INTRODUCTION
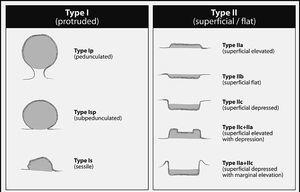
Colorectal adenomas have traditionally been classified in western countries as sessile or pedunculated. However, in 1983 the Japanese Reseach Society for Cancer of the Colon and Rectum also recognized the existence of flat adenomas1. Three types of flat adenomas have been distinguished in relation to their shape: flat-elevated, totally flat or flat-depressed, a classification that has been recently accepted by the WHO2.
In 1985 Muto et al described «small flat adenomas» as lesions ¾ 10 mm in size, flat-elevated, sometimes showing a central redness, and with a significant rate of high grade dysplasia3. Since then, many studies, most of them by Japanese authors, have focused on the clinicopathological characteristics of these lesions, and have determined that approximately 40% adenomas are flat4. Although initial reports from the western world suggested a lower frequency of flat lesions than in the Japanese series, the implementation of chromoendoscopy has improved the detection of non-protruding colorectal neoplasms in western countries5. In fact, several recent prospective studies carried out in the USA, UK and Sweden have reported similar frequencies to the Japanese series6-9.
Several studies have suggested that flat lesions may behave differently to protruding polyps, leading more frequently to high grade dysplasia (HGD) or submucosal invasive carcinoma (SIC)3-5. However, the higher prevalence of HGD and SIC in flat lesions seems to be more relevant in the Japanese population than in western countries suggesting that prognosis of non-protruding adenomas may be influenced by ethnic and environmental factors10,11.
Among the different types of flat adenomas, the recognition of depressed lesions seems to be of paramount importance. Although they are usually small in size, a number of studies have shown a greater risk for HGD or SIC and some authors have suggested that they may follow a different carcinogenic pathway to flat elevated or protruding adenomas9,12-15.
In western countries, data on the characterization of flat adenomas is limited and results are controversial. The aim of the present study was to determine for the first time the frequency of colonic flat adenomas among patients with colorectal polyps and their malignancy risk in a Southern European population.
MATERIAL AND METHODS
Colonoscopic reports of all examinations carried out by the same endoscopist (A.P-B) in a 29 month period were retrospectively reviewed. This endoscopist had received specific training in colonoscopic techniques for endoscopic detection and treatment of flat lesions. All patients with colorectal polyps and histology samples were included. Those patients with familiar adenomatous polyposis, hereditary nonpolypoid colon cancer and inflammatory bowel disease were excluded. One thousand three hundred colonoscopies in 1105 patients were performed during this period. One hundred and ninety-nine patients underwent colonoscopy more than once due to poor tolerance, poor bowel preparation, or incomplete polipectomy. The indications for colonoscopy were follow-up for cancer or adenomas in 375 (28.8%) patients, rectal bleeding in 241 (18.5%), colorectal cancer screening in 199 (15.3%), diarrhoea or constipation in 110 (8.4%), anemia in 98 (7.5%), and others in 277 (21.3%). Patients received a low fibre diet 24 h prior to the colonoscopy and oral bowel preparation with 3 l of polyethylene glycol electrolyte solution (Solución Evacuante Bohm, Laboratorios Bohm S.A, Fuenlabrada, Madrid, Spain) on the same day as the colonoscopy, or two bottles of 45 ml Sodium Phosphate solution (Fosfosoda, Casen Fleet, Utebo, Zaragoza, Spain), one taken the evening before and a second on the morning of the colonoscopy. All patients received 15 mg of Bysacodyle (Dulcolaxo®, Boehringer Ingelheim, S.A., San Cugat del Vallés, Barcelona, Spain), on the day before colonoscopy. Patients were sedated with midazolam and meperidine if necessary. Moreover, hyoscine butylbromide was administrated intravenously in patients with no contraindication in case of bowel spasm. Whenever a flat lesion was suspected (by subtle mucosal changes such as paleness, loss of mucosal vascular net pattern, mucosal deformity, or mucosal erythema), 0.2% indigo carmine dye (5 ml) was applied directly on the area through the biopsy channel, using a syringe9. Pedunculated or sessile polyps larger than 3 mm were treated with the polypectomy snare technique; sessile polyps smaller than 3 mm were removed by cold biopsy or hot biopsy technique; flat-elevated lesions smaller than 5 mm by hot biopsy or endoscopic mucosal resection (EMR); and flat adenomas larger than 5 mm, by EMR. Flat depressed lesions amenable to endoscopic treatment were resected by EMR. In brief, in depressed lesions >= 10 mm in size EMR was not attempted due to the high risk of invasion4. In depressed lesions < 10 mm and in flat elevated lesions, EMR was attempted when there was adequate lifting after submucosal injection of normal saline16. Lesions not amenable to endoscopic resection were tattooed according to the technique described by Fu et al17. Histological samples stained with haematoxilin and eosine of all polyps were retrieved and analysed by two pathologists. The Japanese Research For Cancer of Colon and Rectum Classification was applied in order to classify lesions as mucosal polyps, flat elevated lesions, and flat depressed lesions1 (fig. 1). Morphological characterization of flat-elevated adenomas was done using a modification of the criteria proposed by Sawada et al18 (fig. 2). A flat elevated lesion was defined as one whose diameter was at least twice the height of the lesion; lesions ¾ 10 mm, were diagnosed as flat-elevated only if they clearly showed a plaque-like shape (fig. 3 A-D); otherwise, they were classified as sessile. Lesions were diagnosed as flat depressed when a discrete and extensive depression (with or without a marginal elevation) was observed with indigo carmine stain (fig. 3 G-L)14. Examples of the different types of lesions according to the classification of the Japanese Society for Cancer of the Colon and Rectum are shown in figures 3 and 4. The size of each lesion was estimated by placing the biopsy forceps or the polypectomy snare probe adjacent to the lesion, in order to calculate the width/height ratio. Histopathological diagnosis was performed by two general pathologists (with 40 and 20 years experience respectively) with a co-observation microscope after retrieving the histological slides. Whenever there was a discrepancy in the diagnosis, consensus was reached after discussion. Only the final histopathological diagnosis was recorded in the database. The diagnosis of HGD and SIC was performed according to WHO criteria2. The macroscopic type (protruding or flat) was not assessed specifically by the pathologists. The following data was analysed in flat and protruding polyps: size, location (proximal or distal to splenic flexure), histology (neoplastic or non-neoplastic), HGD and SIC or beyond. Regarding patients with polyps, the following variables were recorded: gender, age, type of polyp (protruding, non-protruding or both), number of polyps, location of the polyps, HGD and SIC.
Fig. 1. Classification of early colorectal neoplasms according to the Japanese Society for Cancer of the Colon and Rectum. Adapted from The Japanese Research Society for Cancer of Colon and Rectum1.
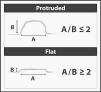
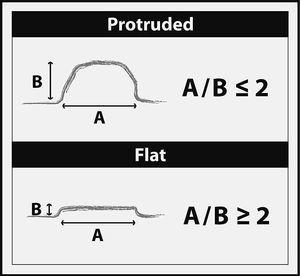
Fig. 2. Criterion for distinguishing between a sessile and a flat elevated lesion. Adapted from Sawada et al18.
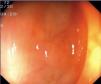
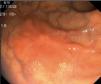
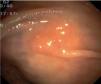
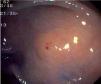
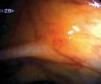
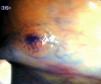
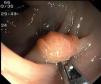
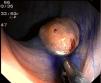
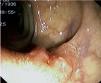
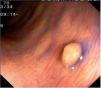
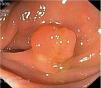
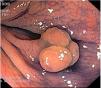
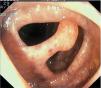
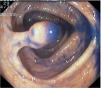
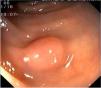
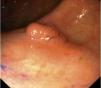
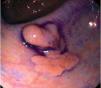
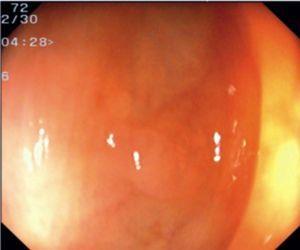
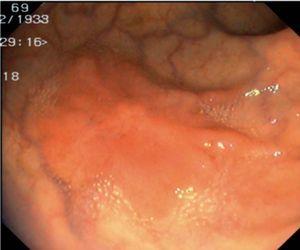
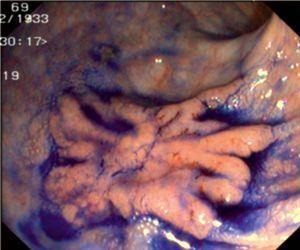
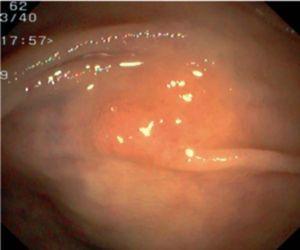
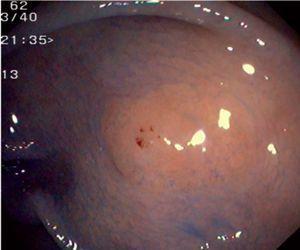
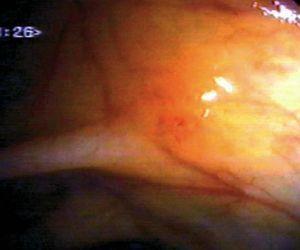
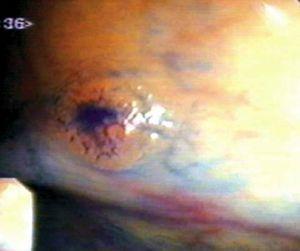
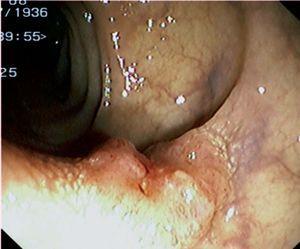
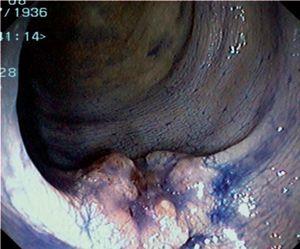
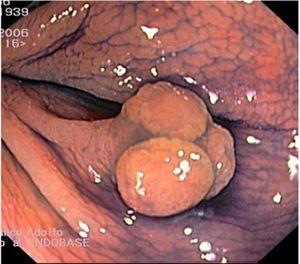
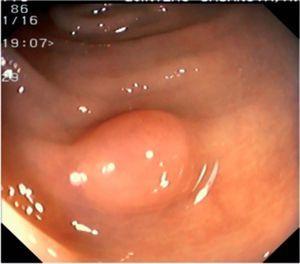
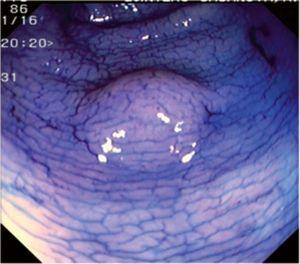
Fig. 3. Examples of flat lesions with conventional examination and after the application of indigo carmine 0.2-0.5%. a, b) Small flat adenoma, 3 mm in size. c, d) Large flat adenoma, 15 mm in size. e, f) Completely flat lesion (IIb in the Japanese classification). g, h) Depressed lesion, 3 mm in size) (IIc in the Japanese classification). I, J) Depressed lesion, 7 mm in size, histopathological study revealed submucosally invasive cancer (traditionally IIc in the Japanese classification, although recently such depressed lesions with a distinct central protrusion indicating submucosal invasion are termed IIc + Is). k, l) Flat elevated lesion with central depression, 10 mm in size, corresponding to a submucosally invasive cancer (IIa + IIc in the Japanese classification).
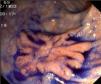
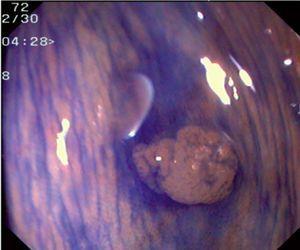
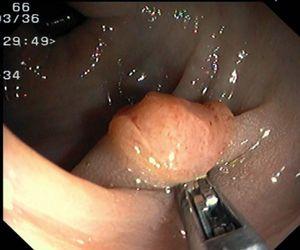
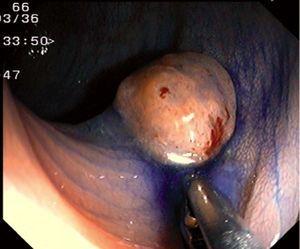
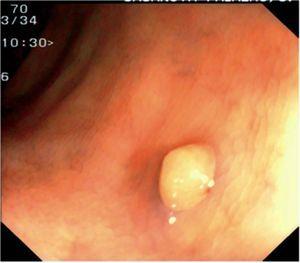
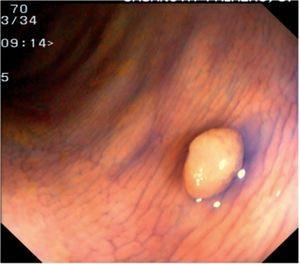
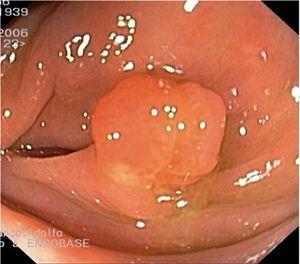
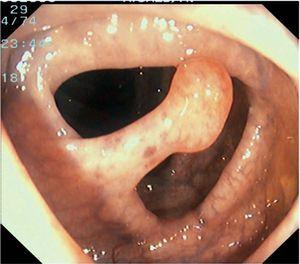
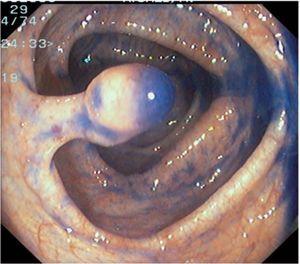
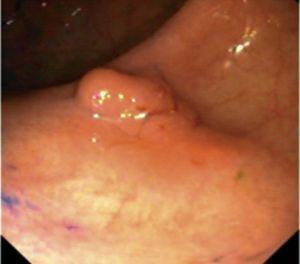
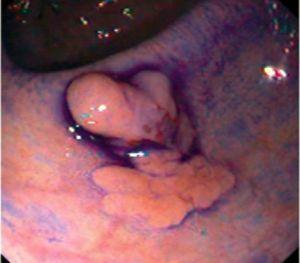
Fig. 4. Examples of protruding lesions with conventional examination and after the application of indigo carmine 0.2-0.5%. a, b) Sessile polyp. c, d) Subpedunculated polyp. e, f) Pedunculated polyp. g, h) Submucosal lesion (indigo carmine shows distinct innominate grooves, ruling out an epithelial lesion). i) Subpedunculated polyp. j) After indigo carmine application, a flat elevated lesion similar in size to the protruding segment is clarified.
Statistical analysis
Quantitative variables between groups were compared with the Student's t-test, whereas the chi-square test and the Fisher exact test were used for comparison of categorical data. A p value lesser than 0.05 was considered statistically significant. Results are expressed as mean ± SD or frequencies. Multivariate logistic regression analysis was performed to identify independent predictors of high-grade dysplasia or SIC. Variables significantly associated with the existence of high-grade dysplasia or SIC were introduced in the regression model.
RESULTS
During the study period 640 polyps were detected in 298/1105 (27.0%) patients. One hundred and eighty two (61.1%) were men and 116 (38.9%) women. The mean age was 62.5 ± 13.3 years. A total of 640 polyps, 144 (22.5%) flat and 496 (77.5%) protruding, were analysed. Four hundred and ninety (76.6%) polyps were neoplastic (including 4 serrated adenomas) whereas 150 (23.4%) were non neoplastic (hyperplastic polyps or mucosal polyps). Adenomatous tissue was found in 114 (79.2%) flat lesions and in 376 (75.8%) protruding polyps. With respect to the macroscopic type, 111 (22.7%) of neoplastic lesions were flat-elevated, 3 (0.6%) flat-depressed and 376 (76.7%) protruding. Thirty-one advanced cancers were detected in the same number of patients during the study period.
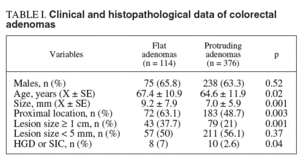
Table I shows the clinical and histopathological data concerning colorectal adenomas. There were no statistically significant differences between flat and protruding adenomas with respect to gender. However, flat adenomas were detected in older individuals, they were predominantly located proximal to the splenic flexure, had a significantly greater mean size, and were more frequently large (>= 1 cm in size), when compared to protruding adenomas.
There were 18 (3.6%) histologically advanced polyps (HGD or SIC). Six (5.3%) flat adenomas and 8 (2.1%) protruding adenomas contained areas of HGD. Two additional patients in each group presented invasive carcinoma (the two flat lesions were depressed, 7 and 10 mm in size respectively). Therefore, the rate of HGD or SIC in flat adenomas (7%) was significantly higher (p < 0.04) than in protruding adenomas (2.6%) (table I). In the multivariate regression analysis, flat or depressed adenomas were associated with an increased risk for HGD or SIC (OR = 2,7; CI, 1,04-7,04; p < 0.05).
In relation to histologically advanced polyps, 44.4% were of the flat type and all except one (flat-depressed, 8 mm), was 1 cm in size or greater. Eight out of 18 (44.4%) of these polyps were located proximal to the splenic flexure without any distal synchronous neoplasm. Among lesions with high grade dysplasia, 3/8 (37.5%) protruding and 5/6 (83.3%) flat were located proximal to the splenic flexure respectively, which was not statistically different (p = 0.09). Among the four invasive lesions, one flat and one protruding were proximal.
Table 2 shows the clinical and histopathological characteristics of patients with colorectal adenomas. No statistically significant differences were found between patients with only flat adenomas (n = 30), only protruding adenomas (n = 158) or both types of lesions (n = 57) with reference to age or gender. Multiple polyps (>= 3) were significantly more frequent (p = 0.001) in the group of patients with both types of lesions (protruding and flat adenomas) when compared with the other two groups. Histologically advanced adenomas were detected in 13 patients. Seven (53.8%) of these patients had flat adenomas (4 unique and 3 combined with protruding polyps). The rate of HGD or SIC was higher (p = 0.056) in patients with only flat adenomas (13.3%) than in those presenting only protruding polyps (3.8%).
Patients with both flat and protruding adenomas had a significantly higher rate of adenomas located proximal to the splenic flexure (71.9%) than patients with only protruding adenomas (48.1%) (p < 0.01). Adenomas were more frequently located proximal to the splenic flexure in the group of patients with only flat adenomas (56.7%) than in those with only protruding adenomas (48.1%) but the difference did not obtain statistical significance. Among the 87 patients diagnosed with flat adenomas, in 31 (35.6%) the lesions were >= 1 cm in size. The mean age of these patients was 66.5±12.2, whereas the mean age of patients whose flat adenomas were < 1 cm was 64.0 ± 12.3 (NS).
DISCUSSION
In this colonoscopy study, flat adenomas represented almost one quarter of all colorectal adenomas, and they showed a higher risk for malignancy, accounting for almost half of the lesions with HGD or SIC.
Flat adenoma detection among reported series is highly variable, and several factors may have influenced those results6-9,12,19-21. Firstly, endoscopical identification and complete removal of flat lesions requires expert endoscopists with a high index of suspicion and, more importantly, knowledge and experience in chromoendoscopic and endoscopic mucosal resection techniques9. In fact, recent evidence suggests that pancolonic application of indigo carmine improves the detection of small flat and depressed neoplasia22-25. Secondly, a suitable bowel preparation is essential for endoscopical identification of flat lesions26. In a recent study we found that detection of flat polyps was influenced by the timing of aality of cleansing on the right side of the large bowel was significantly better and allowed for detection of more flat lesions when the preparation was administered the same day as the colonoscopy. In the present study, using the same bowel preparation schedule in most cases, we corroborated that flat lesions were more frequently located proximal to the splenic flexure, which is in accordance with previous reports6-9,12,21,28,29. This finding suggests that a careful examination of the right colon is needed for the detection of flat lesions during colonoscopy. Thirdly, one likely important source of error when comparing the prevalence of flat adenomas among colonoscopy series is the subjectivity inherent in the application of endoscopic classifications. The most extended classification used by endoscopists has been the one proposed by the Japanese Reseach Society for Cancer of the Colon and Rectum which mostly relies on the endoscopical characteristics of the lesions 1. In order to be more accurate in defining flat lesions, some authors have proposed measuring their size with an objective reference, such as the closed cups of a biopsy forceps situated next to the polyp9. The Paris Endoscopic Classification of superficial neoplastic lesions proposed considering flat elevated lesions (IIa) those whose height is lesser than that of the closed cups of a biopsy forceps (2.5 mm), regardless of the width of the lesions30. It could be speculated that the application of this classification migh result in many minute and clinically irrelevant sessile lesions with a height less than 2.5 mm being called flat-elevated, which theoretically bear a higher malignancy potential, therefore resulting in over-staging, which might lead to a more invasive endoscopic treatment.
We observed that almost two thirds of flat lesions were located proximal to the splenic flexure, in contrast to less than half of protruding polyps. In major european studies a preferential proximal location of flat adenomas was also found, whereas in North American studies less than 50% flat lesions were proximally located5-9,20,21. Flat lesions in our study were histologically more advanced than protruding lesions, accounting for 44.4% of all histologically advanced lesions (HGD/SIC). The increased risk for HGD/SIC in flat compared to protruding neoplasms in the multivariate analysis was 2.7. This finding is in accordance with previous reports from Japan and England in which HGD and SIC were twice as frequent in flat colorectal adenomas than in protruding lesions4,8,9. However retrospective histological review of archival material from the National Polyp Study did not find flat adenomas to be associated with a higher risk for high-grade dysplasia31. In that study 27% of originally sessile adenomas were reclassified as flat, and the risk for high grade dysplasia was compared between that group and protruding adenomas. Nonetheless, no specific methods for the diagnosis of flat adenomas, such as indigo carmine application, seemed to have been employed in the colonoscopies performed in that study. In fact, the lesions were classified endoscopically as sessile or pedunculated, but no category considered depressed lesions. Therefore, the fact that no increased risk for high grade dysplasia in flat adenomas was found does not exclude the possibility that some flat or depressed lesions could have been left unrecognised.
Recently a lot of attention has been focused on flat-depressed neoplastic lesions. Flat and depressed adenomas might progress to flat cancers, and such lesions with a nonpolypoid growth seem to behave more aggresively than those with a polypoid growth32-34.
Of the 3 depressed lesions detected in the present series, 2 (66%) presented SIC despite not being large. In fact, one of them (10 mm in diameter) was an advanced carcinoma. Therefore, although only 0.6% lesions were depressed, they accounted for 50% of the lesions with SIC. These findings are similar to those reported in Japanese, English and French populations4,8,12,19. In another English study very high rates of flat-depressed lesions were reported9. Differences in endoscopic criteria, possibly considering the so-called pseudo-depressed lesions as truly depressed, might account for such discrepancies. Although depressed lesions were a rare finding in most series (1-3%), they account for one-third of the early colorectal neoplasms with SIC4,8. Because of the extremely high malignancy potential of these lesions, special efforts are warranted for their detection, and specific techniques such as chromoendoscopy or magnifying colonoscopy might prove to be effective. The detection of minute flat depressed adenomas seems to be greatly enhanced when indigo carmine is applied in a routine way22.
In the present study flat adenomas were larger than protruding adenomas, and a significant proportion of flat adenomas were >= 1 cm. Although one study from England also found around 40% flat lesions being large, most authors have found flat adenomas to be smaller in size than protruding adenomas4,7,8,9,19. Discrepancies in size of lesions between different colonoscopy studies may be related to patient selection or classification bias, but inter regional differences in the characteristics of flat colorectal polyps can not be ruled out. Additional studies are needed to clarify this issue.
The main potential limitation of the present study is its retrospective condition, which could affect both the accuracy of the endoscopic and histopathological diagnoses. However, one single endoscopist performed all the examinations, which were prospectively registered on a database, indicating in each case the location, shape and size of the polyps according to uniform criteria. Another potential limitation is the fact that the assessment of flat versus protruding is inherently subjective, and one single endoscopist did the assessment of flat versus protruding. However objective measurements were employed to differentiate both types of lesions, and indigo carmine was applied in order to determine the size and shape of the lesions more precisely. Histological evaluation for each lesion was carried out prospectively by two examiners, after retrieving the histological slides, in order to obviate the bias due to multiple observer inherent in retrospective histological analysis. The determination of inter-observer differences in the hystopathological diagnosis of flat lesions, or the correlation between endoscopic and histological diagnoses was not an aim of this study and were not evaluated. One study showed a poor concordance between the endoscopic appearance and the histological criteria of flat neoplasia, as only 20% of flat lesions appeared flat on endoscopy12. Remarkably, in that study chromoendoscopy was not applied, and therefore the message might well be that chromoendoscopy is required to correctly evaluate flat neoplasia. In summary, we believe that the present retrospective study represents a solid body of evidence on the characterization of flat colorectal adenomas in our region.
In conclusion, the present study shows that colorectal flat adenomas are frequently found in a southern European population, accounting for almost one quarter of total adenomas and being detected in about one third of the patients with colorectal adenomas. These lesions are predominantly located proximal to the splenic flexure, a fact that could have implications in the strategies of colorectal cancer screening. The most important clinical relevance of flat adenomas is their higher risk of HGD or SIC, when compared to protruding adenomas. Although flat-depressed adenomas are rarely found, they represented half of all invasive lesions. An increased awareness of flat colorectal neoplasms during colonoscopy seems to be warranted in order to allow for adequate CRC screening and management. Future studies should determine the epidemiological aspects of these lesions (e.g. definition of groups more likely to bear flat adenomas), the endoscopic techniques which can more reliably unmask flat adenomas, and their detectability by non-endoscopic techniques such as CT or MRI virtual colonoscopy.