A proper quantification of the inflammatory activity in Crohn's disease (CD) lesions is needed to establish the appropriate management for each patient. The aim of this study is to evaluate the inflammatory activity of affected segments in small bowel lesions using dynamic studies of magnetic resonance enterography (MRE) in patients undergoing surgery, and their correlation with the level of inflammation and histological fibrosis of the surgical piece.
MethodsA prospective, consecutive, observational, clinical study was conducted that included all the patients with small bowel CD that underwent surgery in this center between March 2011 and September 2013. Diagnosis was established according to Lennard–Jones criteria and the Montreal classification. All the patients underwent MRE within three months before surgery, using a routine protocol involving Liver Acquisition with Volume Acceleration-Extended Volume (LAVA-XV) sequence for the dynamic studies before intravenous administering of gadolinium and 30, 70, 120, and 420s after administering this. The results allowed the designing of graphics with different uptake patterns. The Chiorean classification was used in the histological analysis, as well as a modified version published previously by this study group.
ResultsA total of 28 patients with 47 lesions were analyzed. There was a significant correlation between both curve patterns, including the modified Chiorean classification (P<0.0001) as well as the level of inflammation (P<0.0001) and fibrosis (P<0.002). Inflammatory patterns of dynamic studies are related to histological findings with 80.9% accuracy (sensitivity=75.7%; specificity=100%).
ConclusionThere is a high correlation between dynamic enhancement studies and the level of inflammatory activity. MRE is a suitable tool to differentiate between inflammatory and fibrotic lesions, making it useful to decide the appropriate management of each patient.
Se necesita una cuantificación adecuada de la actividad inflamatoria en las lesiones de la enfermedad de Crohn (EC) para establecer el tratamiento adecuado para cada paciente. El objetivo de este estudio es evaluar la actividad inflamatoria de los segmentos afectados en las lesiones del intestino delgado mediante estudios dinámicos de enterografía por resonancia magnética (ERM).
MétodosEstudio prospectivo, consecutivo, observacional y clínico, que incluye a todos los pacientes con EC del intestino delgado que se sometieron a cirugía en nuestro centro entre marzo de 2011 y septiembre de 2013. El diagnóstico se estableció de acuerdo con los criterios de Lennard-Jones y la clasificación de Montreal. Todos los pacientes se sometieron a una ERM dentro de los 3 meses previos a la cirugía, aplicando el protocolo de rutina y secuencias preestablecidas. Para el estudio dinámico se empleó la secuencia Adquisición hepática con aceleración de volumen-Volumen extendido (LAVA-XV), antes de la administración intravenosa (iv) de gadolinio, y 30, 70, 120 y 420s después de esta administración. Los resultados permiten diseñar gráficos con diferentes patrones de captación. En el análisis histológico se utilizó la clasificación de Chiorean, así como una versión modificada creada por nuestro grupo de estudio.
ResultadosEn total se analizaron 28 pacientes con 47 lesiones. Se detectó una correlación significativa entre ambos patrones de curva, incluyendo la clasificación de Chiorean modificada (p<0,0001), así como el grado inflamatorio (p<0,0001) y de fibrosis (p<0,002). Los patrones inflamatorios de los estudios dinámicos se relacionaron con los hallazgos histológicos con una precisión del 80,9% (S=75,7%; E=100%).
ConclusiónExiste una alta correlación entre los estudios dinámicos y el grado de actividad inflamatoria. La ERM constituye una herramienta adecuada para diferenciar entre lesiones inflamatorias y fibróticas, siendo útil para colaborar en la decisión terapéutica.
Crohn's disease (CD) is a chronic inflammatory pathology that affects the entire digestive tract. The inflammation has a transmural character that involves the entire thickness of the intestinal wall. Despite the progress in the medical treatment of CD, there is still an increased risk of a surgical intervention.
Inflammatory activity in CD is classically measured by clinical, endoscopic and radiological indicators, and by biological markers. Dynamic studies of entero-magnetic resonance imaging (MRE) have a high sensitivity for the detection of wall and extramural lesions and constitute a well-accepted tool for diagnosis and follow-up 1in patients with CD. Some studies have attempted to relate MRI radiological findings with the severity and activity of the CD,2–5 and some have been able to validate these radiological findings with the histology of the surgical specimen.6–8 Usually, morphological changes related to CD activity are assessed, such as wall thickness, contrast uptake, stenosis, mucous membrane distortions, ulcers, cobblestone appearance, edema, hyper vascularization, pathological lymph nodes, abscess, fistulas, adipose-fibrous proliferation, layers enhancement, pre-stenosis dilation, etc. Radiological indicators based on these findings have even been described to evaluate CD activity and intensity.3,5,9 Several publications try to correlate dynamic pattern of wall enhancement with the extent of inflammation in CD, although they all present different methodology and results.10–16 In a very recent systematic review that reviews the state of the art to assess stenosis and fibrotic component in Crohn's disease, the authors conclude that more studies are needed to distinguish fibrosis from inflammation since the studies published to date are not accurate enough to be used in daily clinical practice.17
It would be interesting to know whether dynamic uptake MRI studies allow differentiating early inflammatory processes of CD, which are susceptible to pharmacological treatment, from fibrotic lesions, with low response to medication, which will probably benefit from surgical treatment.
The aim of this study is to assess the inflammatory activity of CD affected areas in small bowel lesions through dynamic studies of entero-magnetic resonance imaging (MRE) in patients undergoing surgery and to explore their correlation with the degree of inflammation and histological fibrosis of the surgical piece.
Materials and methodsThis is a consecutive, prospective, observational clinical 30-month study (March, 2011–September, 2013), carried out at a tertiary hospital. The study protocol was approved by the Hospital Ethics Committee. All the patients accepted to participate in the study by signing an informed consent form. During the study period, all the patients diagnosed with CD, who were older than 14 years old, presented small bowel affection, and needed surgery due to medical treatment failure or complications, were studied by entero-MRI (n=38). A dynamic study was performed in 28 of these patients, and they were therefore considered for this analysis.
CD diagnosis was defined according to the ECCO standard criteria18 and after excluding infectious, ischemic or vascular, malignant and actinic causes. Patients were classified by age at the moment of diagnosis, location and the disease's behavior. Harvey and Bradshaw Index was used to describe the clinical activity of the CD.19 A preoperative colonoscopy was performed in all the patients, to discard colic affection and, when possible, take a biopsy of the terminal ileum. A multidisciplinary committee discussed each case and made all the decisions related to the management of these patients.
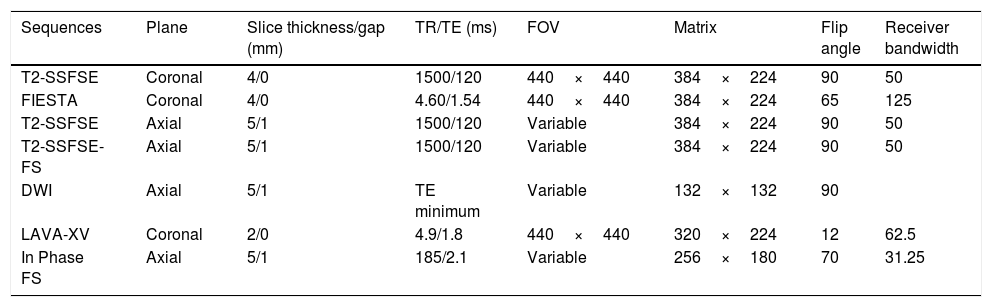
Entero magnetic resonance imagingEvery patient underwent an MRE test during the three months prior to surgery (one month if the patient had received treatment with anti-TNF biological drugs). MRE examinations were performed using a standardized clinical protocol on a 3T magnet (GE MedicalSystems, Milwaukee, WI, USA). Patients fasted for at least 6h and then ingested 1500ml of a 5% mannitol solution over 45min immediately before MRI took place, to distend the small bowel. To reduce bowel peristalsis, 10mg of e.v. hyoscine butylbromide (Buscopan; Boehringer Ingelheim, Ingelheim, Germany) were administered prior to initiating the study, and additional 10mg were administered before administrating the contrast bolus. In case of contraindication (glaucoma, arrhythmia, benign prostatic hypertrophy), 1mg of e.v. glucagon (Glucagen; Novo Nordisk, Bagsvaerd, Denmark) was administered. For the dynamic study, a contrast injection of gadobenate dimeglumine (Multihance; Bracco Diagnostics Inc., Milan, Italy): 0.2ml/kg body weight was administered at a rate of 2ml/s. Images were obtained by placing the patient in prone position. Sequences and parameters of the MRE protocol are detailed in Table 1. Every lesion was listed and measured on a map, starting from the closest to the ileocecal valve. The distance of each lesion from the ileocecal valve, its length and morphological characteristics were precisely measured.
Protocol for magnetic resonance enterography image acquisition.
| Sequences | Plane | Slice thickness/gap (mm) | TR/TE (ms) | FOV | Matrix | Flip angle | Receiver bandwidth |
|---|---|---|---|---|---|---|---|
| T2-SSFSE | Coronal | 4/0 | 1500/120 | 440×440 | 384×224 | 90 | 50 |
| FIESTA | Coronal | 4/0 | 4.60/1.54 | 440×440 | 384×224 | 65 | 125 |
| T2-SSFSE | Axial | 5/1 | 1500/120 | Variable | 384×224 | 90 | 50 |
| T2-SSFSE-FS | Axial | 5/1 | 1500/120 | Variable | 384×224 | 90 | 50 |
| DWI | Axial | 5/1 | TE minimum | Variable | 132×132 | 90 | |
| LAVA-XV | Coronal | 2/0 | 4.9/1.8 | 440×440 | 320×224 | 12 | 62.5 |
| In Phase FS | Axial | 5/1 | 185/2.1 | Variable | 256×180 | 70 | 31.25 |
SSFSE, single-shot fast spin echo; FIESTA, fast imaging employing steady state acquisition; FS, fat saturation; DWI, diffusion-weight imaging; LAVA-XV, Liver Acquisition with Volume Acceleration-Extended Volume.
For the dynamic study, Liver Acquisition with Volume Acceleration-Extended Volume (LAVA-XV) sequences prior to contrast administration were used, and also 30, 70, 120 and 420s after the gadolinium administration. In this study, the area of radiological interest (ARI) was placed in the wall section with maximum initial contrast uptake. Enhancement curves were obtained using FuncTool® in the Windows platform version4.8 (GE Medical Systems, Milwaukee, WI, USA) for the analysis of the dynamic pattern of wall enhancement.
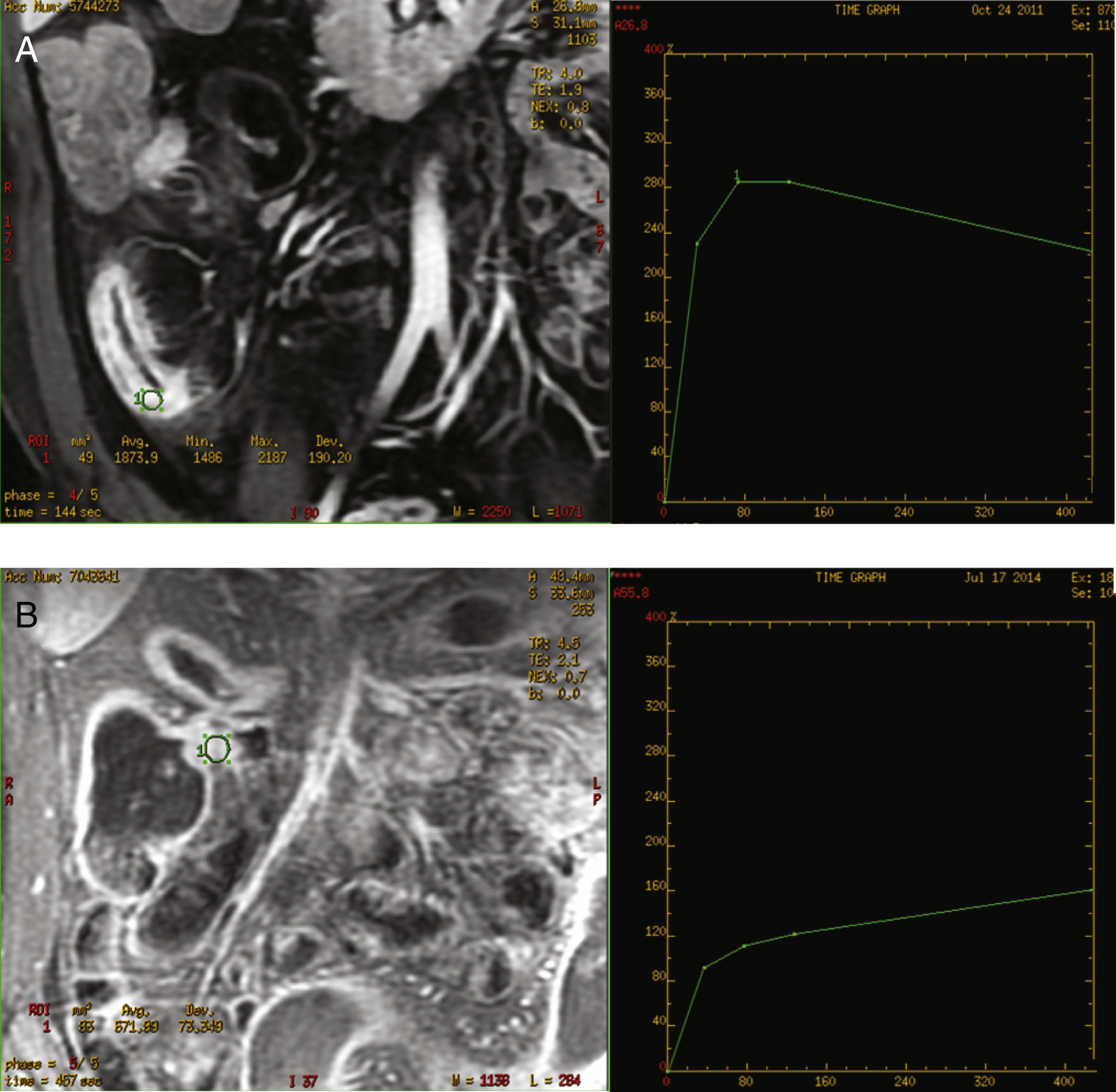
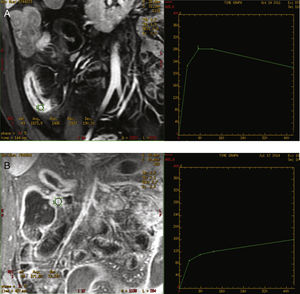
The curves were classified as inflammatory pattern when a steep ascending slope in the early phases of contrast uptake in the dynamic study (above 200%), and a decreasing grade of enhancement at the end of the curve was presented. A fibrosis type pattern was determined when the curve displayed a slowly rising slope during the early phases (always below 200%), reaching a plateau or keeping a progressive increase until the end of the curve with no decrease at the end. The decrease in the parietal enhancement degree at the end of the uptake curve was considered distinctive of the inflammatory pattern (Fig. 1). All the images were evaluated by two radiologists experienced in abdominal imaging who reached a consensus regarding any doubts about the interpretation of the images. Both radiologists were blinded to the clinical and laboratory data.
SurgeryAll the patients were operated on by members of the Colorectal Surgery Unit, under homogenous surgical criteria and applying the same perioperative protocol. Elective laparoscopic ileocaecal or ileocolonic resection with latero-lateral anastomoses was the most common procedure (n=20 patients). All the resected bowel segments were remitted for pathological examination, indicating whether one or more lesions were included, its number and location, specifying its distance from the ileocecal valve. When necessary, a picture describing the location of the resected segments was sent along with the specimens. When a strictureplasty was performed, complete wall samples were obtained for histological study.
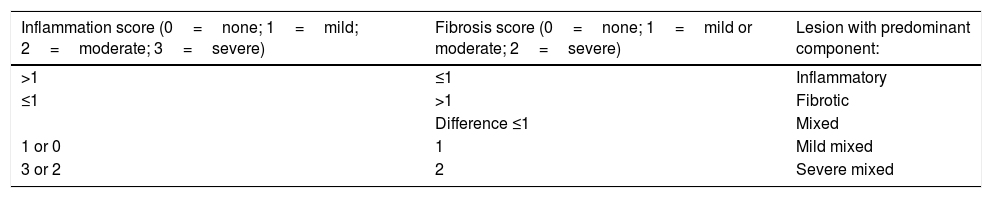
Pathology reportThe freshly excised specimen was photographed before fixation in 10% formaldehyde solution during 24h. Fibrosis and inflammation of the lesions were defined according to the Chiorean criteria.20 A lesion presenting moderate or severe inflammation and none, mild or moderate fibrosis was defined as “Inflammatory”. A lesion displaying severe fibrotic component and none or mild inflammation was defined as “Fibrotic”. In all the other cases lesions were defined as “Mixed”. In this manuscript, a modification of the Chiorean Classification was used dividing the mixed group in mild mixed (no or mild inflammation and mild or moderate fibrosis) and severe mixed (moderate or severe inflammation and severe fibrosis) (Table 2). Two experienced Pathologists, specialized in digestive diseases and part of the multidisciplinary team, evaluated all the specimens. In case of discrepancies, data interpretation was made by consensus.
Histological Chiorean classification modified by the authors.
| Inflammation score (0=none; 1=mild; 2=moderate; 3=severe) | Fibrosis score (0=none; 1=mild or moderate; 2=severe) | Lesion with predominant component: |
|---|---|---|
| >1 | ≤1 | Inflammatory |
| ≤1 | >1 | Fibrotic |
| Difference ≤1 | Mixed | |
| 1 or 0 | 1 | Mild mixed |
| 3 or 2 | 2 | Severe mixed |
Data was analyzed by patients and by lesions. Lesion analysis was accomplished by correlating pathologically assessed surgical specimen's lesions, with the ones identified on the MRE. Unconfirmed lesions during surgery, have been excluded from the analysis.
The Variable Statistics were attained by using the IBM® SPSS® Statistical Program for Social Science version 22.0. In the univariate analysis, continuous variables were expressed using the mean and the standard deviation, while categorical variables were expressed using frequencies and percentages (number of patients, number of lesions, and the percentage). When a non-parametric analysis of categorical variables was necessary, the Pearson's Chi-square test was applied. For the analysis of dichotomous MRI results, a 2×2 table was used and, values of sensitivity, specificity, diagnostic accuracy, positive predictive value (PPV) and negative predictive value (NPV) were obtained by calculating 95% confidence intervals for all estimates. P value of <0.05 was considered statistically significant.
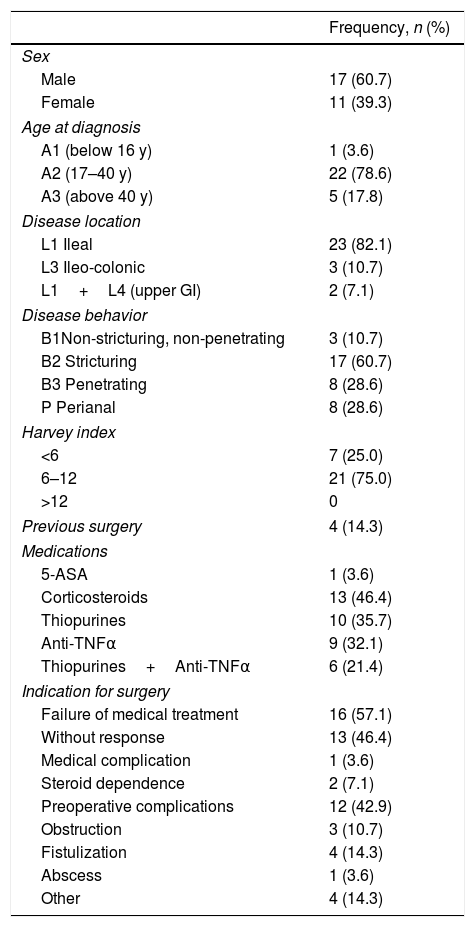
ResultsTwenty-eight CD patients with small bowel lesions were included in the study, identifying 47 lesions. Demographics and clinical characteristics of the patients are shown in Table 3. The ileal location was the most frequent (82.1%) and non-stricturing non-penetrating behavior was present in 60.7%.
Demographic and clinical data of patients (N=28). Age at diagnosis, disease location and behavior were defined according to the Montreal classification.19,20
| Frequency, n (%) | |
|---|---|
| Sex | |
| Male | 17 (60.7) |
| Female | 11 (39.3) |
| Age at diagnosis | |
| A1 (below 16 y) | 1 (3.6) |
| A2 (17–40 y) | 22 (78.6) |
| A3 (above 40 y) | 5 (17.8) |
| Disease location | |
| L1 Ileal | 23 (82.1) |
| L3 Ileo-colonic | 3 (10.7) |
| L1+L4 (upper GI) | 2 (7.1) |
| Disease behavior | |
| B1Non-stricturing, non-penetrating | 3 (10.7) |
| B2 Stricturing | 17 (60.7) |
| B3 Penetrating | 8 (28.6) |
| P Perianal | 8 (28.6) |
| Harvey index | |
| <6 | 7 (25.0) |
| 6–12 | 21 (75.0) |
| >12 | 0 |
| Previous surgery | 4 (14.3) |
| Medications | |
| 5-ASA | 1 (3.6) |
| Corticosteroids | 13 (46.4) |
| Thiopurines | 10 (35.7) |
| Anti-TNFα | 9 (32.1) |
| Thiopurines+Anti-TNFα | 6 (21.4) |
| Indication for surgery | |
| Failure of medical treatment | 16 (57.1) |
| Without response | 13 (46.4) |
| Medical complication | 1 (3.6) |
| Steroid dependence | 2 (7.1) |
| Preoperative complications | 12 (42.9) |
| Obstruction | 3 (10.7) |
| Fistulization | 4 (14.3) |
| Abscess | 1 (3.6) |
| Other | 4 (14.3) |
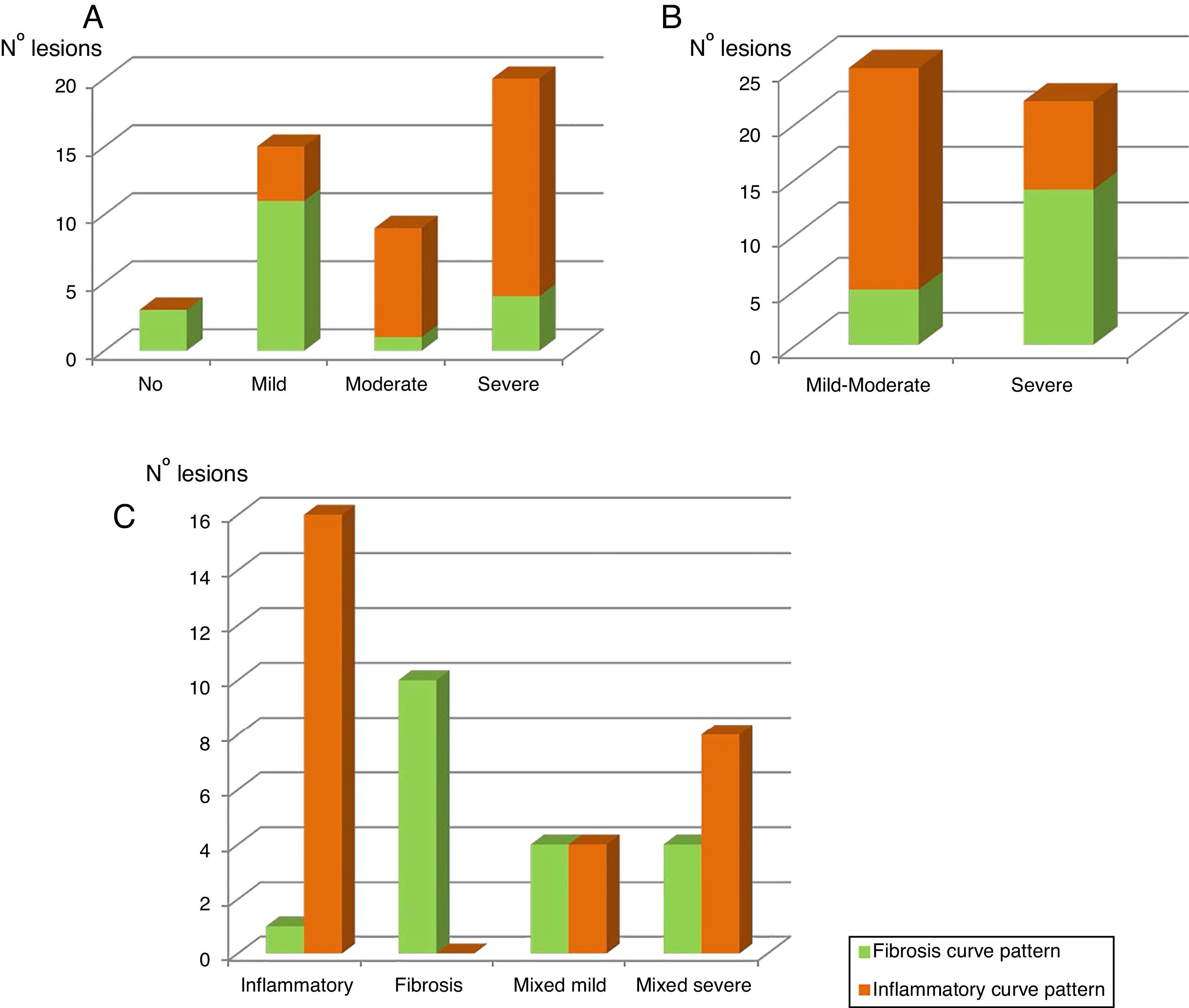
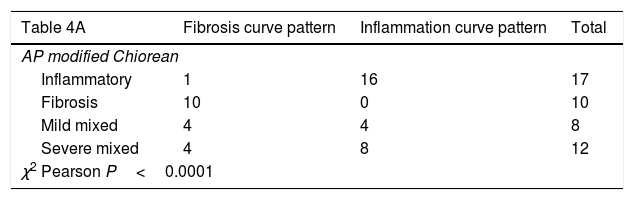
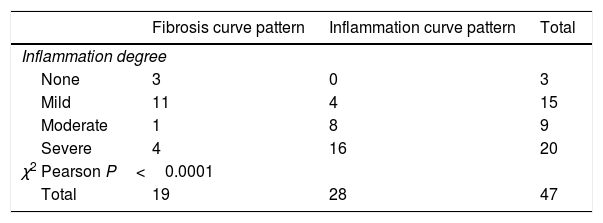
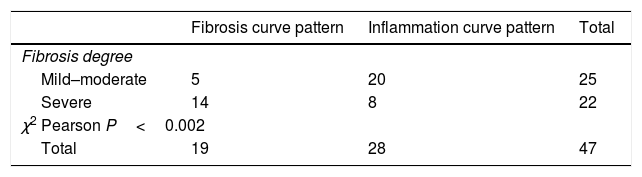
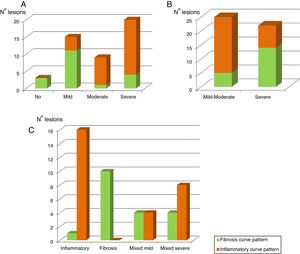
Table 4A shows the significant correlation between both curve patterns and the modified Chiorean classification. Tables 5 and 6, show correlation between inflammation degree and fibrosis.
Results of correlation between dynamic curve pattern with histological study.
| Table 4A | Fibrosis curve pattern | Inflammation curve pattern | Total |
|---|---|---|---|
| AP modified Chiorean | |||
| Inflammatory | 1 | 16 | 17 |
| Fibrosis | 10 | 0 | 10 |
| Mild mixed | 4 | 4 | 8 |
| Severe mixed | 4 | 8 | 12 |
| χ2 Pearson P<0.0001 | |||
All 28 resected bowel segments that showed inflammatory uptake curve in the dynamic MRE presented inflammation in the histological study (segments classified as inflammatory or mixed) in 100% cases (Accuracy 80.9%; Sensitivity 75.7%; Specificity 100%, PPV 100%; NPP 52.6%) (Table 4B). None of the pieces classified as fibrotic in the histological study (n=10) presented an inflammatory enhancement curve (sensitivity 100%, specificity 75.7%).
Figure 2A shows the correlation between the histological inflammation degree and the inflammatory enhancement pattern. As the inflammation degree increases, so does the possibility of finding an inflammatory enhancement curve. This effect is stronger when comparing the patients with non-or mild inflammation with moderate or severe inflammation cases. The same effect happens, but on the contrary, with the severity of fibrosis and the fibrosis enhancement curve (Fig. 2B).
The graphic representation of both enhancement patterns and their correlation with the modified Chiorean's classification is shown in Figure 2C.
DiscussionThis series is part of a global study carried out on 120 consecutive patients in which an enterography was going to be performed for clinical criteria. An important number of clinical parameters, serological data (including calprotectin and PCR), MRI-related variables (activity indices and other analytes) and data related to the surgical piece and its anatomopathological study have been analyzed and published during these years.21,22 This is one of the reasons why the study is now published. In any case, we consider that the study is still valid due to the following comments in the following discussion.
The therapeutic management of CD patients is currently established with the combination of clinical and biological data, along with radiological findings. The correct identification of the extension and activity of the disease and its inflammatory activity degree is essential to decide the most appropriate medical or surgical treatment, for each patient.
Therefore, it is crucial to establish the disease's behavior, identifying the lesions and confirming the inflammatory degree, to decide whether the patient can benefit from medical treatment, or to select surgical treatment or endoscopic dilations, in case that a predominant fibrotic component is demonstrated. In the case of patients with established fibrosis, they could suffer deterioration in their quality of life when subjected to intensification of medical treatment; increasing the possibility of complications, medication's side effects and raising healthcare costs. Clinical and endoscopic indicators described to assess the activity degree of the disease do not have good clinical applicability. In recent years, MRI has been successfully employed for diagnosis, phenotype identification and CD's patient's follow-up. Dynamic contrast-enhanced MR with quantitative perfusion analysis of small bowel,23 wall bowel thickness and layered enhancement24 have shown a good correlationwith lical degree of Crohn's disease inflammation activity. The anatomical vision that MRE offers surgeons is superior to that of the one provided by ultrasound, allowing us to obtain a “preoperative surgical map”.
In 2007 Chiorean's Classification allows rating both the inflammatory as well as the fibro-stenotic characteristics, and constitutes the simplest and most practical among those that have been described.20 It classifies lesions in mainly inflammatory, mainly fibrotic or mixed. However, the mixed group is rather frequent and contains lesions of very different behavior. That is why subdividing the mixed group in mild mixed and severe mixed seems to be appropriate. This modified Chiorean's Classification has practical implications, since the behavior of the mild mixed subgroup is similar to fibrosis and the behavior of the severe mixed is similar to predominantly inflammatory. In a recent systematic review of publications, authors found a lack of validated histopathologic scoring systems for assessment of fibromuscular stenosis.25 This fact is obviously a limitation for this and any study that claims comparisons with histopathological measures.
The demographic characteristics of patients analyzed in this study are similar to those of larger series of patients undergoing surgery.26 The clearest and most accepted surgical indication in CD is established fibrotic stenosis. The ECCO guidelines advise against overtreatment in cases with no response to steroids, immunosuppressant or biological drugs.18 However, most strictures may not be exclusively fibrotic and every case might have a certain inflammatory factor.
The initial description of kinetic parameters using in quantitative perfusion analysis such as the volume transference coefficient (kTrans or transference constant) and the fraction of extracellular volume (Ve) 27have allowed different authors to describe more parameters related to the inflammatory activity such as uptake slope, maximum enhancement time, enhancement percentage, ratio between maximum and final enhancement.10,13,14,28,29
These parameters present important limitations since there are no standard references, the measurements of signal's intensity have limited value, different devices are used, the amount of necessary liquid for distension varies, and movement might create artifacts.29 Several authors believe that enhancement percentages based on static images and the thickness of the wall are the best parameters to evaluate activity, therefore, dynamic studies do not offer relevant information.10
However, semi-quantitative analysis of several studies conclude that inflamed segments present increased contrast enhancement, greater initial area beneath the curves and steeper uptake slope.28 Dynamic MRE allows the quantitative analysis of contrast uptake, describing several parameters and, graphically, obtaining intensity enhancement–time curves. The curves intensity-time have been determined semi-automatically in the work station. This enabled us to obtain the curve, by calculating the same area of radiological interest in all the series of the dynamic study. In our study, we manually selected the areas of interest of the affected bowel segment wall, and consequently, detect any inflammatory activity.
Both types of obtained curves have been correlated with a high CD degree of activity, as well as with a high sensitivity and specificity. Giusti et al. also perform dynamic studies, although the curves obtained are not similar to ours, since they compare inflammatory segments with healthy bowel segments.13 In our study, the inflammatory enhancement pattern was always related to an active inflammation at histological analysis. These curves were represented by a slow progressive contrast uptake, with maximum enhancement at 420s, in the later phase. Delayed enhancement pattern of the fibrotic affected segment in CD has also been recently described in another study,30 with findings similar to our own study. However, this study only compares endoscopic and radiological findings, offering no pathological data about the correlation between the enhancement curves and the lesions, whereas our study shows the relation between the enhancement curves and the pathology report. These curves could be very useful to identify patients who could benefit from endoscopic treatment with dilations with or without stent or from surgical treatment.
In our study, the area of radiological interest (ARI) was placed on the wall region with the maximum initial contrast enhancement, however, it would be interesting to place several ARIs on the same lesion to obtain different quantitative parameters and curves that would allow us to assess whether placing the ARI is as important as it seems.
One of the highlights of our study is the fact of having compared the types of curve obtained for each lesion with the histological study of the whole wall of such lesion. Recently Wagner et al. retrospectively assessed MRE with diffusion weighted imaging in 27 patients who underwent surgery.31 They assessed the value of MRE for the characterization of histopathological tissue composition of small bowel CD, including inflammation, fibrosis, and smooth muscle hypertrophy. Even though the degree of fibrosis and inflammation on histopathology was graded using a self-developed system, when assessing the bowel wall thickness and differentiating fibrosis from muscular hypertrophy a sensitivity of 61% and a specificity of 89% were achieved.
MRE imaging studies reported high accuracies to detect fibrosis, the applied reference standard for fibrosis scoring varied considerably and simple dichotomous scoring systems were used to classify fibrosis in surgical resection specimens. While MRE has excellent capability to assess the degree of inflammation, fibrosis detection is likely problematic.32 The fact of going deeper into the measurement of parameters such as those analyzed in our study, improving and validating histological indices and carrying out studies in a greater number of patients are all reasons to help improve current data.
In conclusion, the performance of dynamic studies can become a useful tool to solve the conventional difficulties of MRE to determine the degree of inflammatory activity, and therefore the response to medical treatment. Nevertheless, it is early and there is a lack of studies to position its role in daily clinical practice and it should, for the moment, be used as complementary measures.
Conflict of interestThe authors declare no conflict of interest.