Endoscopic retrograde cholangiopancreatography (ERCP) is the technique of choice for the treatment of biliopancreatic pathology. However, fluoroscopic imaging does not always allow an adequate diagnosis. On the other hand, some large stones cannot be removed by the usual methods. In these situations, cholangioscopy has proven to be an essential tool for the diagnosis of biliary strictures and the treatment of large stones. Its role in pancreatic pathology is also increasing. The development of a single-operator, disposable cholangioscope has made it possible to expand the technique to a large number of hospitals that perform ERCP. For this reason, the Spanish Society of Digestive Endoscopy has developed this consensus document on the use of the SpyGlass-DS cholangioscope. The document has been prepared by a group of endoscopists with expertise in cholangioscopy, reviewing the scientific evidence on the main current indications for cholangiopancreatoscopy.
La colangiopancreatografía retrógrada endoscópica (CPRE), es la técnica de elección para el tratamiento de la patología biliopancreática. Sin embargo, las imágenes fluoroscópicas no siempre permiten un diagnóstico adecuado. Por otra parte, algunos cálculos de gran tamaño no se pueden extraer con los métodos habituales. En estas situaciones, la colangioscopia ha mostrado ser una herramienta fundamental para el diagnóstico de las estenosis biliares y el tratamiento de los cálculos de gran tamaño. Su papel, además en la patología pancreática está en creciente aumento. El desarrollo de un colangioscopio de un único operador y desechable, ha permitido expandir la técnica entre buena parte de los hospitales que realizan CPRE. Por este motivo, la Sociedad Española de Endoscopia Digestiva ha desarrollado este documento de consenso sobre la utilización del colangioscopio SpyGlass-DS. El documento ha sido elaborado por un grupo de endoscopistas expertos en colangioscopia, revisando la evidencia científica de las principales indicaciones actuales de la colangiopancreatoscopia.
Endoscopic retrograde cholangiopancreatography (ERCP) is an essential tool for the treatment of biliopancreatic diseases. However, one of its main limitations is the suboptimal definition of fluoroscopic images in the diagnosis of biliopancreatic duct diseases.
Cholangiopancreatoscopy not only allows direct macroscopic evaluation of the interior of the bile duct and pancreas, but also allows us to obtain targeted biopsies of lesions or perform targeted treatment such as lithotripsy on difficult stones.
With the introduction of the SpyGlass single-use cholangiopancreatoscopy system (Boston Scientific, Natick, MA, USA) in 2007, the technique has gained interest among professionals who perform ERCP, achieving widespread use because, with a single operator, it facilitates easy access to the biliopancreatic duct, it is a disposable device, with good manoeuvrability, with an independent accessory channel for irrigation and, due to its calibre, it enables exploration of the bile duct even if it is not dilated. Other cholangioscopy systems, such as 'mother-baby' cholangioscopes, have had less acceptance, due to their fragility and the cost of repairs, and the fact that two operators are required to perform the examination. As a result, these systems are only available in a few referral hospitals worldwide. Finally, although cholangioscopy through the mouth using ultrathin endoscopes yields better image quality, it has limited manoeuvrability, especially in intrahepatic and narrow calibre bile ducts.
The second generation of SpyGlass, the SpyGlass DS System (SG-DS), benefits from a significant increase in image quality, while manoeuvrability and ease of use are considerably improved over the optical Spyglass system. This system incorporated a reusable fibre-optic probe, with poor image quality. The current DS system incorporates a digital chip that improves the image and facilitates its use, as the entire system is disposable. The new DS-II version further improves the image, depth of field and illumination within the bile duct, avoiding the appearance of reflections and areas of shadow.
This consensus document pertains to the use of the SpyGlass DS System (Boston Scientific Corp.), the only one currently approved in Spain for cholangioscopy, and the most widely used cholangioscopy system worldwide.
This article complements the article by Dolz Abadía et al.,1 previously published by this same team.
Our objective is to define the indications and determine the possibilities and questions that the application of this technology poses for the endoscopist.
MethodThe work team was made up of 13 endoscopists from all over Spain who carried out 30 or more procedures with SG-DS at their place of work per year.
We divided the theme into seven blocks representing the main indications for using SG-DS:
- -
Indeterminate bile duct stricture.
- -
Difficult stone treatment.
- -
Guidewire advancement through difficult strictures.
- -
Diagnosis of intraductal papillary mucinous neoplasm of the pancreas.
- -
Evaluation of dilation/stricture of the pancreatic duct.
- -
Pancreatic intraductal stones.
- -
Other indications.
The final recommendations were established by applying the Delphi method, including in the document the recommendations that reached the necessary degree of agreement (DA). Recommendations that did not reach the appropriate DA were reformulated and subjected to a new evaluation (they are indicated with an*). If this agreement was not reached, they were dismissed.
The recommendations prepared by the group of experts present the level of evidence (LE) and the grade of recommendation (GR) according to the Oxford Centre for Evidence-Based Medicine,2 and the DA among the experts.
Recommendations and proposalsBlock 1: Indeterminate bile duct stricture (IBS)1. What is the role of SG-DS in the evaluation of IBS?
The use of cholangioscopy by SG-DS is recommended in the evaluation of indeterminate bile duct stricture (IBS) after inconclusive cytology and/or biopsy.
(LE: High; GR: D; DA: 91%)
Comment: In IBS, brush cytology has a very low sensitivity (45%), as does blind intraductal biopsy (48.1%).3 When these patients are operated on based on suspicion of neoplasia, 15–24 % have benign pathology.4
2. What is the expected technical success in evaluating an IBS with SG-DS?
The technical success is greater than 98%, which is dependent on the technical success of the ERCP. This technical success rate may be lower in those cases in which the IBS is distal or affects intrahepatic ducts.
(LE: High; GR: D; DA: 100%)
Comment: The SG-DS system has a high technical success rate in this context, and the few technical failures are mainly related to very distal lesions, in which exploration with SG-DS is difficult.
3. At what point in the diagnostic process should SG-DS be used?
The use of SG-DS is recommended in IBS cases in which the information provided by visual inspection, with or without pathological anatomy, leads to a change in patient management.
(LE: High; GR: D; DA: 91%)
4. Is visual diagnosis by SG-DS sufficient in IBS?
The diagnostic yield of visual evaluation in the characterisation of indeterminate strictures is high. The presence of dilated and tortuous vessels is highly suggestive for a diagnosis of malignancy. Despite the high efficiency of visual evaluation, taking biopsies is mandatory, when technically feasible, due to their high positive predictive value (PPV).
(LE: High; GR: D; DA: 100%)*
Comment: SG-DS has a sensitivity for visual diagnosis of 95.5–96.6%, with a specificity of 93.3–94.5 % and a negative predictive value (NPV) of 96.3–97.7%. However, the sensitivity of histological diagnosis is lower (57.7–86.2%).3,5
5. What is the diagnostic yield of biopsy guided by SG-DS in IBS?
The diagnostic yield of biopsy guided by SG-DS is currently lower than visual evaluation due to its limited sensitivity. A minimum of four biopsies is recommended to increase the yield.
(LE: High; GR: D; DA: 100%)*
6. What are the criteria for malignant imaging?
The most powerful predictive criterion for malignancy is the presence of dilated and tortuous vessels, with a PPV of up to 100% in some studies.
(LE: High; GR: D; DA: 100%)
Comment: It is now known that the presence of abnormal vessels or spider veins in the stricture, together with villous papillary projections, is a sufficient criterion to define the existence of malignancy, with a specificity and PPV of 100%. The presence of abnormal vessels shows a sensitivity of 94% and a specificity of 63%, a PPV of 75% and a NPV of 90% for the diagnosis of malignant stricture.6
For the diagnosis of bile duct strictures, the Robles-Medranda et al. classification7 was proposed and the Mendoza criteria8 were subsequently defined. The diagnostic accuracy of this classification is 77% (64–88%).
7. What is the utility of SG-DS in the diagnosis of endoluminal spread of extrahepatic cholangiocarcinoma?
The use of cholangioscopy by SG-DS is suggested in the study of the spread of extrahepatic cholangiocarcinoma in those patients who are candidates for surgery, in whom imaging studies are ineffective in defining the endoluminal spread of the lesion.
(LE: High; GR: D; DA: 100%)*
Comment: In cases where the information has an impact on the clinical management of the patient. Note: In stricture of the distal intrapancreatic section, the SG-DS study may be compromised due to the degree of stricture and proximity to the papilla.
8. What is the role of SG-DS in the evaluation of patients with primary sclerosing cholangitis (PSC)?
The use of SG-DS is recommended as the method of choice for the diagnosis of cholangiocarcinoma in patients with PSC, as long as the finding through imaging methods (CT/MRI) is not conclusive.
(LE: High; GR: D; DA: 82%)
SpyGlass is effective and safe in the endoscopic management of patients with PSC. In a recent study, it detected the presence of cholangiocarcinoma in 18% of patients with dominant strictures.9
9. What is the utility of SG-DS in the evaluation of radiofrequency treatment of biliary lesions?
There are no data to recommend the use of SG-DS in the evaluation of radiofrequency treatment of biliary lesions.
(LE: Low; GR: D; DA: 91%)
10. Is SG-DS useful in the study of intraductal spread of ampullary lesions?
There are no data to recommend the use of SG-DS in the evaluation of intraductal spread of ampullary lesions.
(LE: Low; GR: D; DA: 91%)
11. What is the role of SG-DS in evaluating other cholangiographic findings, such as contrast filling defects of uncertain nature?
The use of cholangioscopy with SG-DS is suggested as an effective method to evaluate filling defects of uncertain nature seen on cholangiography.
(LE: High; GR: D; DA: 100%)*
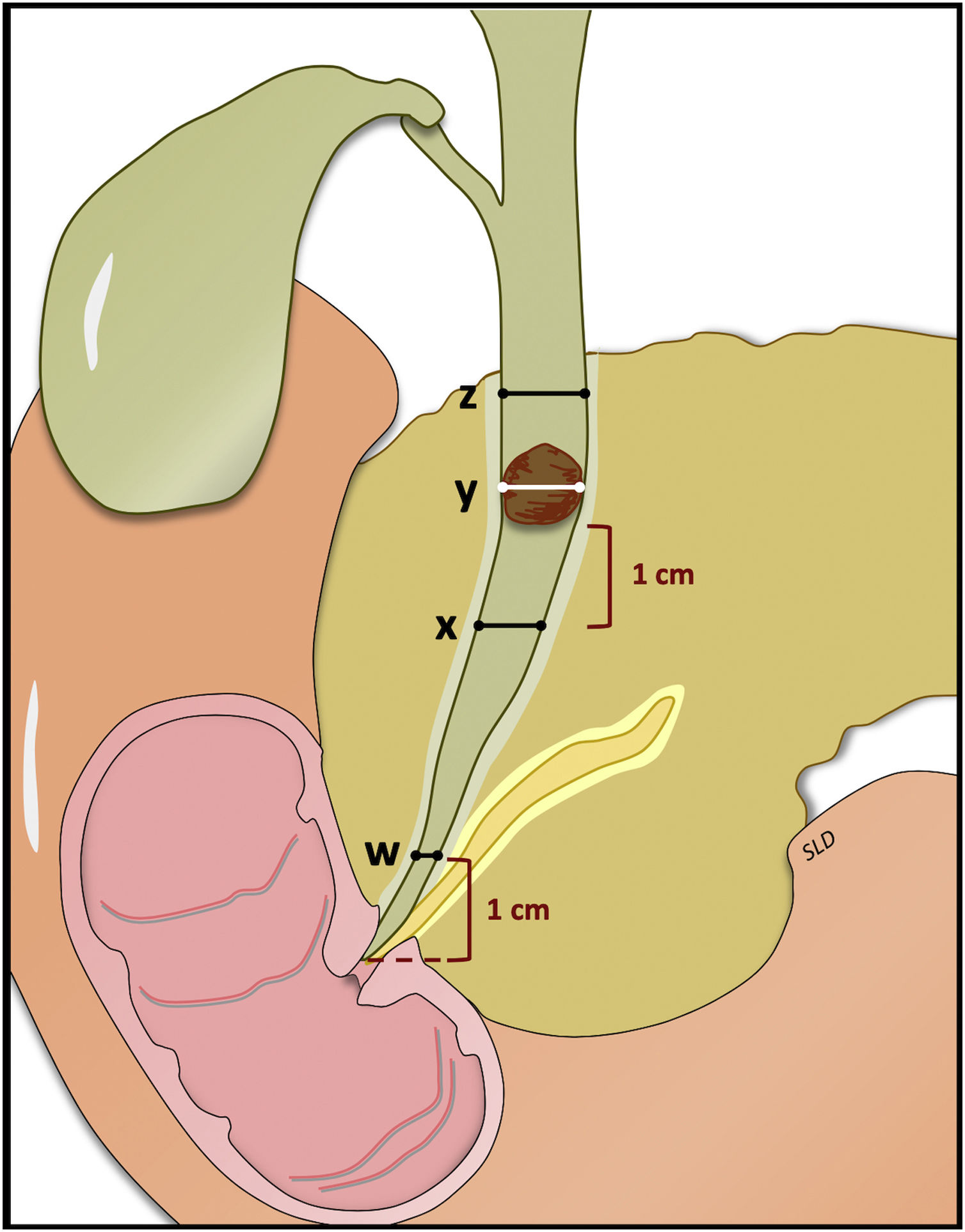
Block 2: Treatment of difficult stones (DS)The classic characteristics that define a difficult stone (DS) are: size larger than 15mm, more than three simultaneous stones larger than 10mm, disproportion between the size of the stone and the distal bile duct, faceted morphology and suprastenotic location, in the cystic or intrahepatic duct10 (Table 1). We could also talk about DS when extraction is not achieved after applying conventional techniques (sphincterotomy, sphincteroplasty, retrieval basket and/or mechanical lithotripter).11
Difficult stones in the bile ducts.
| Category | Conditions | Reasons |
|---|---|---|
| Stone characteristics | Large stones (>15mm) | Need for lithotripsy and difficulty capturing the stone with a basket |
| Multiple stones (>3 stones, size >10mm) | ||
| Hard stones | ||
| Strange shaped stones | ||
| Stone location | Intrahepatic duct stone | Difficult access |
| Stones above a stricture | ||
| Stone impacted in bile duct/cystic duct | ||
| Mirizzi syndrome | ||
| Anatomical situation | Altered anatomy | Difficulty in biliary access and limitation of the endoscope/accessory |
| Billroth II/Roux-en-Y gastric bypass anatomy | ||
| Periampullary diverticulum | ||
| Patient factors | Old age/poor general condition | Risk of adverse events |
| Unstable vital signs | ||
| Tendency to bleed | ||
| Paradoxical response |
1. What is the role of SG-DS in the treatment of DS?
a) The use of SG-DS cholangioscopy-assisted lithotripsy is recommended as an effective and safe treatment of DS.
(LE: Moderate; GR: D; DA: 100%)
Comment: Between 10% and 15% of gallstones12,13 cannot be removed by the usual means and it is necessary to resort to special techniques such as mechanical lithotripsy (ML), large diameter balloon dilation (LDBD) or the placement of stents, with stone extraction reattempted in subsequent ERCPs.
A systematic review suggests that laser lithotripsy (LL) is more effective in terms of stone clearance and fragmentation than external shock wave lithotripsy (SWL) and is also associated with fewer adverse effects.14
Several studies compare lithotripsy with SG-DS with ML and/or LDBD.14–16 From these studies it can be deduced that both electrohydraulic lithotripsy (EHL) and LL by SG-DS are an effective treatment for the management of DS, although it seems advisable to reserve their use to after the failure of conventional methods. However, in large stones (>15mm) that are impacted, located in a cyst, proximal to a stricture, or with a funnel-shaped bile duct, where sphincteroplasty can have significant complications,17 lithotripsy using SG-DS may be the initial treatment of choice. In these cases, the placement of a biliary stent to decompress the bile duct may be an option if cholangioscopy is performed later.18
b) Is transluminal SG-DS feasible for the treatment of DS?
DS lithotripsy with SG-DS is feasible through prior creation of an EUS-guided transluminal access, whenever transpapillary access is not possible.
(LE: High; GR: D; DA: 100%)*
Comment: The application of lithotripsy with SG-DS through EUS-guided biliary access has been described in DS cases that cannot pass through the dilated papilla. Only one pilot study of lithotripsy with SG-DS through endoscopic ultrasound-guided transluminal access has been published, showing that it was feasible to perform DS lithotripsy in eight cases.19
c) Does lithotripsy with SG-DS have any advantages over other cholangioscopy techniques?
Lithotripsy with SG-DS is easier to use than direct cholangioscopy with ultrathin endoscopes or the classic “mother-baby” cholangioscopy.
(LE: Low; GR: D; DA: 91%)
Comment: Access to the bile duct for lithotripsy in DS15,16 using cholangioscopy has been performed using three different methods: directly through an ultrathin endoscope, with the “mother-baby” system (ultrathin cholangioscope passed through a wide channel duodenoscope) and, more recently, with the SG-DS system. The difference between the three systems can be seen in Table 2.
Three types of cholangioscopy system.
| “Mother-baby” dual operator cholangioscopy | Single operator cholangioscopy (SpyGlass DS System) | Direct peroral cholangioscopy (ultrathin endoscope) | |
|---|---|---|---|
| Endoscopists | 2 | 1 | 1 |
| Additional endoscope system | Yes | Yes | No |
| Scope diameter, mm | 3.3–3.5 | 3.6 | 5–6 |
| Accessory channel, mm | 1.2 | 1.2 | 2 |
| Irrigation channel | No | Yes | No |
| Cost | High | High | Low |
| Image quality | High | Moderate | High |
2. When should SG-DS lithotripsy be performed in the management of DS?
The current position of SG-DS lithotripsy in the DS treatment algorithm would be in cases where conventional techniques have failed. The group of experts recommends the early incorporation of LL/EHL with SG-DS in the management of DS.
(LE: Low; GR: D; DA: 82%)
Comment: The indication for lithotripsy with SG-DS for the management of DS can be inferred from the different studies summarised in the recent meta-analysis16 (Table 3).
Efficacy and safety of peroral cholangioscopy: cumulative and comparative meta-analysis.20,21
| Cumulative data for peroral cholangioscopy | Data from individual techniques for peroral cholangioscopy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All therapies | Randomised controlled trials and prospective studies | Mother-baby system | FIRST-generation direct visualisation choledochoscope for a single operator | SECOND-generation direct visualisation choledochoscope for a single operator | ||||||
| Combined rate (95% Cl) | Heterogeneity (I²) | Combined rate (95% Cl) | Heterogeneity (I²) | Combined rate (95% Cl) | Heterogeneity (I²) | Combined rate (95% Cl) | Heterogeneity (I²) | Combined rate (95% Cl) | Heterogeneity (I²) | |
| Overall fragmentation success | 91.15% | 64.00% | 88.88% | 51.89% | 89.31% | 62.11% | 89.99% | 54.45% | 95.03% | 31.87% |
| (95% CI 87.97 to 93.55) | (95% CI 83.94 to 92.44) | (95% CI 81.53 to 94.05) | (95% CI 82.09 to 94.63) | (95% CI 91.86 to 97.01) | ||||||
| 33 studies, n=1,718 | 17 studies, n = 697 | 10 studies, n = 366 | 10 studies, n = 321 | 6 studies, n=629 | ||||||
| [Supp Ref:1,3-34] | [Supp Ref:9-11,13,14,16,17,19-23,25-27,29,33] | [Supp Ref:1,5,15,16,25-27,29,30,32] | [Supp Ref:3,4,6,11,13,15,17,18, 28,31] | [Supp Ref:7-10,24,34] | ||||||
| Success of single session fragmentation and duct clearance | 76.81% | 74.05% | 76.87% | 35.58% | 66.75% | 72.91% | 80.64% | 81.19% | 80.44% | 60.94% |
| (95% CI 70.79 to 80.85) | (95% CI 70.80 to 82.00) | (95% CI 53.95 to 77.48) | (95% CI 65.51 to 90.13) | (95% CI 73.26 to 86.06) | ||||||
| 27 studies, n=1,472 | 11 studies, n = 451 | 7 studies, n = 278 | 9 studies, n = 304 | 5 studies, n=581 | ||||||
| [Supp Ref:1,3-8,10-15,18-22,24,26-32,34] | [Supp Ref:10,11,13,14,16,20-22,26,27,29] | [Supp Ref:1,5,26,27,29,30,32] | [Supp Ref:3,4,6,11,13,15,18,28, 31] | [Supp Ref:7,8,10,24,34] | ||||||
| Adverse events | 8.90% | 62.05% | 7.50% | 47.50% | 13.51% | 48.80% | 9.75% | 4.87% | 4.14% | 0.00% |
| (95% CI 6.40 to 12.26) | (95% CI 4.74 to 11.69) | (95% CI 8.54 to 20.72) | (95% CI 6.48 to 14.43) | (95% CI 2.67 to 6.37) | ||||||
| 27 studies, n=1,512 | 11 studies, n = 451 | 7 studies, n = 331 | 8 studies, n = 275 | 3 studies, n=481 | ||||||
| [Supp Ref:1-12,14,16,19-23,25-33] | [Supp Ref:10,11,13,14,16.20-22,26,27,29] | [Supp Ref:1,5,25-27,30,32] | [Supp Ref:2-4,6,11,21,28,31] | [Supp Ref:7,8,10] | ||||||
In recent years, LDBD has replaced ML with significant reductions in the total rate of adverse effects.22–27 The safety of these techniques is related to the size of the stone and the anatomy of the bile duct.28
External SWL on DS reports a bile duct clearance capacity of 78–90%,27,28 while this figure is 75–100% in cholangioscopy-assisted lithotripsy (EHL/LL).16 In both cases, more than one session is often required to achieve complete clearance of the bile duct.29,30
In patients with DS, in whom the classic techniques of ML and LDBD have a lower clearance rate and a greater number of complications, lithotripsy (EHL/LL) with SG-DS could be a substitute as the first choice indication.31
3. What is the utility of SG-DS in the detection of residual stones?
The SG-DS residual stone detection rate is 34%, most of them less than 7mm, and more frequently when lithotripsy has been previously performed.32,33
(LE: Low; GR: D; DA: 91%)
4. What is the recommended treatment regimen for the management of DS?
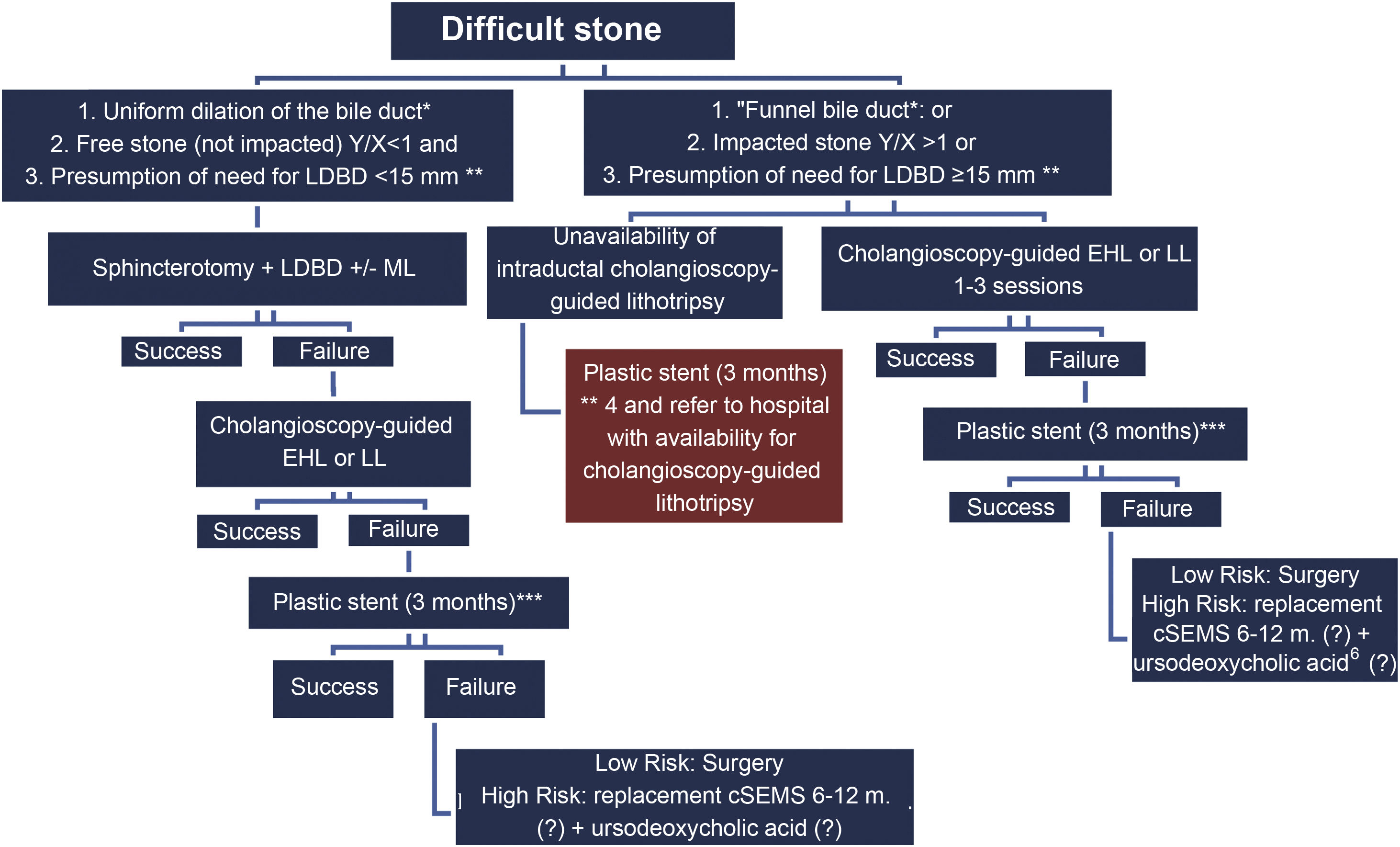
It is recommended to see Fig. 1 and the algorithm shown in Fig. 2.
Algorithm.
cSEMS: covered self-expanding metal stent; EHL: electrohydraulic lithotripsy; LDBD: large diameter balloon dilation; LL: laser lithotripsy; ML: mechanical lithotripsy.
*Uniform dilation of the bile duct: ratio of bile duct diameter (1cm over papilla) (w)/widest proximal bile duct diameter (z) 1≥0.5; “Funnel bile duct”: Ratio of bile duct (1cm over papilla) (w)/widest proximal bile duct diameter (z) 1<0.5. **Extraction will be possible with a balloon (LDBD) <15mm. ***Two 7F double-pigtail stents in parallel are recommended.
Depending on a) the anatomical characteristics of the bile duct, b) the presence of free or impacted stones, and c) the presumed diameter of the dilatation balloon to be used, LDBD or lithotripsy with SG-DS is recommended in the first session. If bile duct clearance is not obtained with LDBD, lithotripsy with SG-DS is recommended. If stone clearance is not achieved or SG-DS is not available, it is recommended to insert a plastic stent for three months34 and attempt a new lithotripsy, or surgery in low-risk cases. When all options fail in high-risk patients, a covered self-expanding metal stent (cSEMS) is recommended with replacements every 6–12 months and the possibility of adding continued treatment with ursodeoxycholic acid.
5. What is the recommended lithotripsy method?
a) Intraductal lithotripsy using either of the two available techniques (LL and EHL) is a very effective treatment for the management of complex lithiasis.
(LE: High; GR: D; DA: 100%)
Comment: Although most studies do not show a clear superiority between LL and EHL,35 in a systematic review, LL achieved greater stone clearance than EHL and ML (95.1%, 88.4% and 84.5%, respectively; p<0.001).12
Laser probes should probably be selected only in cases of very large, multiple, or hard stones, to overcome the lifespan limitation of the EHL probe.36
b) LL and EHL have proven to be effective in the extraction of DS as the first-choice technique and as a rescue technique for other methods (ML and the combination of LDBD/ML).14,37
(LE: High; GR: D; DA: 100%)*
Comment: For ML, when the stone is >25mm, is impacted or located at the confluence of the main hepatic ducts, the success rate does not exceed 68%.38
c) There is currently not enough scientific evidence to determine which is the best method of intraductal lithotripsy. As such, studies specially designed to compare LL with EHL must be conducted.
(LE: Low; GR: D; DA: 100%)
6. What is the economic impact of the use of SG-DS in the management of patients with DS?
Intraductal lithotripsy with SG-DS can be cost-effective when used early39 and when other methods are expected to fail, avoiding the repetition of new ERCP sessions and surgical rescue.
(LE: High; GR: D; DA: 100%)*
Comment: Intraductal lithotripsy would result in a 27% reduction in the number of procedures and, ultimately, an 11% reduction in the economic cost.39
The only study that included a cost study did not find significant economic differences between the LDBD method and intraductal LL, although it presented a higher bile duct clearance rate (72.7% vs 93.9%; p=0.021).40
Block 3: Selective guidewire advancement through difficult strictures1. What is the utility of the SG-DS in guidewire advancement through difficult bile duct strictures?
The use of SG-DS is suggested as a method for the passage of guidewires through difficult bile duct strictures, selective cannulation of a hepatic or cystic duct, when conventional methods fail.
(LE: Low; GR: D; DA: 91%)
SG-DS facilitates guidewire advancement in bile duct strictures in liver transplant patients when conventional methods fail.
(LE: Low; GR: D; DA: 82%)
Comment: One study,41 using direct visualisation with SG-DS, managed to pass the guidewire through the stricture in 70% of cases: in 88.2% of benign strictures and in 46.2% of malignant strictures, p=0.02. In another study with transplant patients, the authors managed to pass the guidewire using SG-DS in all cases, with estimated savings in this type of patient of between $6,240 and $66,540.42 The rest of the studies43–47 generally show that, in transplant patients, the efficacy seems greater in benign strictures.
Cystic duct cannulation with SG-DS is useful for transcystic drainage in patients with acute cholecystitis.48
Block 4: Diagnosis of intraductal papillary mucinous neoplasm (IPMN) of the pancreas1. What is the role of SG-DS in the diagnosis of main-duct IPMN?
Pancreatoscopy with SG-DS is useful in the diagnosis of IPMN of the main pancreatic duct in patients with dilated duct in whom the diagnosis could not be confirmed by standard imaging methods (CT, MRI, EUS).
(LE: Moderate; GR: D; DA: 91%)
Comment: Two studies49,50 show that the technical success is greater than 90%, but the sensitivity for the diagnosis of IPMN is very disparate. The specificity is 100% for the diagnosis of malignancy. Pancreatoscopy leads to a change in therapeutic decision making in three out of four cases.49
2. What role does SG-DS play in the diagnosis of preoperative spread of main-duct IPMN?
Preoperative pancreatoscopy may be helpful in defining the spread of IPMN and the presence of synchronous lesions in the pancreatic duct.
(LE: Low; GR: D: DA: 91%)
Comment: the sensitivity of SG-DS to define the resection margin is 85.7%51 but the efficacy of taking biopsies proximal and distal to the lesion (mapping) for the histological evaluation of the spread of the IPMN has not been investigated.
3. What findings are indicative of malignancy?
The presence of vessels in the papillary structures of the IPMN, as well as the presence of a nodule, infiltrative stricture and irregular margins are associated with in situ or invasive carcinoma.
(LE: Low; GR: D; DA: 91%)
Comment: The presence of vessels is associated with in situ or invasive carcinoma in 89% of cases, while the villous or vegetative type is associated in 91–92% of cases.52
4. Is SG-DS useful in the evaluation of patients with secondary branch IPMN?
Pancreatoscopy with SG-DS is useful in patients with side branch IPMN when involvement of the main duct is suspected (mixed IPMN) to confirm or rule out involvement of the main duct, in order to establish the best therapeutic approach.
(LE: High; GR: D; DA: 100%)*
Comment: The usefulness of pancreatoscopy with SG-DS in patients with secondary branch IPMN49 shows a sensitivity of 78%.
Block 5: Evaluation of dilation/stricture of the pancreatic duct1. What is the role of SG-DS in the evaluation of patients with dilation of the pancreatic duct?
The use of pancreatoscopy using SG-DS is suggested in the evaluation of patients with significant dilation of the pancreatic duct with a cause not diagnosed by the usual imaging techniques.
(LE: High; GR: D; DA: 100%)*
2. What role does SG-DS play in the evaluation of indeterminate strictures of the pancreatic duct?
It is feasible to use pancreatoscopy with SG-DS and take biopsies in the evaluation of indeterminate strictures or abnormalities of the pancreatic duct in the absence of a diagnosis by other imaging techniques, provided that the diameter of the pancreatic duct distal to the lesion allows the passage of the SG-DS. However, the risk of post-exploration acute pancreatitis should not be ignored, so this should be kept in mind in asssessing the risk/benefit balance.
(LE: High; GR: D; DA: 100%)*
Block 6: Pancreatic intraductal stones1. What is the indication for performing SG-DS lithotripsy in patients with chronic pancreatitis and intraductal stones?
The indication would be for the presence of intraductal stones that cannot be extracted by conventional technique (preferably balloon), generally stones larger than 5mm.
(LE: Low; GR: D; DA: 100%)
Comment: The location is not a limitation, although due to its lesser difficulty it is preferable if it is located in the head or distal body. For stones close to the papilla, it may be more difficult to perform lithotripsy using SG-DS.
2. Which lithotripsy method is best for pancreatic intraductal lithotripsy?
a) It is suggested that LL or EHL applied via pancreatoscopy with SG-DS is feasible, with effects probably superior to standard balloon extraction and comparable to external SWL.
(LE: Low; GR: D; DA: 100%)
b) No differences were observed between the use of LL or EHL.53
(LE: Low; GR: D; DA: 91%)
3. What is the role of lithotripsy using SG-DS vs extracorporeal lithotripsy in the treatment of chronic pancreatitis?
It is suggested that LL or EHL through pancreatoscopy with SG-DS can be substituted in hospitals where external SWL is not available or not easily accessible, with a similar level of efficacy and safety profile.
(LE: Low; GR: D; DA: 100%)
Comment: In two recent meta-analyses,53,54 similar efficacy and safety were observed between external SWL and intraductal lithotripsy using pancreatoscopy. Therefore, and until there is more evidence, both techniques can be equally recommended and their use will depend more on their availability and experience with each of them.
Block 7: Other indications for SG-DS1. Is SG-DS useful in the evaluation of patients with haemobilia?
The use of SG-DS in the evaluation of patients with haemobilia is not recommended, especially in cases of significant haemobilia where it may delay treatment.55
(LE: Low; GR: D; DA: 100%)
2. What role does SG-DS play in the management of foreign bodies lodged in the bile and pancreatic ducts?
It is suggested that SG-DS may be useful in the extraction of foreign bodies in the bile duct when conventional methods fail.
(LE: Low; GR: D; DA: 91%)
Comment: The utility of SG-DS in the extraction of foreign bodies lodged in the bile duct only has been described in published clinical cases.56–61 Most cases use the biopsy forceps (SpyBite) for extraction. Recently, a retrieval snare (Spysnare) and basket (Spybasket) have been marketed that may be more useful for the extraction of foreign bodies.
3. Can SG-DS replace fluoroscopy for ERCP in some situations?
The use of SG-DS is suggested as an alternative method to fluoroscopy in those situations in which this cannot be used or is contraindicated for the treatment of uncomplicated gallstones.
(LE: Moderate; GR: D; DA: 91%)
Comment: The possibility of performing fragmentation of uncomplicated choledocholithiasis and bile duct clearance without the need to use fluoroscopy or greatly reducing its use is confirmed.62–64
FundingTo discuss the consensus document, Boston Scientific Spain financed the face-to-face meetings and facilitated the online meetings of the signing authors.
Conflicts of interestJR Aparicio Tormo Ramón is a consultant for Boston Scientific. J Vila Costas is a consultant for Boston Scientific and has participated as a speaker in courses and educational activities sponsored by Cook Endoscopy, Pentax, Olympus, Cassen and Norgine. J Gornals Soler is a consultant for Boston Scientific. E Domínguez-Muñoz has received fees as a speaker and for attending consulting meetings for Boston Scientific. F González Huix was an Advisory Board speaker or participant for Olympus, Ambu and Izasa until 2021 and currently remains on Advisory Boards for Boston Scientific. V Pons Beltrán is a consultant for Boston Scientific. The other authors have no conflicts of interest to declare.