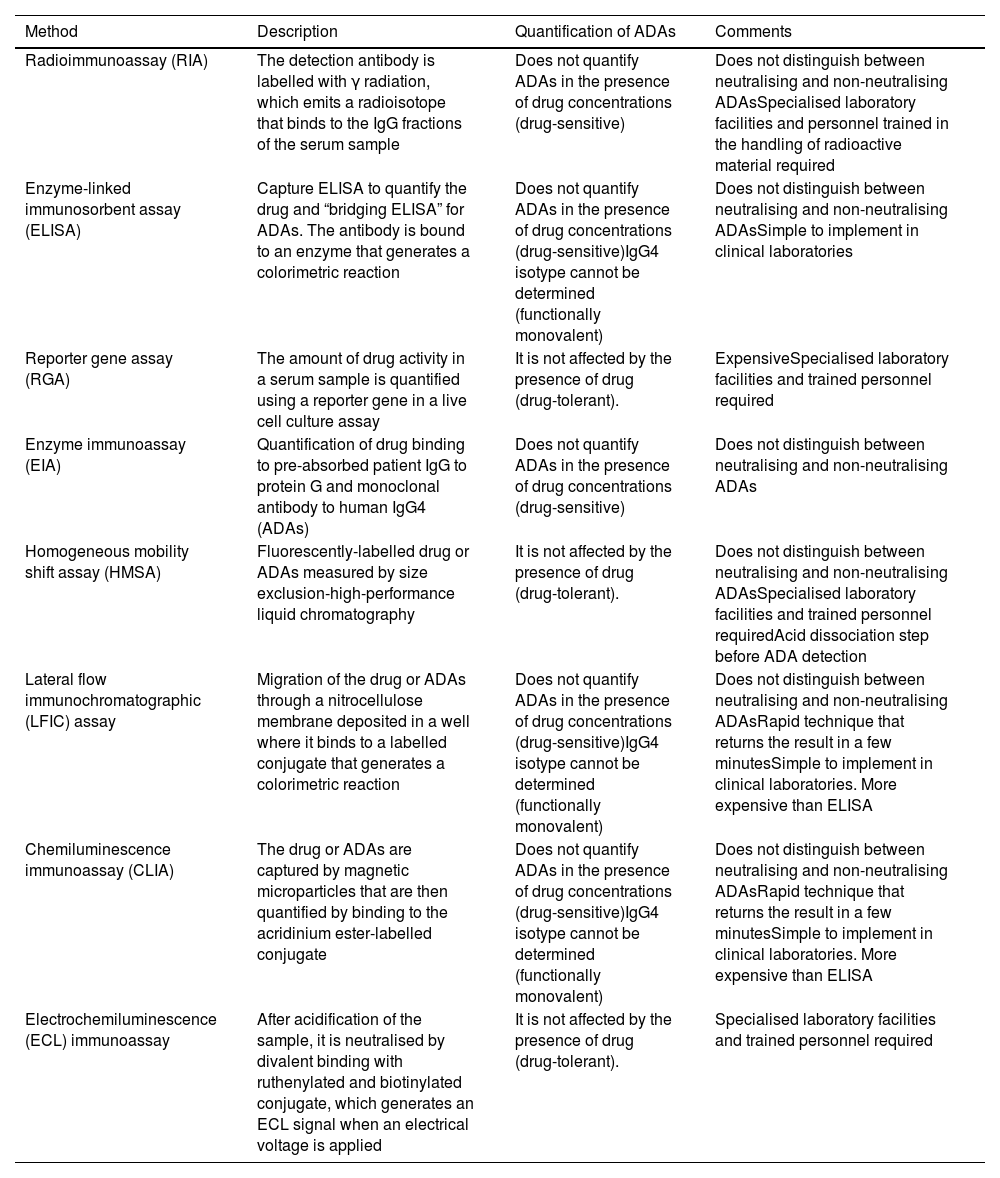
Table 1 summarises the technical basis and the main operational characteristics for each of the methods used.
Characteristics of the main analytical methods to determine the concentrations of biological medicinal products and Ab indicated in inflammatory bowel disease.
| Method | Description | Quantification of ADAs | Comments |
|---|---|---|---|
| Radioimmunoassay (RIA) | The detection antibody is labelled with γ radiation, which emits a radioisotope that binds to the IgG fractions of the serum sample | Does not quantify ADAs in the presence of drug concentrations (drug-sensitive) | Does not distinguish between neutralising and non-neutralising ADAsSpecialised laboratory facilities and personnel trained in the handling of radioactive material required |
| Enzyme-linked immunosorbent assay (ELISA) | Capture ELISA to quantify the drug and “bridging ELISA” for ADAs. The antibody is bound to an enzyme that generates a colorimetric reaction | Does not quantify ADAs in the presence of drug concentrations (drug-sensitive)IgG4 isotype cannot be determined (functionally monovalent) | Does not distinguish between neutralising and non-neutralising ADAsSimple to implement in clinical laboratories |
| Reporter gene assay (RGA) | The amount of drug activity in a serum sample is quantified using a reporter gene in a live cell culture assay | It is not affected by the presence of drug (drug-tolerant). | ExpensiveSpecialised laboratory facilities and trained personnel required |
| Enzyme immunoassay (EIA) | Quantification of drug binding to pre-absorbed patient IgG to protein G and monoclonal antibody to human IgG4 (ADAs) | Does not quantify ADAs in the presence of drug concentrations (drug-sensitive) | Does not distinguish between neutralising and non-neutralising ADAs |
| Homogeneous mobility shift assay (HMSA) | Fluorescently-labelled drug or ADAs measured by size exclusion-high-performance liquid chromatography | It is not affected by the presence of drug (drug-tolerant). | Does not distinguish between neutralising and non-neutralising ADAsSpecialised laboratory facilities and trained personnel requiredAcid dissociation step before ADA detection |
| Lateral flow immunochromatographic (LFIC) assay | Migration of the drug or ADAs through a nitrocellulose membrane deposited in a well where it binds to a labelled conjugate that generates a colorimetric reaction | Does not quantify ADAs in the presence of drug concentrations (drug-sensitive)IgG4 isotype cannot be determined (functionally monovalent) | Does not distinguish between neutralising and non-neutralising ADAsRapid technique that returns the result in a few minutesSimple to implement in clinical laboratories. More expensive than ELISA |
| Chemiluminescence immunoassay (CLIA) | The drug or ADAs are captured by magnetic microparticles that are then quantified by binding to the acridinium ester-labelled conjugate | Does not quantify ADAs in the presence of drug concentrations (drug-sensitive)IgG4 isotype cannot be determined (functionally monovalent) | Does not distinguish between neutralising and non-neutralising ADAsRapid technique that returns the result in a few minutesSimple to implement in clinical laboratories. More expensive than ELISA |
| Electrochemiluminescence (ECL) immunoassay | After acidification of the sample, it is neutralised by divalent binding with ruthenylated and biotinylated conjugate, which generates an ECL signal when an electrical voltage is applied | It is not affected by the presence of drug (drug-tolerant). | Specialised laboratory facilities and trained personnel required |
Ab: antibodies; ADAs: anti-drug antibodies.
The techniques used to determine anti-TNF levels (Table 1) show an excellent linear correlation between them (R2 = 0.97 to 0.99)7 and, when comparing them with each other, small differences have been reported (between 0.48 and 1.55 μg/ml).8–11 There are significant variations, however, between the concentrations obtained over the course of a day, on different days or between individuals.4 There are very few comparative studies between analytical techniques for ustekinumab and vedolizumab, but from the available data, they should not be considered interchangeable.12
What are neutralising and non-neutralising anti-drug antibodies?The biological medicinal products used in IBD are monoclonal antibodies with a G1 immunoglobulin (Ig) structure. These proteins are immunogenic and induce an Ab response against them, as the body detects them as foreign molecules.1,2,4,5,13
The clinical repercussions of Ab formation against biologicals depend on the ADA epitope. Neutralising Ab bind to the active site of the drug, inhibiting its mechanism of action. With non-neutralising Ab, however, their epitope is the constant region of the Ig, so they bind to the drug without neutralising it. In both cases, the kinetics of the drug are modified, as the formation of drug-ADA immune complexes accelerates its elimination, affecting therapeutic efficacy.14 The prevalence of ADAs in response to different drugs is variable and depends on the analytical technique used. Molecules with a chimeric structure (infliximab) have higher rates (17–70%) than those with a humanised or human structure: 12.7–25.0% for adalimumab; 2.8–21.8% for golimumab; 4.0–8.0% for vedolizumab; and 2.3–5.7% for ustekinumab.5,6,15 Other factors that increase the development of ADAs are subcutaneous (SC) administration, high body mass and having low drug plasma concentrations.16,17
What techniques are used to measure anti-drug antibodies?The ideal technique should be sensitive, specific and capable of detecting ADAs in the presence of the drug14 (Table 1). There are two groups of techniques based on their ability to detect ADAs in the presence of detectable concentrations of the drug. Drug-tolerant techniques quantify ADAs when the biological medicinal product is in measurable concentrations (for example, ELISA techniques using an anti-human lambda chain).18Drug-sensitive techniques do not detect ADAs in the presence of the drug because of interference due to competitive inhibition or immune complex formation.2,11,14,19–21 Drug tolerance tests could be useful during induction, due to their ability to detect ADAs when the concentration of anti-TNF is high.22 ADA levels in this period are not identifiable by drug-sensitive tests, but they predict PNR and secondary LOR. During maintenance, the use of a drug-sensitive assay would be sufficient, as ADAs could be non-neutralising or transient, although their clinical relevance is still not fully understood.22
How are biological medical product and antibody concentrations interpreted?The ELISA assay is the most used analytical technique and is easy to implement in clinical practice due to its high sensitivity, low cost and operational characteristics. Furthermore, it is the technique used in the majority of studies that characterise the therapeutic range of these drugs.2,5,12,13 Most commercial ELISA kits have demonstrated acceptable precision, specificity, reproducibility, interclass correlation and concordance with target values.23–25 Its limitations are that it is a drug-sensitive technique and is unable to detect monomeric Ab. For this reason, the ELISA technique produces a high percentage of false-negative ADA results. This may not have clinical relevance because these are only important (at least in maintenance) with a high titre and these high values are detectable by a drug-sensitive assay.21
When interpreting the results, we have to be aware that no universal standards are available and the findings of ADA concentrations are not comparable between the different analytical methods.2–6,12,13,26 Furthermore, it is recommended to perform multiple quantifications to identify transient or persistent Ab, as only those that persist over time have clinical repercussions.14
One strategy for detecting and managing immunogenicity when a drug-tolerant technique is not available could be the application of population pharmacokinetic (PK) models and Bayesian estimation because, as part of their development, the influence of ADAs in plasma clearance is quantified.27–32
What role do rapid assays play in biological medicinal product monitoring?One limitation of biological medicinal product monitoring is the amount of time between sample extraction and obtaining the result, which delays any necessary clinical action.33–35 This is because ELISA techniques require pooling a large number of samples to improve the efficiency of the kits.33–36
This limitation could be avoided with the use of rapid point-of-care (POC) assays10,37–39 that provide the result in minutes.8,10,40 The downside is that they require serum instead of whole blood and laboratory infrastructure. The dried blood spot testing technique enables drug and ADA concentrations to be quantified quickly from capillary blood obtained by skin prick. It could be used as a rapid test without the need for additional equipment.41–44 However, it requires further studies to determine correlation and comparability with traditional methods.
The performance of therapeutic monitoring would improve by combining rapid POC assays with population pharmacokinetics (PK) and Bayesian dosage adjustment, optimising the precise drug dose in real time and an immediate and personalised reaction on the actual exposure to the drug.6,45 A multidisciplinary approach where the skills of IBD specialist doctors are combined with experts in clinical PK could contribute to better comprehensive patient management.6,46,47
The cost of the POC assay is higher than conventional ELISA so, for reasons of efficiency, it should be reserved for situations where a delay in the results is not acceptable (for example, secondary LOR or induction management).6
Biological medicinal product reference levelsMonitoring of biologicals during inductionAlthough most studies have been conducted in the maintenance phase, there is emerging interest in the use of proactive monitoring during the induction stage with the aim of preventing primary failure.48 The recent adoption of strategies aimed at achieving more demanding therapeutic goals (endoscopic or histological remission) means that appropriate drug levels vary depending on the goal in question.49
Biological medicinal product concentrations during inductionOne essential aspect, although the subject of much debate, is to establish what should be considered to be adequate levels of the drug for making therapeutic decisions (Table 2). Reference values for measuring drug levels and ADAs are, even today, difficult to define as the studies are very heterogeneous. They depend on the technique used, the goal to be achieved and, above all, measurement timing.
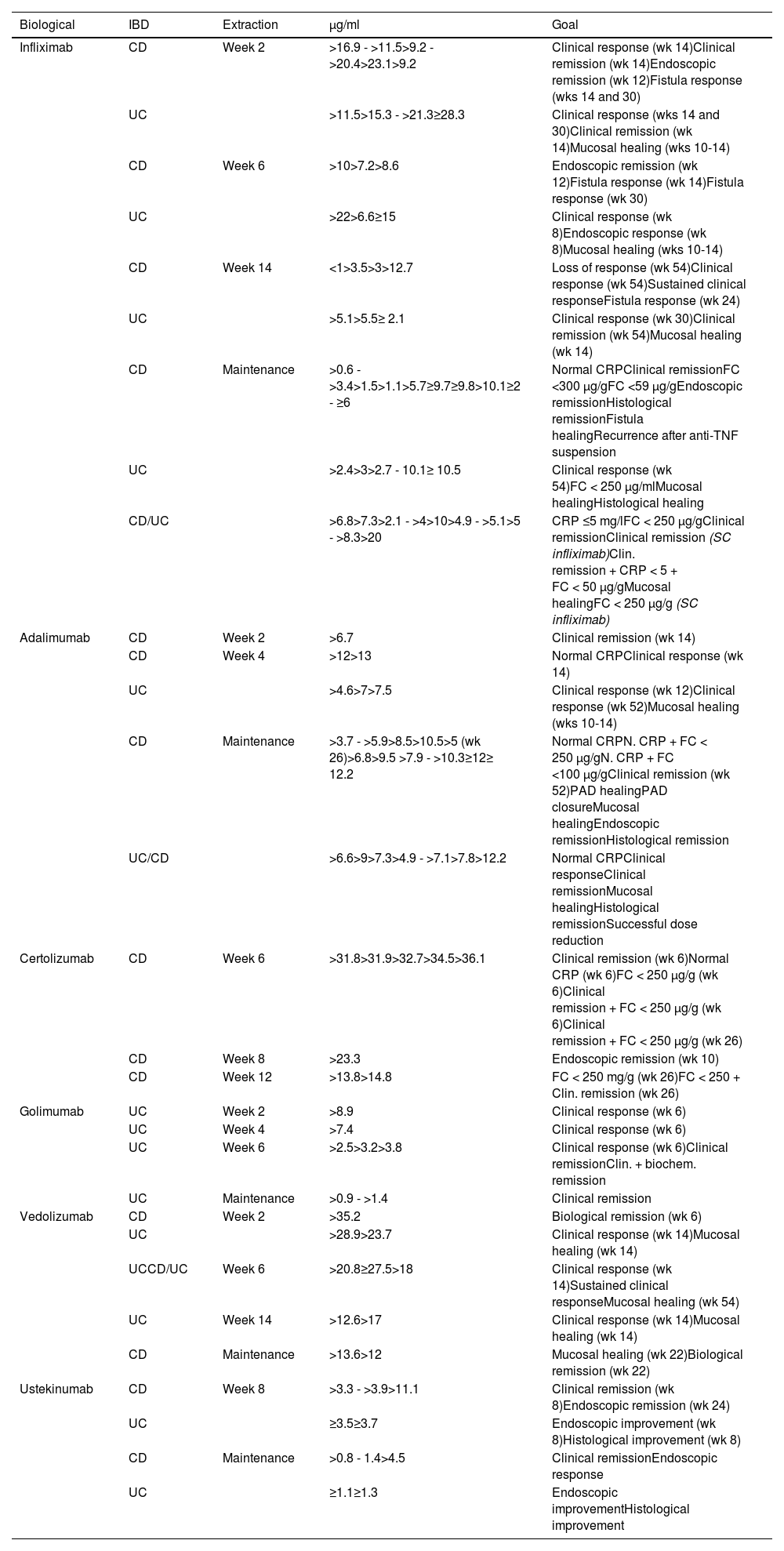
Association of trough concentrations of biologicals and therapeutic targets during induction and maintenance therapy in inflammatory bowel disease.13,141,186,267
| Biological | IBD | Extraction | µg/ml | Goal |
|---|---|---|---|---|
| Infliximab | CD | Week 2 | >16.9 - >11.5>9.2 - >20.4>23.1>9.2 | Clinical response (wk 14)Clinical remission (wk 14)Endoscopic remission (wk 12)Fistula response (wks 14 and 30) |
| UC | >11.5>15.3 - >21.3≥28.3 | Clinical response (wks 14 and 30)Clinical remission (wk 14)Mucosal healing (wks 10-14) | ||
| CD | Week 6 | >10>7.2>8.6 | Endoscopic remission (wk 12)Fistula response (wk 14)Fistula response (wk 30) | |
| UC | >22>6.6≥15 | Clinical response (wk 8)Endoscopic response (wk 8)Mucosal healing (wks 10-14) | ||
| CD | Week 14 | <1>3.5>3>12.7 | Loss of response (wk 54)Clinical response (wk 54)Sustained clinical responseFistula response (wk 24) | |
| UC | >5.1>5.5≥ 2.1 | Clinical response (wk 30)Clinical remission (wk 54)Mucosal healing (wk 14) | ||
| CD | Maintenance | >0.6 - >3.4>1.5>1.1>5.7≥9.7≥9.8>10.1≥2 - ≥6 | Normal CRPClinical remissionFC <300 µg/gFC <59 µg/gEndoscopic remissionHistological remissionFistula healingRecurrence after anti-TNF suspension | |
| UC | >2.4>3>2.7 - 10.1≥ 10.5 | Clinical response (wk 54)FC < 250 µg/mlMucosal healingHistological healing | ||
| CD/UC | >6.8>7.3>2.1 - >4>10>4.9 - >5.1>5 - >8.3>20 | CRP ≤5 mg/lFC < 250 µg/gClinical remissionClinical remission (SC infliximab)Clin. remission + CRP < 5 + FC < 50 µg/gMucosal healingFC < 250 µg/g (SC infliximab) | ||
| Adalimumab | CD | Week 2 | >6.7 | Clinical remission (wk 14) |
| CD | Week 4 | >12>13 | Normal CRPClinical response (wk 14) | |
| UC | >4.6>7>7.5 | Clinical response (wk 12)Clinical response (wk 52)Mucosal healing (wks 10-14) | ||
| CD | Maintenance | >3.7 - >5.9>8.5>10.5>5 (wk 26)>6.8>9.5 >7.9 - >10.3≥12≥ 12.2 | Normal CRPN. CRP + FC < 250 µg/gN. CRP + FC <100 µg/gClinical remission (wk 52)PAD healingPAD closureMucosal healingEndoscopic remissionHistological remission | |
| UC/CD | >6.6>9>7.3>4.9 - >7.1>7.8>12.2 | Normal CRPClinical responseClinical remissionMucosal healingHistological remissionSuccessful dose reduction | ||
| Certolizumab | CD | Week 6 | >31.8>31.9>32.7>34.5>36.1 | Clinical remission (wk 6)Normal CRP (wk 6)FC < 250 µg/g (wk 6)Clinical remission + FC < 250 µg/g (wk 6)Clinical remission + FC < 250 µg/g (wk 26) |
| CD | Week 8 | >23.3 | Endoscopic remission (wk 10) | |
| CD | Week 12 | >13.8>14.8 | FC < 250 mg/g (wk 26)FC < 250 + Clin. remission (wk 26) | |
| Golimumab | UC | Week 2 | >8.9 | Clinical response (wk 6) |
| UC | Week 4 | >7.4 | Clinical response (wk 6) | |
| UC | Week 6 | >2.5>3.2>3.8 | Clinical response (wk 6)Clinical remissionClin. + biochem. remission | |
| UC | Maintenance | >0.9 - >1.4 | Clinical remission | |
| Vedolizumab | CD | Week 2 | >35.2 | Biological remission (wk 6) |
| UC | >28.9>23.7 | Clinical response (wk 14)Mucosal healing (wk 14) | ||
| UCCD/UC | Week 6 | >20.8≥27.5>18 | Clinical response (wk 14)Sustained clinical responseMucosal healing (wk 54) | |
| UC | Week 14 | >12.6>17 | Clinical response (wk 14)Mucosal healing (wk 14) | |
| CD | Maintenance | >13.6>12 | Mucosal healing (wk 22)Biological remission (wk 22) | |
| Ustekinumab | CD | Week 8 | >3.3 - >3.9>11.1 | Clinical remission (wk 8)Endoscopic remission (wk 24) |
| UC | ≥3.5≥3.7 | Endoscopic improvement (wk 8)Histological improvement (wk 8) | ||
| CD | Maintenance | >0.8 - 1.4>4.5 | Clinical remissionEndoscopic response | |
| UC | ≥1.1≥1.3 | Endoscopic improvementHistological improvement |
Ab: antibody; CD: Crohn’s disease; CRP: C-reactive protein; FC: faecal calprotectin; IBD: inflammatory bowel disease; UC: ulcerative colitis.
Overall, 30% of patients do not respond to induction therapy with infliximab and are considered PNR at week 14. Retrospective studies have shown that low levels of infliximab being found at weeks 2 and 6 were associated with PNR at week 14.50 Different studies have shown that PNR have significantly lower infliximab levels at weeks 6 and 14 compared to responders (week 6: 7.3 vs 11.2 μg/ml and week 14: 1.5 vs 4.7 μg/ml, respectively).48,51 A post-hoc analysis of the ACT 1 and 2 studies demonstrates that infliximab levels >22 μg/ml at week 6 are associated with clinical response at week 8, while levels >5.1 μg/ml at week 14 predict response at week 30.52
Mucosal healing is now a therapeutic goal. The levels required to obtain this goal are higher than those for clinical remission. In the ACT 1 and 2 trials, endoscopic remission was associated with infliximab levels ≥18.6 μg/ml at week 2, ≥10.6 μg/ml at week 6 and ≥34.9 μg/ml at week 8.53 In the study by Papamichael et al.,54 the infliximab values in UC were higher in the induction phase when the goal was endoscopic remission, at week 2 (22.9 vs 19.3 mg/ml, p: 0.018), week 6 (17.6 vs 10,3 mg/ml, p: 0.001) and week 14 (7.4 vs 4 mg/ml, p: 0.014), compared to patients who did not achieve mucosal healing.
In CD, it seems that the levels required to achieve the therapeutic goals are lower,55,56 with the exception of perianal CD57 (Table 2). Analysis of the ACCENT 1 study58 concludes that levels >3.5 μg/ml at week 14 predict an adequate long-term response. In the PANTS study, primary failure occurred in 21.9% of patients treated with infliximab and only low infliximab levels at week 14 were associated with PNR and non-remission at week 54. The optimal level of infliximab, at week 14, linked to remission in that week and at week 54, was 7 μg/ml.59
In a clinical practice study, the cut-off point of 15 μg/ml at week 6 predicted mucosal healing at weeks 10–14.48 In a post-hoc analysis of the induction phase of the TAILORIX study, infliximab levels >23.1 μg/ml at week 2 and >10.0 μg/ml at week 6 predicted endoscopic remission at week 12.60
Recommendation: we recommend measuring infliximab levels at week 14 in all patients.
The minimum trough concentration for clinical remission is >5 μg/ml and for endoscopic healing, 7–10 μg/ml.
In subjects with a greater baseline inflammatory burden, such as in severe UC and perianal CD, it is recommended to measure levels early during induction with the aim of achieving higher drug concentrations: 20–25 μg/ml at week 2; and 10–15 μg/ml at week 6.
Although some studies establish an association between adalimumab levels and PNR, due to the heterogeneity of their design, it is difficult to establish a solid trough level at induction. In the PANTS study, PNR (week 14) occurred in 26.8% of patients treated with adalimumab and only adalimumab levels at week 14 were associated with PNR.59Post-hoc analysis of the CLASSIC I and II clinical trials found that adalimumab levels at week 4 were higher in responders than in PNR (8.1 vs 5.1 μg/ml, respectively).61 Adalimumab levels >12 μg/ml at week 4 were associated with biological remission. Prospective clinical practice studies have observed that, as early as week 2, levels are linked to clinical remission at week 14 (6.8 vs 4.8 μg/ml), establishing the cut-off point for adalimumab levels at >6.7 μg/ml (area under the curve [AUC] = 0.73).48
In UC, cohort studies have also revealed an association between adalimumab levels, clinical remission and early mucosal healing in the induction phase.48 Adalimumab concentrations at week 4 of ≥4.58 μg/ml and ≥7.5 μg/ml represent an independent factor linked to clinical remission and early mucosal healing at week 12, respectively.48,62
It should be noted that detectable ADAs (determined by a drug tolerance test) is common and early with adalimumab (32% at week 44, of which 55% appear at week 2 and 79% at week 14) and their presence is associated with both PNR and secondary LOR in patients with IBD.
GolimumabGolimumab levels are not easily available in routine practice. In the induction phase of the PURSUIT study, the median concentrations of golimumab at week 6 in patients with clinical remission and mucosal healing were 3.14 μg/ml and 3.14 μg/ml, respectively.63
In a clinical practice study with 21 patients with moderate-to-severe UC, the mean concentrations of golimumab at weeks 2 and 6 were 8 (5.3–10.3) μg/ml and 4.3 (2–6.9) μg/ml, respectively, and levels of 2.6 μg/ml were associated with clinical response at week 14, data similar to those found in the PURSUIT study.64
Clinical trial results suggest a trough concentration of golimumab at week 6 and maintenance ≥2.5 μg/ml and >1.4 μg/ml, respectively,63 although insufficient data are available to make a recommendation.
VedolizumabAnalyses by quartiles of vedolizumab levels in pivotal studies establish a clear relationship between exposure and response.65,66 The median trough concentration at week 6 is higher in patients in clinical remission in both UC (34.7 vs 23.7 μg/ml) and CD (26.8 vs 23.5 μg/ml), although the overlap of results in this last group is high.65,66 The exposure-response relationship is clearer at week 10 (than at week 6) in subjects with CD, suggesting that the efficacy of vedolizumab in this group could be later.67
Clinical practice studies have analysed the exposure-response relationship of vedolizumab in IBD.48,68 Levels >28.0 μg/ml at week 6 predict a sustained clinical response in patients with UC (AUC: 0.723). In subjects with CD, trough levels at week 2 > 29.8 μg/ml are associated with clinical and biological remission at week 6. Considering people with IBD overall, it is concluded that clinical remission and endoscopic improvement at week 14 are related to vedolizumab concentrations >30 μg/ml in week 2, >24–37 μg/ml at week 6 and >14–20 μg/ml at week 14.13,69
Recommendation: there are insufficient data to make a formal recommendation regarding the utility of vedolizumab. However, the reference values, with the current data in patients with IBD, clinical remission/response and endoscopic improvement at week 14 are associated with vedolizumab concentrations >30 μg/ml at week 2, 24 μg/ml at week 6 and >14 μg/ml at week 14.
Data from the UNITI-1 and UNITI-2 induction clinical trials in CD confirm the relationship between exposure and clinical efficacy for ustekinumab. At week 8 post-induction, the median concentrations of ustekinumab are 2.1 and 6.4 μg/ml for doses of 130 mg and 6 mg/kg, respectively, and correlate with clinical remission at that same time point. Based on the analysis by quartiles, only the 6 mg/kg dose is linked to an increase in clinical remission, with the trough concentration being 3.2 to 3.9 μg/ml. Using the week 8 ustekinumab serum concentration to correlate with remission at that point, AUC analysis identified a cut-off of 3.3 μg/ml.63,70 Different observational studies of clinical practice in CD have also established a favourable exposure-response relationship.48,70 Most of these analyse ustekinumab concentrations at week 8 after administration of 6 mg/kg and relate it to its efficacy. The pooled analysis of these studies allows us to estimate a weighted average of 3.9 μg/ml for clinical remission (range 2 to 7.3 μg/ml) and 11.1 μg/ml for endoscopic remission.70,71
However, although the evidence on monitoring ustekinumab levels in the early phase is limited, it has been found that levels at weeks 2 and 4 post-induction could help estimate the likelihood of response at week 24.71 In fact, ustekinumab concentrations obtained as early as one hour after intravenous infusion (>105 μg/ml) identify patients with a greater likelihood of achieving endoscopic remission, with performance similar to the determination at weeks 2 and 4.71 Analysis at such an early stage would make it possible to bring forward the first SC dose.
Data from phase 2 and 3 clinical trials in patients with UC (UNIFI 1 and 2) determine that ustekinumab concentrations are dose-proportional and are not affected by prior exposure to other biologicals or concomitant IMS, with a well-defined exposure-response relationship. Analysis of the Receiver-Operating-Characteristic (ROC) curves reveals that the trough concentration associated with clinical response at week 8 is ≥3.7 μg/ml; for other therapeutic goals (clinical remission, endoscopic and histological improvement), it ranges from 3.5 to 3.7 μg/ml.72
Recommendation: although we believe that there is limited information on the utility of ustekinumab levels for making a formal recommendation, levels could be measured at week 8 in patients with CD. The reference levels at week 8 are 3.9 μg/ml (range 2 to 7.3 μg/ml) for clinical remission and >11.1 for endoscopic remission.
In subjects with UC, the trough concentration associated with clinical response at week 8 is ≥3.7 μg/ml.
Numerous studies and clinical guidelines have been published pertaining to patients on maintenance therapy, especially with anti-TNF. They propose intervals and cut-off points for interpreting secondary LOR.
InfliximabTrough concentrations >5 μg/ml are associated with a lower risk of immunogenicity.73 The different clinical guidelines recommend target maintenance levels between 3.5 and 7 μg/l for clinical remission.74–76 After secondary LOR, levels >10 μg/ml should be achieved.13
However, one of the main goals for IBD is mucosal healing and the levels required to achieve this are higher than those previously described (in this case ≥9.7).77,78 Maintenance levels for fistulising perianal CD are also higher (≥13 μg/l).57,79,80
With regard to SC infliximab, a recent cross-sectional study in patients with IBD on maintenance therapy identified a concentration of >10 μg/ml to be associated with clinical remission and >20 μg/ml with biological remission (faecal calprotectin [FC] <250 μg/g).81
AdalimumabThe guidelines of the scientific societies recommend levels for maintaining clinical remission of 5–12 μg/ml.75,76 The proportion of patients not in remission with a minimum adalimumab threshold ≥5 μg/ml is 17%, falling to <10% with levels >7.5 μg/ml.75,82 The upper limit of adalimumab’s therapeutic range to achieve clinical remission is poorly defined. However, for endoscopic remission, the recommended trough levels of adalimumab are ≥12 μg/l; 90% of subjects who maintain these levels achieve endoscopic remission.83 A similar cut-off point (≥12.2 μg/l) has been identified as a predictor of histological remission.84
VedolizumabThere are very few studies and virtually no data for a reactive strategy. Higher concentrations of vedolizumab are associated with higher response rates, and increasing the dose to infusions every four weeks increases clinical remission rates.85 The BRIDGe group13 recommends measuring vedolizumab levels in patients with PNR or secondary LOR.
A recent study estimated that subjects with secondary LOR and levels <7.4 μg/ml responded better to dose escalation than those with vedolizumab at that level or higher before escalation.86 Another clinical practice study87 analysed 141 measurements of levels, most of them (75.9%) in maintenance therapy. A range between 5 and 15 μg/ml was accepted as appropriate for achieving remission. However, although only 44% of the patients had levels measured because of secondary LOR, the majority of them were in this range or even above it. Some data suggest that secondary LOR to vedolizumab is not due to PK causes, so in the event of an incomplete response or secondary LOR, in-range trough levels would not rule out intensification of the biological medicinal product.88
UstekinumabData are very limited and those that are available are inconsistent. However, different analyses of clinical trials confirm that higher drug concentrations correlate with higher response rates in both CD63 and UC.89
Levels >1.5 μg/ml (week 40) were associated with clinical remission.63 At week 26, concentrations >4.5 μg/ml achieved higher endoscopic response rates.90 Meanwhile, after secondary LOR, patients who responded to intensification attained figures higher than those mentioned above (>6 μg/ml).91 Therefore, it may be useful to take the patient’s previous levels in remission as a reference point and, if they decrease (and secondary LOR occurs), that would be the target level.
Reactive monitoring strategySecondary LOR to biological therapy is defined as the development of symptoms attributable to disease activity (assessed by C-reactive protein [CRP], faecal calprotectin [FC], endoscopy or imaging techniques) in patients previously in remission. A meta-analysis of 39 studies with adalimumab92 and a systematic review of 16 studies with infliximab93 established an annual risk of secondary LOR of 20.3% and 13% per patient-year, respectively. Secondary LOR frequently leads to treatment suspension,94 so it is crucial to detect it and optimise therapy accordingly as early as possible.
The most important factors involved in secondary LOR include exposure to subtherapeutic levels of the drug and the formation of ADAs.95–97
In clinical practice, drug trough levels are determined when secondary LOR is suspected with confirmed active disease. Numerous studies have demonstrated the utility of combining drug trough levels and ADAs, compared to empirical treatment, in the management of secondary LOR.98–102 Their results have shown that:
- 1)
Patients with subtherapeutic drug levels without ADAs achieve better results with intensification compared to switching to another anti-TNF.
- 2)
Switching to a drug with a different mechanism of action should be considered when levels are in the therapeutic range.
- 3)
When ADAs are present, switching to another anti-TNF is effective, although if ADA titres are low and/or transient, the drug may be intensified and/or an IMS added.52,103,104
Management of secondary LOR based on drug trough levels is more cost-effective than empirical treatment.24–26 In a Danish multicentre, randomised study, 69 patients with CD and secondary LOR to infliximab were assigned to empirical intensification (5 mg/kg/4 weeks) or to a treatment algorithm based on trough levels and ADAs. Both strategies achieved similar clinical outcomes (responses of 58% and 53% in the level-based management group and the empirically intensified group, respectively) but the cost of therapy was substantially lower (34%) in the group with the level-based approach.105,106 These results have been corroborated in subsequent studies and in a meta-analysis.107
In conclusion, secondary LOR to anti-TNF drugs is a common clinical scenario. Measuring drug levels and ADAs has proven to be efficient and cost-effective in decision making in clinical practice.
Secondary loss of response with low drug levels. Pharmacokinetic failureFor cases of secondary LOR with low drug levels, intensifying the treatment by doubling the dose, shortening the administration interval, or both, is recommended.108 There is little evidence on which of these is the best strategy to recover the response.
A total of 28% of patients on infliximab maintenance therapy in the ACCENT I trial required dose escalation to 10 mg/kg/8 weeks, with recovery of response in 88% of cases, although the authors did not report the duration of the response and there was no comparison with any other strategy.109
A mathematical model in patients with rheumatoid arthritis110 and some population PK models in IBD111,112 suggest that shortening the interval may be superior to increasing the infliximab dose. In contrast, a retrospective multicentre study in 169 individuals with secondary LOR to infliximab compared the effectiveness of infliximab intensification by doubling the dose to 10 mg/kg/8 weeks or shortening the interval with doses of 5 mg/kg/4 weeks.113 There were no significant differences in terms of efficacy, although doubling the dose meant a reduction in direct costs so, in the absence of validation in specifically designed studies, this strategy could be preferable.
In the case of adalimumab, the most recommended intensification strategies108 are the administration of 40 mg/week or 80 mg/2 weeks. In this case, controlled clinical studies with adalimumab advocated shortening the interval between injections rather than increasing the dose.114,115
There are no studies that compare the clinical efficacy of intensification of adalimumab 40 mg/week with 80 mg/2 weeks in patients with IBD and secondary LOR. One prospective study included 62 subjects with IBD in sustained clinical remission after intensification of adalimumab to 40 mg/week in which the treatment was modified to 80 mg/2 weeks. There were no differences in the PK of the drug, none of the patients needed to return to the 40 mg/week regimen and it was the regimen preferred by the participants. Therefore, it is feasible to propose changing adalimumab 40 mg/week to 80 mg/2 weeks.116
A prospective, multicentre, observational study evaluated the efficacy of a second intensification of adalimumab up to 80 mg/week, obtaining a response of 50% and clinical remission of 33%, without observing significant side effects.117
Lastly, re-induction could be an effective strategy comparable to intensification and more economical.118 However, it should be noted that the information available on the effectiveness of re-induction is very limited, which is why more studies are necessary.
Secondary loss of response in the presence of anti-drug antibody. Immunogenic failureOne of the main causes of secondary LOR is the development of ADAs.17,94,97,119 This immunogenicity can appear as early as the second week of treatment.120 It appears to be related to clinical,121 genetic122–125 and pharmacological factors,59,126–128 as well as concomitant conventional IMS treatment. The development of ADAs at low titres, not detectable by conventional methods, has also been described. The significance of this is unknown. It has been found that, although (in a high percentage of cases) they disappear over time, this has occurred in a context of proactive intensification.20 Their presence has been related to shorter survival of the drug.129
Low levels of anti-TNF are associated with a greater risk of immunogenicity.59,95,126,127,130 However, intensification of anti-TNF treatment (increasing the dose, decreasing the administration interval or a re-induction regimen) is capable of recovering response in patients with secondary LOR.73,118,131–136 Regarding the indication for intensifying therapy, a Danish multicentre, randomised study on 69 patients with CD who had experienced secondary LOR to an anti-TNF demonstrated that the personalised regimen (based on assessing drug levels and ADAs) was more cost-effective in the short term (12 weeks) than empirical, clinically-guided intensification.105,137
Treatment with anti-TNF combined initially with IMS (thiopurines or methotrexate) is more effective than the anti-TNF regimen in monotherapy, especially in the case of infliximab.85,138 The benefit of combined treatment seems to be related to a lower proportion of patients who develop ADAs.97,128,137–144 No relationship has been observed with the dose of IMS,145 although an association has been found with thioguanine levels.146 In subjects who receive monotherapy with anti-TNF and experience secondary LOR, adding an IMS could be an effective strategy for recovering therapeutic response. However, although widely used in clinical practice, there are no randomised studies with this strategy as objective.132,147–149 There appears to be no benefit from adding azathioprine in terms of immunogenicity in people with high levels of infliximab.146 However, in a randomised trial in subjects with CD treated with infliximab,150 premedication with conventional steroids administered intravenously (IV) immediately before each infusion of infliximab was shown to reduce the proportion of patients with ADAs, and is a common clinical practice. However, this protective effect of steroids (demonstrated in on-demand infliximab regimens) is not so clear with the current infliximab induction and maintenance strategy.151
Lastly, it is advisable to change the molecule in patients with ADAs at high titres and/or in individuals with side effects related to immunogenicity. The effectiveness of a second anti-TNF is highly variable depending on the reason for changing the first drug, and is greater in patients who change for intolerance (around 60%) than in those with secondary LOR (45%) or primary failure, in whom the effectiveness is 30%.152 In any case, it is very possible that the decision on the next drug to use in people with secondary LOR should be based on assessing drug and ADA levels.153,154
How can we manage the genetic risk of developing immunogenicity?There is evidence that suggests an innate risk of developing immunogenicity. Patients with ADAs in response to the first anti-TNF are up to 11 times more likely to develop them in response to the second.155,156 Human leukocyte antigen(HLA) genotypes have been described in rheumatoid arthritis and hidradenitis suppurativa, associated with a lower risk (HLA-DQB1*05, HLA-DRB1*01 and HLA-DRB1*07) and higher risk (HLA-DRB1*03 and HLA-DRB1*01) of immunogenicity.157
The PANTS cohort study prospectively included 1,240 CD patients on anti-TNF treatment based on a genome-wide association studies (GWAS) analysis, with the aim of identifying genetic risk markers for developing immunogenicity, determined by a drug-tolerant assay. At 12 months, 44% developed ADAs. The risk was higher for infliximab than for adalimumab and higher in monotherapy than in combination therapy with IMS. It was found that carriers of the HLA-DQA1*05 allele (present in 40% of Europeans) had double the risk of developing immunogenicity regardless of anti-TNF or IMS treatment. The patients at highest risk were carriers of the unfavourable haplotype in monotherapy with infliximab (likelihood of 92% at 12 months). In contrast, those at lower risk were those without the unfavourable haplotype treated with adalimumab in combination therapy (10% at 12 months).216 Similar results were found in a retrospective cohort study of 262 patients with IBD exposed to infliximab. Carriers of this variant had a higher risk of secondary LOR and treatment interruption (hazard ratio [HR] = 2.27).217 The magnitude of the risk identified has been very similar in other observational studies.122,123,158,159
However, strategies that reduce immunogenicity (such as the addition of an IMS122,123 or proactive monitoring of levels160) have been shown to reduce the impact of the presence of HLA-DQA1*05 in the risk of immunogenicity.
Recommendation: pending robust evidence, it seems prudent in patients carrying the haplotype HLA-DQA1*05 and who require treatment with an anti-TNF to suggest combination therapy with an IMS (particularly with infliximab) and consider using strategies that improve drug persistence (such as proactive monitoring) or using an alternative target.
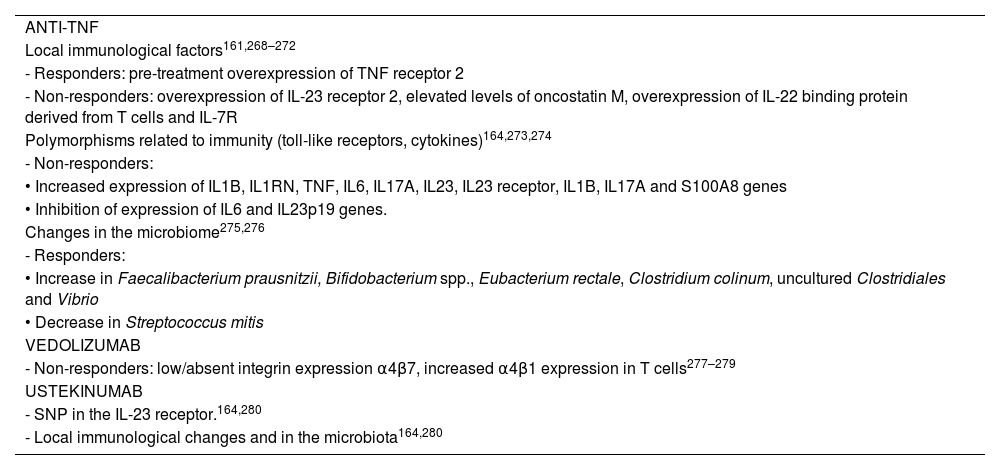
Pharmacodynamic failure is defined as primary or secondary failure of biological treatment in the presence of adequate levels of the drug,13,153,161 which could be explained by the activation of inflammation pathways not mediated by the target of the treatment.153,162 A number of mechanisms have been identified that could explain non-response to biological medicinal products153,163,164 (Table 3).
Proposed mechanisms for pharmacodynamic failure.
| ANTI-TNF |
| Local immunological factors161,268–272 |
| - Responders: pre-treatment overexpression of TNF receptor 2 |
| - Non-responders: overexpression of IL-23 receptor 2, elevated levels of oncostatin M, overexpression of IL-22 binding protein derived from T cells and IL-7R |
| Polymorphisms related to immunity (toll-like receptors, cytokines)164,273,274 |
| - Non-responders: |
| • Increased expression of IL1B, IL1RN, TNF, IL6, IL17A, IL23, IL23 receptor, IL1B, IL17A and S100A8 genes |
| • Inhibition of expression of IL6 and IL23p19 genes. |
| Changes in the microbiome275,276 |
| - Responders: |
| • Increase in Faecalibacterium prausnitzii, Bifidobacterium spp., Eubacterium rectale, Clostridium colinum, uncultured Clostridiales and Vibrio |
| • Decrease in Streptococcus mitis |
| VEDOLIZUMAB |
| - Non-responders: low/absent integrin expression α4β7, increased α4β1 expression in T cells277–279 |
| USTEKINUMAB |
| - SNP in the IL-23 receptor.164,280 |
| - Local immunological changes and in the microbiota164,280 |
If it is suspected that a patient with IBD has not responded to anti-TNF due to persistence of symptoms in weeks 12–14, activity must be confirmed and infection or other complications must be ruled out, as well as ensuring that the initial diagnosis is correct.
Optimal levels of anti-TNF (Table 2) will define the existence of PNR of pharmacodynamic origin.153 In these cases, switching to a drug with a different mechanism of action is recommended102,153,165 given that the efficacy of a second anti-TNF in this scenario is very low.166,167 Patients with primary failure to anti-TNF are intrinsically more difficult to treat with second-line biologicals.166 With regard to people with UC, more than 50% of those with PNR to infliximab will require surgery.168 Therefore, there is a need to validate biomarkers capable of determining the order in which different biological treatments should be indicated and identifying subjects who will be refractory to one or another biological treatment, establishing the basis of personalised medicine.
Proactive monitoring strategyWhat does a proactive monitoring strategy involve?Proactive therapeutic drug monitoring could theoretically improve clinical outcomes, reducing PNR and secondary LOR rates48; improving the patient’s quality of life and achieving remission more quickly. It would also help achieve adequate drug levels more rapidly, reducing immunogenicity, and increase the useful time of the first biological, with reduced reliance on intensification and subsequent de-escalation. Last of all, it could be cost-effective and improve the safety of these drugs by increasing the administration interval and/or avoiding the combination with IMS in cases of supratherapeutic levels.169
One retrospective study analysed 264 patients on infliximab treatment with follow-up for 10 years. They identified that the proactive strategy was associated with greater durability of the drug, less need for surgery or hospitalisations and less risk of immunogenicity or serious infusion reactions.170–173
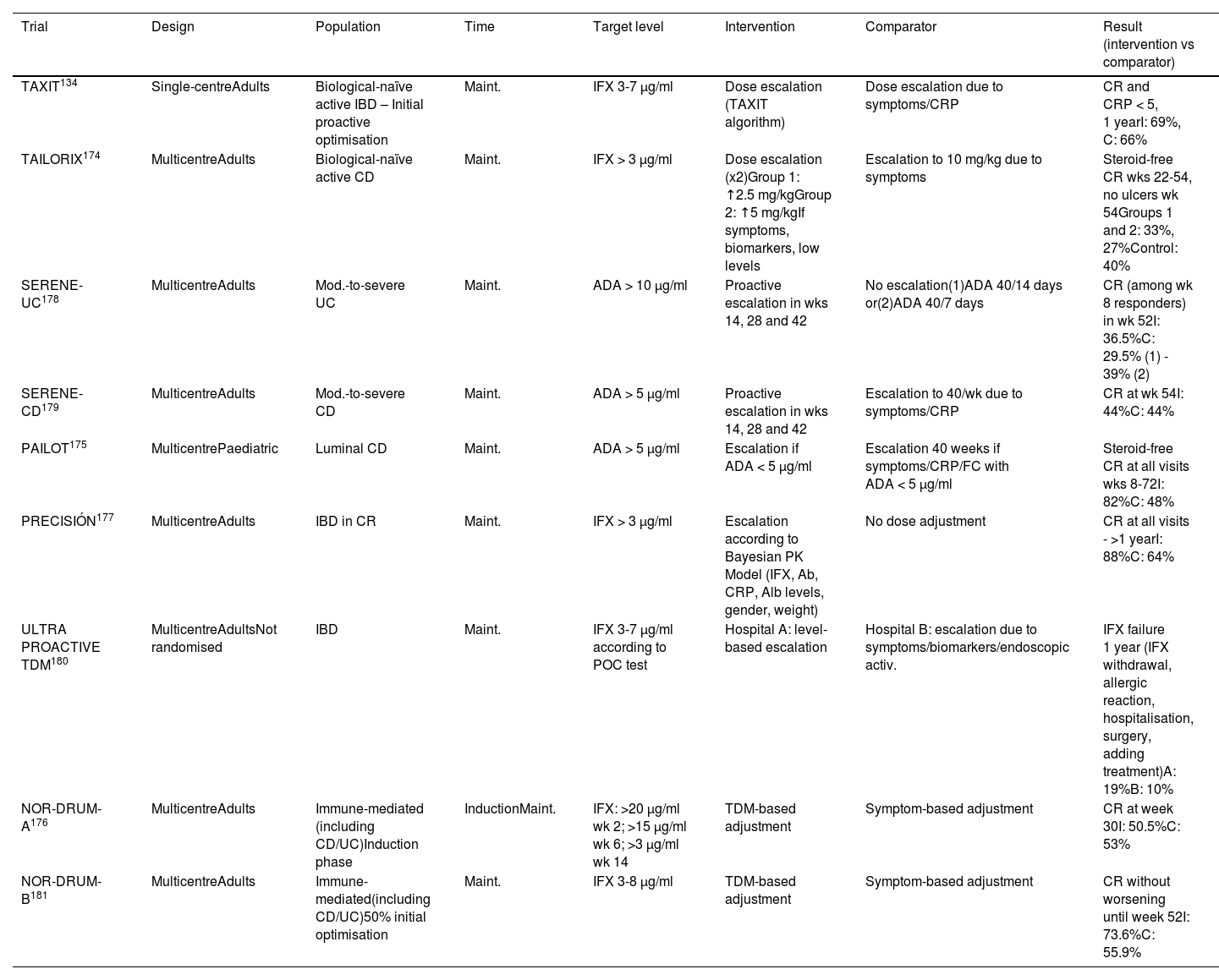
To date, nine clinical trials have been published134,174–181 that compare a proactive strategy with a conventional (or reactive) strategy, but with contradictory results (Table 4). A recent meta-analysis that includes 26 studies (nine clinical trials) concludes that the proactive strategy is associated with a higher rate of clinical and endoscopic remission, as well as a lower rate of hospitalisation and surgery.182 Other meta-analyses,183,184 however, suggest that the proactive strategy, overall, does not improve the rate of clinical remission in maintenance therapy, with a 56% increase in the likelihood of intensification of the anti-TNF dose, without finding either a lower rate of immunogenicity or of adverse effects. We cannot therefore currently recommend this monitoring strategy routinely.
Clinical trials that have evaluated a proactive strategy.
| Trial | Design | Population | Time | Target level | Intervention | Comparator | Result (intervention vs comparator) |
|---|---|---|---|---|---|---|---|
| TAXIT134 | Single-centreAdults | Biological-naïve active IBD – Initial proactive optimisation | Maint. | IFX 3-7 µg/ml | Dose escalation (TAXIT algorithm) | Dose escalation due to symptoms/CRP | CR and CRP < 5, 1 yearI: 69%, C: 66% |
| TAILORIX174 | MulticentreAdults | Biological-naïve active CD | Maint. | IFX > 3 µg/ml | Dose escalation (x2)Group 1: ↑2.5 mg/kgGroup 2: ↑5 mg/kgIf symptoms, biomarkers, low levels | Escalation to 10 mg/kg due to symptoms | Steroid-free CR wks 22-54, no ulcers wk 54Groups 1 and 2: 33%, 27%Control: 40% |
| SERENE-UC178 | MulticentreAdults | Mod.-to-severe UC | Maint. | ADA > 10 µg/ml | Proactive escalation in wks 14, 28 and 42 | No escalation(1)ADA 40/14 days or(2)ADA 40/7 days | CR (among wk 8 responders) in wk 52I: 36.5%C: 29.5% (1) - 39% (2) |
| SERENE-CD179 | MulticentreAdults | Mod.-to-severe CD | Maint. | ADA > 5 µg/ml | Proactive escalation in wks 14, 28 and 42 | Escalation to 40/wk due to symptoms/CRP | CR at wk 54I: 44%C: 44% |
| PAILOT175 | MulticentrePaediatric | Luminal CD | Maint. | ADA > 5 µg/ml | Escalation if ADA < 5 µg/ml | Escalation 40 weeks if symptoms/CRP/FC with ADA < 5 µg/ml | Steroid-free CR at all visits wks 8-72I: 82%C: 48% |
| PRECISIÓN177 | MulticentreAdults | IBD in CR | Maint. | IFX > 3 µg/ml | Escalation according to Bayesian PK Model (IFX, Ab, CRP, Alb levels, gender, weight) | No dose adjustment | CR at all visits - >1 yearI: 88%C: 64% |
| ULTRA PROACTIVE TDM180 | MulticentreAdultsNot randomised | IBD | Maint. | IFX 3-7 µg/ml according to POC test | Hospital A: level-based escalation | Hospital B: escalation due to symptoms/biomarkers/endoscopic activ. | IFX failure 1 year (IFX withdrawal, allergic reaction, hospitalisation, surgery, adding treatment)A: 19%B: 10% |
| NOR-DRUM-A176 | MulticentreAdults | Immune-mediated (including CD/UC)Induction phase | InductionMaint. | IFX: >20 µg/ml wk 2; >15 µg/ml wk 6; >3 µg/ml wk 14 | TDM-based adjustment | Symptom-based adjustment | CR at week 30I: 50.5%C: 53% |
| NOR-DRUM-B181 | MulticentreAdults | Immune-mediated(including CD/UC)50% initial optimisation | Maint. | IFX 3-8 µg/ml | TDM-based adjustment | Symptom-based adjustment | CR without worsening until week 52I: 73.6%C: 55.9% |
ADA: adalimumab; C: control; CD: Crohn’s disease; CR: clinical remission; CRP: C-reactive protein; I: intervention group; IBD: inflammatory bowel disease; IFX: infliximab; TDM: therapeutic drug monitoring; UC: ulcerative colitis.
However, one issue that clinical trials have not yet resolved is the scenarios in which the patient has a higher risk of underexposure to the biological and, in theory, could benefit from a more intensive and personalised dose escalation strategy. For this reason, the various current clinical guidelines have not established firm recommendations on the proactive use of the levels, although the trend is to introduce some specifications and scenarios in which these determinations can be used.13,76,185,186 These scenarios include the induction period (already discussed previously), perianal disease, severe UC, situations with a higher risk of immunogenicity (for example, monotherapy or HLADQA1*05 carrier status), paediatric disease and prior to de-escalation/treatment withdrawal.
Clinical situations of high inflammatory burden with greater elimination of the drug that may require a more adjusted dosage at an early stage must also be taken into account, such as a severe UC flare-up or severe CD.51,187–189
Several studies also propose that proactive monitoring can avoid the need for concomitant therapy with IMS, maintaining levels >5 μg/ml.190,191 In fact, in a post-hoc study of the SONIC clinical trial, stratification by infliximab levels showed a similar outcome regardless of the use of IMS.79,192 This could be more relevant with IV infliximab193 than with SC infliximab194 or adalimumab,139,195 where the utility of combination therapy during maintenance is less evident. Similarly, proactive monitoring after IMS withdrawal could prolong the life of the biological and have an efficacy comparable to that of combination therapy.196
The paediatric population is special, due to greater variability in exposure to biologicals. In this case, high levels of adalimumab and infliximab at induction are associated with clinical remission at week 52.197,198 The only trial published in this cohort (PAILOT trial) demonstrated that proactive monitoring with intensification of adalimumab (target level >5 μg/ml) resulted in higher rates of corticosteroid-free remission than the reactive strategy (82% vs 48%, p: 0.002).175
Recommendation: based on the current evidence, a generalised proactive monitoring strategy is not recommended. However, pending additional information, use of such a strategy should be assessed during or at the end of the induction phase, especially in difficult-to-manage clinical scenarios (such as perianal disease or high inflammatory burden), during drug maintenance, if there is a risk of secondary LOR (monotherapy, second anti-TNF, carrier of HLA-DQA1*05) and for de-escalation or reintroduction strategies after a prolonged suspension.
Reducing the dose of anti-TNF during maintenance therapy appears to have little impact on disease remission, as long as the trough levels of the drug remain within those considered “adequate” and as long as the patient is in deep remission. De-escalation by reducing the dose of the biological or increasing the interval between doses could reduce costs, although it has not been confirmed whether this reduces adverse events in subjects with IBD.199,200 Current data for IBD do not demonstrate that higher concentrations of anti-TNF are associated with greater toxicity, unlike spondyloarthritis, where infliximab concentrations >20.3 μg/ml were related to an increased risk of infections.201
Before reducing (or withdrawing) the dose of any maintenance therapy in IBD, the disease must be re-assessed and clinical, biological, endoscopic and/or radiological and even histological remission confirmed.199,202 The history, severity and extent of the disease are important factors to consider.202 Studies not guided by therapeutic drug monitoring in patients who have required a previous dose increase due to secondary LOR report that subsequent de-escalation is associated with a high short-term relapse rate and, furthermore, only a third of them will respond to the new treatment escalation.202–204
Dose reduction based on symptoms and CRP levels in IBD patients in clinical remission with infliximab is associated with a high risk of relapses, while management based on therapeutic monitoring of drug levels could prevent a significant proportion of these relapses.76,134,205 Proactive drug level monitoring allows safe de-escalation in subjects with sustained response and high serum drug concentrations, reducing unnecessary drug exposure and costs.76,200,205 The TAXIT algorithm demonstrated the effect of therapeutic drug monitoring in optimising and reducing the cost of treatment.134 De-escalation was carried out by reducing the dose to 5 mg/kg (if they were previously on 10 mg/kg) or increasing the interval between infusions by two weeks (up to a maximum interval of 12 weeks). De-escalation of the infliximab dose led to a 28% reduction in the costs of medications in patients with IBD who had initially had a complete or partial response to maintenance therapy, without resulting in significant clinical changes.134 A recent study analysed the profitability of de-escalation of biosimilar infliximab and found that in two years, the reduction guided by therapeutic drug monitoring resulted in savings of 6%, about 25.4 million euros per 10,000 patients.206
Other observational and clinical practice studies support the use of therapeutic drug monitoring in dose de-escalation.207–209 In patients in clinical and biological remission with infliximab concentrations >7 μg/ml, reducing the dose did not lead to a greater risk of relapse.207 If, after de-escalation, the subjects showed levels <2.4 μg/ml, they were at increased risk of secondary LOR.208 Progressive reduction of the dose to the original 5 mg/kg or until achieving a concentration of 3–7 μg/ml led to high long-term remission rates.209
Papamichael et al. recommend reducing the dose of infliximab when concentrations are repeatedly >15 μg/ml. Given that no greater toxicity has been associated with high levels of anti-TNF, there will be cases in which high doses can be continued, especially in those patients who have persistent IBD activity despite having high drug concentrations, or in those who took longer to achieve remission.1
Lengthening the interval between doses has also proven useful in de-escalating patients treated with adalimumab.210 One study retrospectively evaluated 40 subjects with CD who began receiving the drug every three weeks. Of them, 65% remained in clinical remission with adalimumab trough concentrations >4 μg/ml and it was associated with the resolution of side effects (skin manifestations, arthralgia and mild infections) in half of the individuals.210
However, the data are not homogeneous. A study in 25 people with IBD in deep remission showed that anti-TNF levels prior to de-escalation were not predictors of failure.211 A total of 64% remained in remission at 12 months after de-escalation, and re-escalation was effective in all cases.211 This study offers hope that anti-TNF dose reduction can be implemented successfully and safely in IBD under close follow-up. However, prospective studies with a larger number of patients are necessary to investigate this scenario.212
Should levels be determined before withdrawing biological therapy?Discontinuation of drugs in a patient with IBD, especially biological therapy, should be personalised and take into account the individual’s preference. In general, when it comes to combination therapy, withdrawal of IMS is preferred because it is associated with a low likelihood of clinical relapse (compared to withdrawal of anti-TNF).213 The possibility of relapse after halting biological therapy is high: 19% per patient-year and 40–50% over a two-year period after discontinuation of anti-TNF.213–215 A recent clinical trial in subjects with CD found a relapse rate of 49% after discontinuation of infliximab (as opposed to 0% among those who continued).216 However, reintroduction of the drug also restores the response in 80%.217 The risk-benefit ratio of any IMS must also be considered in the context of age and comorbidities.
Several studies have analysed predictive factors of relapse. The clinical factors identified are early age at the onset of IBD, being male, extensive disease, previous surgery, perianal disease and early use of anti-TNF and/or IMS.213,218 Laboratory markers predictive of relapse after discontinuation of anti-TNF in CD include CRP, elevated leukocytes, low haemoglobin and elevated FC. A model in the STORI trial219 incorporated these laboratory markers (haemoglobin ≤14.5 g/dl, leukocytes ≥6 × 109, CRP ≥ 5 mg/dl, FC ≥300 μg/g), along with being male and the absence of surgical resection. It was concluded that patients with ≤2 factors (29% of the study population) had a 15% risk of relapse at one year. Lastly, evidence of mucosal healing either on endoscopy or imaging, and therefore low levels of FC, are associated with a reduced risk of relapse in CD.220
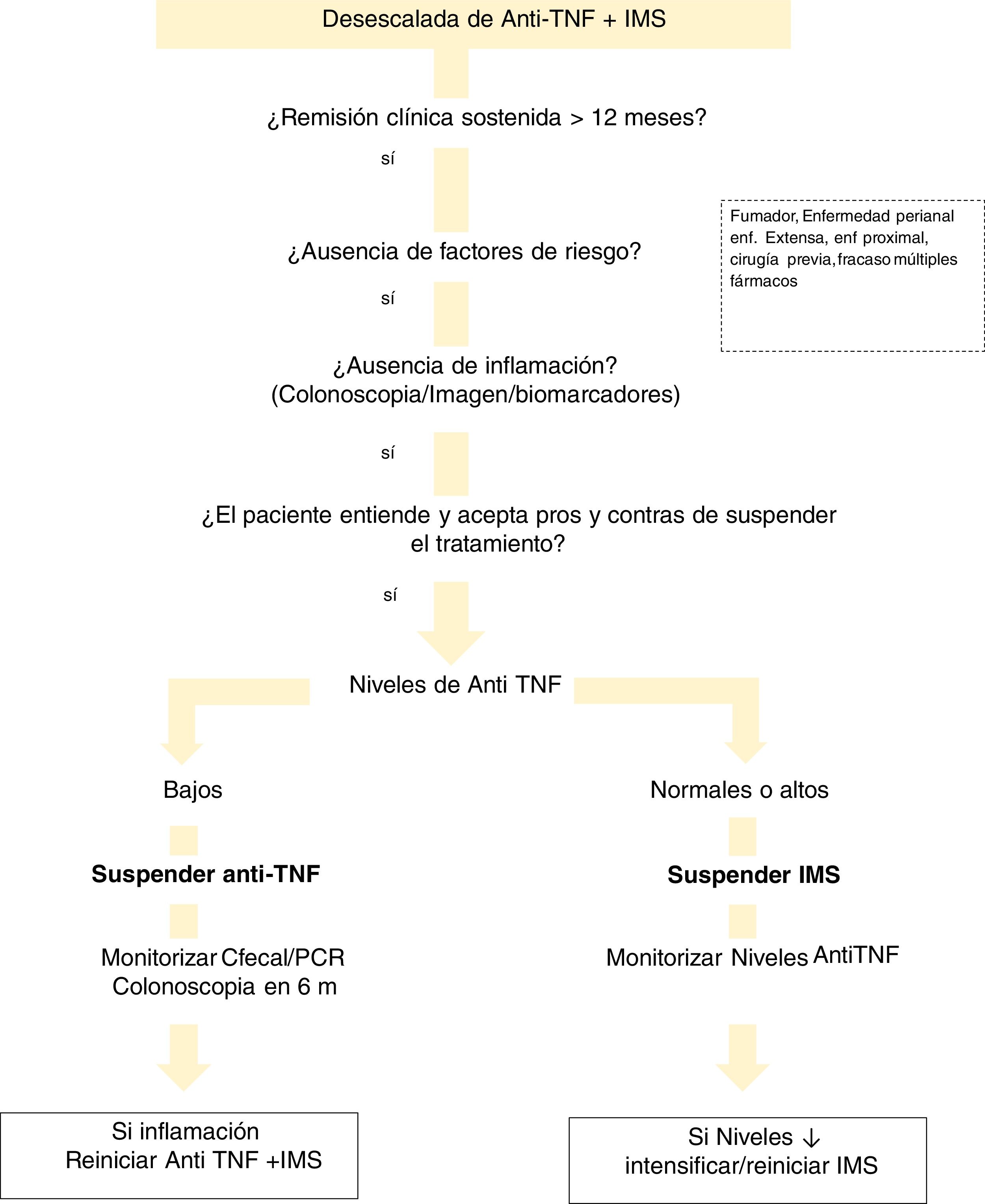
With regard to drug levels, there are various situations that need to be evaluated (Fig. 1). If the patient is on combination therapy and has high levels of anti-TNF, we propose withdrawal of the IMS. In the event that the biological medicinal product levels are low or undetectable, we recommend discontinuing the biological while maintaining the IMS. This is justified by the finding that patients with undetectable concentrations of the drug have a significantly lower risk of relapse after discontinuing anti-TNF compared to those who discontinued it with detectable levels.221 This can be explained by the hypothesis that remission in subjects with low or undetectable levels of anti-TNF drug is maintained, regardless of the continuation of anti-TNF, due to the natural history of their disease or due to the IMS. Discontinuation of the IMS (while maintaining anti-TNF) shows a lower risk of relapse if anti-TNF concentrations were >5 μg/ml.191 This general idea of withdrawing anti-TNF would also be applicable in cases where the patient is not on IMS.
There are few studies that analyse the drug level to consider withdrawing the biological. A lower risk of relapse has been found in patients with anti-TNF levels <6 μg/ml.222 However, the STORI trial219 concluded that concentrations <2 μg/ml were required to make this strategy safer.
There are no studies addressing the withdrawal of non-anti-TNF drugs.
Measuring antibody levels when reintroducing anti-TNF after drug holidaysEpisodic therapy increases ADA levels and infusion reactions, especially in patients without IMS therapy or on prolonged pauses.223
A subsequent study in a small cohort of 27 patients re-treated with infliximab or adalimumab after a suspension of at least four months found that 3/5 subjects with ADAs had an infusion reaction, compared to 11/22 without ADAs, which does not suggest great utility in this context. Follow-up was performed on the 22 with ADAs. ADA negativity was found one year after discontinuation of infliximab and somewhat later for adalimumab.224
The absence of ADAs after re-exposure to the drug (before the second dose) is associated with a better short-term response, while detectable ADAs is associated with higher rates of infusion reactions.225
A meta-analysis that included 1,351 patients showed that the risk of infusion reaction and severe infusion reaction was higher in subjects with detectable ADAs (relative risk [RR] 2.4 and RR 5.8, respectively). The risk decreased in those on combination therapy.226
Pharmacokinetic modelsWhat are pharmacokinetic models and what do they offer?Pivotal clinical trials establish the effectiveness of a drug and the initial way of using it, which will be reflected in the summary of product characteristics. The recommended doses are the same for all patients or are adjusted to anthropometric variables. However, drug exposure is very different from one person to another. The proportion of adults with low infliximab levels (<3 μ/ml) may be higher than 40%227,228 and, in the paediatric population, up to 68%.229 With regards to adalimumab, the variable of SC absorption and administration in fixed doses is added, so its variability is greater. After a 160-mg dose of adalimumab, concentrations on day 7 range from 7.1 to 26.8 μg/ml.31 This variability could be critical in induction, as low levels (<2 μg/ml for adalimumab and <4 μg/ml for infliximab) in week 2 identify patients who will develop immunogenicity and will therefore have a greater likelihood of PNR or secondary LOR. This occurs in up to 22% of those treated with adalimumab and 26% with infliximab.230
Population PK models try to simplify the complex processes that a drug goes through in the body by attempting to predict its behaviour through mathematical equations. In clinical practice, they allow the dosage of a medication to be personalised (to achieve maximum therapeutic efficacy while minimising adverse effects) and identify patients at risk of insufficient exposure. A population PK model is developed based on the study of a specific population. From this, drug levels are measured at different times and it can be determined which covariates influence PK and best explain interindividual variability. It is then validated in that group and in a different population.231 A Bayesian probability system is used to improve the predictions, as there is an appreciable rate of variability not explained by the population PK models (from 28% to 51%).232,233
What are the available pharmacokinetic models? (Appendix A, Table 1 annex)InfliximabThere are numerous published population PK models, many of them validated.234,235 With a single measurement of the drug concentration, the dose can be calculated to obtain a target level. Their predictive capacity increases if more than one drug concentration value is available for the specific patient. They help correctly identify >80% of subjects who will have low levels after intensification.28,235 Immunogenicity reduces their predictive capacity.235 The covariates included in most population PK models are weight or body mass index (BMI), albumin, detectable ADAs and gender.27,236,237 Some models include biomarkers of inflammation such as FC,47,232 CRP233,238,239 or erythrocyte sedimentation rate (ESR)240 or clinical indices.232,233 There are specific paediatric population PK models238,240 whose covariates are similar to those of adults. A two-compartment infliximab SC population model241 has recently become available that includes weight, albumin and detectable ADA levels. The trough serum concentrations of the SC drug are higher with SC administration than with IV administration, but without differences in efficacy. It should be noted that the trough concentration of SC infliximab does not fully explain its efficacy, and its cumulative exposure (the AUC) should probably be taken into account.81 The trough levels of IV and SC infliximab cannot therefore be extrapolated.
AdalimumabThere are fewer published population PK models.29–32,242,243 SC absorption is the factor with the greatest inter-individual variability and it can lead to lower bioavailability.244,245 The variables included are the presence (or suspected presence) of ADAs,243 body weight or BMI and the dose used.30,31,243 One model includes FC and pen type (40 mg vs 80 mg).116
UstekinumabAs yet, monitoring ustekinumab levels has not been recommended due to lack of evidence, with the exception of a recent published consensus proposing its use in secondary LOR.186 Although therapeutic levels are not sufficiently defined,63,72,90,246 intensification in patients with partial response or secondary LOR is effective.247 Those who achieve endoscopic remission have drug levels higher than those recommended.91,248 Bayesian models could help achieve greater drug exposure and therefore improve efficacy.249 There are two models published in IBD - one in UC and the other in CD - which include albumin and weight as covariates.250,251 There are no studies exploring the utility of these models in clinical practice but they could have a relevant role in induction, as the determination of levels as early as one hour after the initial infusion correlates with endoscopic remission at week 24.71
VedolizumabFour population PK models of vedolizumab have been published,252–255 constructed from the pivotal trials. They identify as covariates extreme values of weight, albumin253,254 and ADA titre.253 A model based on data from the LOVE-CD study also uses prior exposure to anti-TNF, which increases the clearance of vedolizumab by 25%.252
There is a clear relationship between drug exposure and efficacy.256 However, in the case of secondary LOR, increased levels with the intensification of treatment does not correlate with the likelihood of response.88 For this reason, the utility of population PK models in treatment with vedolizumab has yet to be determined.
How to apply predictive models?Population PK models help identify patients with a greater likelihood of therapeutic failure due to inadequate exposure to the drug and to choose the best dosage regimen in induction and maintenance for each specific subject.
Population prediction (prior to measuring levels in the patient) with a population PK model does not have optimal precision. However, it can help identify those with a worse prognosis due to greater clearance of the drug.257 This is the only independent factor associated with colectomy in some studies in severe UC.189
In a retrospective analysis of the patients included in the ACT studies, it was found that a baseline clearance <0.397 l/day was associated with clinical remission at week 8.53 Recently, a decision tool258 developed from the ACT studies has been proposed to identify UC subjects who achieve mucosal healing at weeks 8 and 30. The calculation includes initial clearance and achieves good diagnostic performance (sensitivity 82–90%, specificity 80–87%). It is available online with free access (www.premedibd.com).
Finally, calculating clearance dynamically enables us to identify patients highly suspected of having immunogenicity, but cannot be detected if we do not have drug tolerance tests when significant increases in clearance occur.238 Elevated clearance predicts the possibility of low levels and therefore immunogenicity.240 In fact, it has been estimated that the increased risk of ADAs is 61% for every 0.1 l/day increase in clearance.259
How useful are pharmacokinetic models in maintenance therapy?They seek to achieve a target level of the drug, which means adopting a proactive strategy. The chosen cut-off point can vary from one study to another and should probably be adapted to the chosen treatment goal and the circumstances of the specific patient.186 In perianal disease, the required trough levels will be higher,260 and probably also in the small intestine.261
The models allow us to approximate the percentage of patients who will achieve the target level with the dosages listed in the summary of product characteristics and identify subgroups at a higher risk of not achieving it; in the paediatric population, only 24.2% of subjects are reported to reach a level ≥5 μg/ml240 with the usual doses of infliximab. Adalimumab models262 enable us to approximate that with the usual regimen, <75% of patients will achieve a trough level >8 μg/ml and that those with a BMI > 30 or high FC (>200) will not achieve the target level with the conventional regimen.243
They will also help optimise dosage, choosing the regimen with the greatest likelihood of achieving high levels. The administration interval can be shortened instead of increasing the dose of infliximab,111,112 as higher levels will be achieved with the same amount of drug used.263
Several studies support the use of the Bayesian model. The Precision clinical trial177 included 80 patients with IBD in remission treated with infliximab for an average of four years, randomised to adjustments (from 3 to 5 μg/ml) according to a commercial model (i-Dose) compared to a control group in which no adjustments were permitted. At one year of follow-up, 88% of those assigned to the model were in sustained remission compared to 64% in the control group (p: 0.017). The main limitation, apart from the intervals chosen or not performing endoscopy, was that intensifying treatment was not permitted in the control group, which in no way reflects what would happen in routine clinical practice. The open series by Eser et al.264 compared patients with IBD being treated with infliximab who received adjustments according to a Bayesian model to those whose adjustments were made at the discretion of the physicians (blind to the model proposal), finding greater durability of the drug (51.5 months vs 4.6 months; p: 0.00004) in the first group. A Spanish series227 retrospectively compared a drug level management strategy based on the TAXIT study algorithm134 to the use of a Bayesian model in 47 subjects with IBD treated with infliximab. The percentage of people who achieved adequate levels was significantly higher with the model (odds ratio [OR]: 8.94; 95% confidence interval [CI]: 2.24–35.6) and this type of adjustment is the only factor identified in the multivariate analysis. The main limitation, in addition to the number of patients included, is the temporal difference between one cohort and another.
Therefore, although the use of population PK models in clinical practice is promising, more studies are needed to support the theoretical advantages over the strategies that tend to be used in clinical practice (empirical, reactive and proactive).
Are population pharmacokinetic models applicable in induction therapy?There is little published data on the utility of models in induction. In a pharmacokinetic-pharmacodynamic model on a retrospective cohort of patients with UC, the cumulative AUC of infliximab was the parameter that best correlated with mucosal healing at week 12.233 The model proposes an initial dose of 10 mg/kg/day with tiered dose adjustment at week 2. However, universal high doses for all patients have not been shown to be more effective with adalimumab265 and were not useful in another model from the same group for those with CD.232
Mould et al.266 performed a simulation with real paediatric patients (from the Precision study) comparing the dosing with the population PK model i-Dose to the 5- and 10-mg/kg regimens during induction. The proportion with levels <10 μg/ml at week 14 was 29.4% among those dosed with the population PK model and 94.6% and 82.5% when using fixed doses of 5 and 10 mg/kg, respectively.
ConclusionTherapeutic monitoring of biological medicinal product levels has emerged as a valuable tool in personalising the management of patients with IBD. Although it clearly does not answer all questions, it provides additional and complementary information to the clinical, biological and morphological data. Therapeutic drug monitoring is now considered well justified in the management of secondary LOR and in the optimisation of treatment during induction to reduce the risk of secondary PNR. Although the results show a lack of consistency, there is a growing interest in the utility of therapeutic drug monitoring as a proactive strategy in maintenance therapy (particularly, for example, in risk groups for secondary LOR, second anti-TNF, HLADQA1*05 carriers, perianal disease and monotherapy) and to guide the de-escalation or withdrawal of biological treatments.
FundingNo funding was received for this paper.
Conflicts of interestThe authors have received funding for research, training, attendance at scientific meetings and consultancy from the following companies:
F. Rodríguez Moranta: Janssen, MSD, AbbVie, Pfizer, Takeda, Lillie Ferring, Faes Farma and Falk Pharma.
J. Hinojosa: MSD, AbbVie, Ferring, Pfizer, Janssen, Takeda and Kern Pharma.
M. Iborra: MSD, AbbVie, Pfizer, Takeda, Janssen, Galapagos, Kern Pharma, Amgen and Sandoz.
M.D. Martín Arranz: MSD, AbbVie, Hospira, Pfizer, Takeda, Janssen, Shire Pharmaceuticals, Tillotts Pharma and Faes Farma.
L. Menchén: MSD, AbbVie, Pfizer, Janssen, Takeda, Biogen, Sandoz, Dr. Falk-Pharma, FAES, Ferring, General Electric and Medtronic.
F. Muñoz: Tillotts Pharma, Kern Pharma, AbbVie, Janssen, Pfizer and Takeda.
E. Ricart: MSD, AbbVie, Janssen, Takeda, Ferring, Pfizer, Amgen and Fresenius Kabi.
T. Valdés-Delgado: MSD, AbbVie, Pfizer, Takeda, Janssen, Galapagos, Kern Pharma, Amgen and Sandoz.
J. Guardiola: Roche, MSD, AbbVie, Kern Pharma, Takeda, Janssen, Pfizer, Ferring, Chiesi and GE Healthcare.
M. Barreiro-de Acosta: MSD, AbbVie, Hospira, Pfizer, Kern Pharma, Biogen, Takeda, Janssen, Sandoz, Celgene, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceutical and Vifor Pharma.
M. Mañosa Ciria: MSD, AbbVie, Kern, Takeda, Janssen, Pfizer, Ferring, Faes Farma, Dr. Falk Pharma, Tillotts Pharma and Adacyte.
Y. Zabana: Shire, Ferring, AbbVie, MSD, Takeda, FAES Farma, Falk, Adacyte, Janssen, Almirall, Amgen, Tillotts and Pfizer. Has also received consulting fees from Tillotts, Janssen and Ferring.
A. Gutiérrez: AbbVie, MSD, Kern, Ferring, FAES, Amgen, Roche, Sandoz, Janssen, Pfizer, Dr. Falk Pharma, Tillotts and Galapagos.
F. Argüelles-Arias: MSD, AbbVie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Tillotts Pharma, Galápagos and Chiesi.
JG Sánchez-Hernández: no conflicts of interest.