Alterations in the molecular mechanisms of specific amino acids (AAs) may be implicated in the pathophysiology of schizophrenia (SZ). However, little is known about antipsychotic drugs influence on levels of AAs. This study aimed to further explore antipsychotics' effects on AAs and serum lipid levels in first-episode SZ.
MethodsEighty subjects with the International Classification of Diseases, Tenth Edition (ICD-10) criteria-defined SZ were enrolled. The levels of 31 AAs were measured in plasma samples using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS).
ResultsTen AAs (i.e., citrulline, sarcosine, tyrosine, leucine, proline, hydroxyproline, kynurenine, tryptophan, valine and isoleucine) were observed to be higher and three AAs (i.e., GABA, aminobutyric acid and asparaginic acid) were lower in 80 patients with first-episode SZ after various antipsychotics treatment. In addition, there were 1 out of 31 AAs altered after olanzapine treatment and there were only 2 out of 31 AAs altered after risperidone treatment. Furthermore, serum triglyceride (TG) was markedly upregulated after olanzapine treatment, while Apolipoprotein A1 (ApoA1) was generally upregulated after risperidone treatment in patients with first-episode SZ.
ConclusionsTaken together, antipsychotic treatment can affect the plasma levels of AAs in patients with first-episode SZ, and olanzapine and risperidone have differential effects on the levels of AAs.
Schizophrenia (SZ) is a group of systemic mental diseases with complex pathogenesis and unknown cause. It has a variety of mental disorders such as emotion, will, behavior and perception, and is mainly characterized by inconsistency between mental performance and the real environment.1,2 Amino acids (AAs) are extremely abundant in the human body, and they are the most basic units for the human body to perform life activities. They can participate in a series of glycolipid biotransformation processes of the body, and play a crucial role in metabolic activities such as energy metabolism, neurotransmission and lipid transport.3,4 Accumulating evidence suggests that disturbance in AAs levels is linked to pathophysiology of SZ.5-8 One study showed that 11 AAs and their derivatives were significantly altered in the schizophrenic group compared to the healthy control group, which may represent underlying pathophysiology, including but not limited to changes in excitatory and inhibitory neurotransmission, dysregulated protein-production processes, urea cycle and ammonia metabolism. This suggests that AAs hold promise as potential biomarkers for SZ.7 Antipsychotic drugs can inhibit the progression of the disease, but long-term use of antipsychotic drugs can lead to varying degrees of weight gain, and at the same time prone to abnormal glucose and lipid metabolism, hyperlipidemia, coronary heart disease, hypertension, and increase in the proportion of type 2 diabetes.9-11 In recent years, the focus of most studies exploring antipsychotics' metabolic side effects has been on changes in glucose levels and lipid metabolism syndrome, but antipsychotics' influence on AAs levels is little understood. Our research sought clarification regarding antipsychotics' effects on AAs levels in first-episode SZ. It also compared the AAs and serum lipid levels before and after olanzapine treatment and risperidone treatment, respectively. These results provide further important insight into the metabolic effects of use of antipsychotics in patients with first-episode SZ.
Materials and methodsParticipantsEighty schizophrenic patients aged 16 to 65 years who were first admitted to the Third People's Hospital of Huai'an, Jiangsu province were collected. The study was performed between February 2019 and November 2019. The patients were diagnosed as first-episode SZ according to the International Classification of Diseases, Tenth Edition (ICD-10) criteria and had no history of antipsychotic treatment. Patients who met the following criteria were excluded from the study: co-existing psychiatric disorders, major physical illness (cardiovascular disease, encephalopathy), and prior treatment with antipsychotic drugs. The initiation of antipsychotics is a part of patient's treatment protocol and duly approved by the hospital. This study was approved by the Medical Ethics Committee of the Third People's Hospital of Huai'an with the approval number of 2021–35. All subjects gave informed consent and signed informed consent.
Plasma samplesAll blood samples (≈4 mL) were collected from subjects in the first days of hospitalization before initiation of antipsychotic treatment and the day of discharge after at least 3 weeks of treatment, respectively. The maximum period of time between blood collections was 107 days and the average time was 42.05 days. Sample extraction was also associated with part of their treatment. Peripheral blood was drawn by venous puncture from all subjects during the day and collected into 5 mL EDTA-coated tubes. Blood samples were centrifuged for 10 min at 3500 × g at 4 °C to isolate the plasma; the plasma was stored at −80 °C until undergoing liquid chromatography analysis.
Instruments and reagentsWaters XEVO-TQD Mass Spectrometer was purchased from Waters Corporation (Milford, MA, USA). ST16R Table Top High Speed Refrigerated Centrifuge was purchased from Thermo Fisher Scientifc (Waltham, MA, USA). G560E vortex mixer was purchased from Scientific Industries (Bohemia, NY, USA). MTN-2800 W Nitrogen Blow Concentrator was purchased from Ottersines Instrument Co., Ltd. (Tianjin, China). High Performance Liquid Chromatography (HPLC)-grade formic acid and acetonitrile were supplied by Merck KGaA (Darmstadt, Germany). Pure water was purchased from A.S. Watson Group Ltd. (Hong Kong, China). AAs release agent were obtained from Baichen Medical Inspection Institute Co., Ltd (Hangzhou, China).
UPLC and MS parametersChromatographic separation was performed on Waters HSS T3 column (2.1 mm × 50 mm, 1.8 µm particle size). The column temperature was set for 40 °C. Mobile phase A consisted of 0.1% formic acid (FA) in liquid chromatography-tandem mass spectrometry (LC–MS) water and mobile phase B was 0.1% formic acid (FA) in acetonitrile. The gradient program was as follows: 1% B (0–2.5 min), increased to 3% B (2.5–3.2 min), to 13% B (3.2–4 min), to 18% B (4–4.8 min), to 33% B (4.8–5.7), to 35.5% B (5.7–7.7), to 50% B (7.7–8.1), returning to 1% B (8.1–9.8 min) and re-equilibrating (9.8–10.5 min). Total run time was 10.5 min. The flow rate was 0.5 mL min−1, and the injection volume was 5 µL. The mass spectrum parameters are set as follows: the ion source was electrospray ionization ion source (ESI, positive ion mode) and the capillary voltage was 3.2 kV. The solvent removal temperature was 400 °C, the flow rate of solvent removal was 800 L·h-1, the reverse blowing velocity of the taper hole was 50 L·h-1 and the source temperature was 150 °C. The solvent removing gas and taper hole back blowing gas were nitrogen (purity 99.9%) and the collision gas was argon (purity 99.999%). The scanning mode was multiple reactive ion monitoring (MRM).
Statistical analysisIBM SPSS 13.0 statistical software was used for analysis. Differences in levels of AAs between the groups were tested using Wilcoxon tests, and serum lipid were tested using paired-samples T tests. P-values less than 0.05 were regarded as statistically significant.
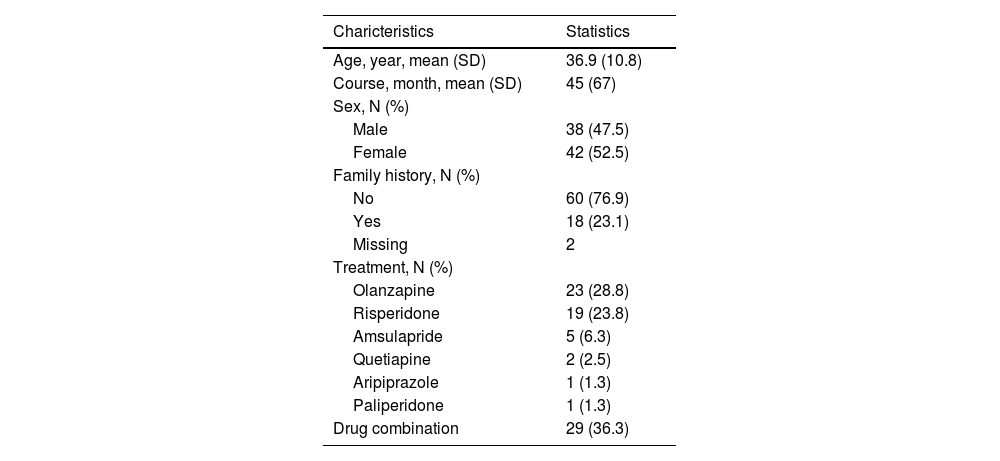
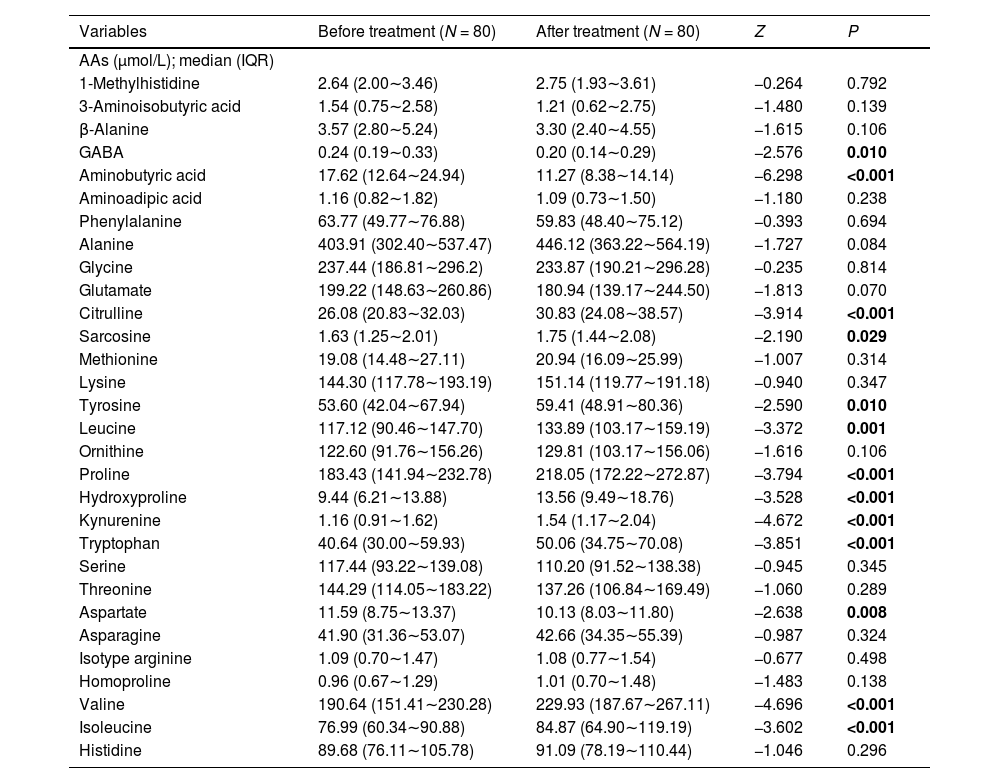
ResultsEffects of various antipsychotics on AAs levels in 80 patients with first-episode SZClinical and demographic features of all the participants included in the study are summarized in Table 1. The average age of the participants was 36.9 ± 10.8. Drug use after admission: 23 cases of olanzapine, 19 cases of risperidone, 5 cases of amsulapride, 2 cases of quetiapine, 1 case of aripiprazole, 1 case of paliperidone, 29 cases of combination drug use. The concentration of 31 AAs obtained from plasma of subjects with first-episode SZ before and after antipsychotic treatment are presented in Table 2. There were 13 out of 31 AAs altered after antipsychotic treatment. Ten AAs displayed a higher concentration in subjects, including citrulline, sarcosine, tyrosine, leucine, proline, hydroxyproline, kynurenine, tryptophan, valine and isoleucine. Conversely, three AAs were demonstrably lower in concentration, including GABA, aminobutyric acid and asparaginic acid.
Demographic and clinical features of study participants.
Levels of AAs in patients with first-episode SZ before and after antipsychotic treatment.
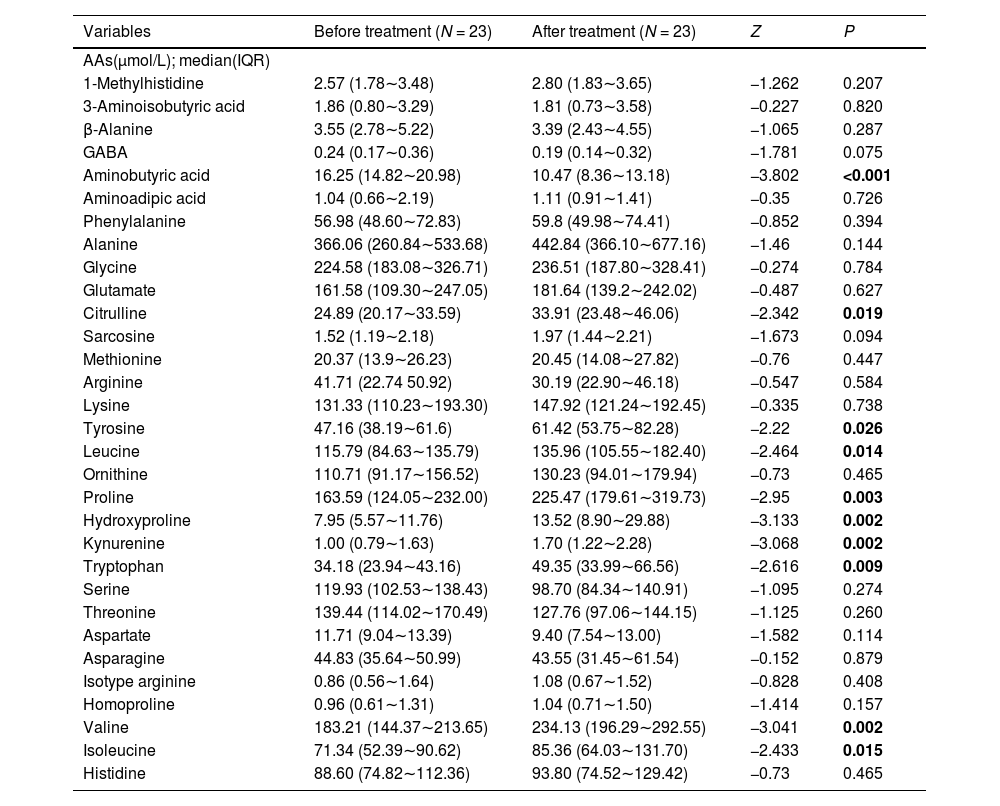
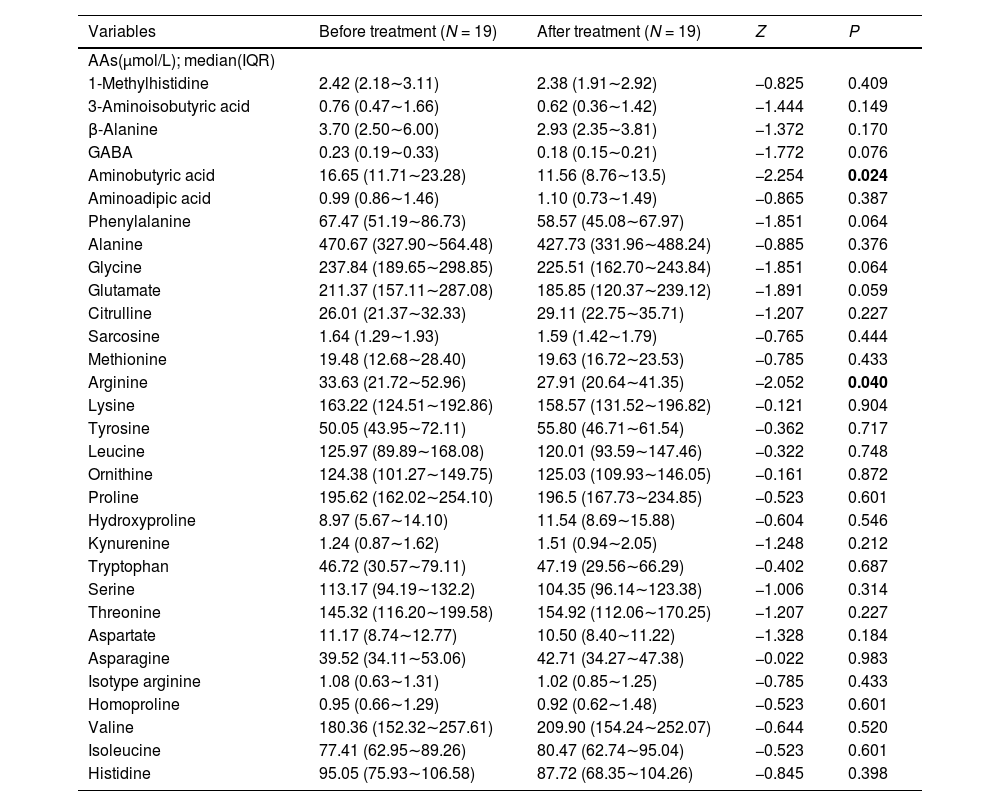
The concentration of 31 AAs obtained from plasma of subjects with first-episode SZ before and after olanzapine treatment and risperidone treatment are respectively presented in Tables 3 and 4. We found that there were 10 out of 31 AAs altered after olanzapine treatment and there were 2 out of 31 AAs altered after risperidone treatment. Our results showed that citrulline, tyrosine, leucine, proline, hydroxyproline, kynurenine, tryptophan, valine and isoleucine were significantly increased and aminobutyric acid were remarkably decreased after olanzapine treatment. However, only two AAs (aminobutyric acid and arginine) displayed a lower concentration after risperidone treatment. There was no significant difference in age and sex between these two groups (Supplementary Table S1).
Levels of AAs in patients with first-episode SZ before and after olanzapine treatment.
Levels of AAs in patients with first-episode SZ before and after risperidone treatment.
The levels of 7 serum lipid acquired from subjects with first-episode SZ before and after olanzapine treatment and risperidone treatment are respectively presented in Supplementary Table S2 and S3. We noticed that TG was markedly upregulated after olanzapine treatment, while ApoA1 was generally upregulated after risperidone treatment.
DiscussionA large number of studies have shown that AAs can be involved in the protein synthesis, glucose and lipid metabolism, excitatory and inhibitory neurotransmitter transport/biosynthesis, ammonia metabolism and urea cycle of mental diseases, resulting in a wide range of metabolic disorders.12-14 That means the occurrence and development of SZ is not only related to a single AAs defect, and may be associated with a variety of AAs metabolism imbalance. It has been reported that elevations of GABA levels in both the medial prefrontal cortex and in the dorsal caudate of first-episode psychosis (FEP) patients can be normalized in both regions following 4 weeks of oral risperidone treatment.15 The evidence is consistent with our findings. Previously, comparison of AAs levels before and after antipsychotic treatment revealed that the levels of four AAs (i.e., alanine, proline, tyrosine and valine) were significantly increased after 7-month treatment. Only the level of aspartate was lower.12 Moreover, significantly decreased level of aspartate was observed in the FEP patients after 5.1-year antipsychotic treatment.16 These trends are consistent with our findings. Evidence suggests that citrulline, leucine, tryptophan, and isoleucine did not change significantly after antipsychotics treatment, while these four AAs were apparently increased in our findings.16 This may be due to differences in study cohort size and follow-up duration. The current study found after the antipsychotic treatment, plasma hydroxyproline level was significantly increased, which is consistent with our study.17 New research shows that sarcosine was associated with significant reductions of symptom severity in the subgroups of people with chronic SZ after six weeks of oral treatment at a dose of 2 g of sarcosine per day.18 This finding may indicate that sarcosine therapy has a positive effect on the treatment of patients with SZ. Herein, we identifed sarcosine level was significantly elevated after antipsychotic treatment, which may provide a basis for the follow-up mechanism exploration of sarcosine in the treatment of SZ. The kynurenine pathway is postulated to play various roles in immune system dysregulation of SZ. A meta-analysis showed that the kynurenine levels were higher in subjects with SZ after antipsychotic treatment when compared with baseline, which is consistently observed in our study.19 The evidence provides valuable insight of the potential underlying involvement of the kynurenine pathway in the pathogenesis of SZ.
Antipsychotics form the mainstay of treatment for patients with SZ, but many are associated with weight gain, lipid disturbance, and glucose dysregulation, thereby contributing to the development of metabolic syndrome.20 Studies in children have shown that antipsychotic drugs can increase susceptibility to weight gain. In addition, it can cause central obesity, insulin resistance, dyslipidemia and systemic inflammation.21 A recent meta-analysis showed that olanzapine was more likely to cause metabolic syndrome than risperidone.22 Furthermore, insulin resistance index (IRI), fasting blood glucose (FBG) and fasting insulin (FINS) levels were significantly increased in Chinese patients treated with olanzapine compared with those treated with risperidone.23 In our study, for the first time, we compared the changes of AAs caused by olanzapine and risperidone treatment. The results show that olanzapine caused more changes in AAs types, which may also indicate that olanzapine treatment leads to greater metabolic side effects related to AAs. In addition, we also explored the changes of seven blood lipids after treatment with these two antipsychotic drugs.
In a previous meta-analysis of the effect of olanzapine on lipid profile in SZ patients, the researchers found that TG, total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) increased, while in this study, TG also increased after olanzapine treatment.24 Besides that, our results indicated that ApoA1 was elevated obviously in patients with first-episode SZ treated with risperidone, which provide a basis for further study on the relationship between antipsychotic drugs and apolipoprotein (APO).
The study has several limitations. The sample size of this study was too small to fully elaborate the effects of antipsychotic medication on AA levels in SZ patients. In future studies, large sample studies will be conducted to investigate the mechanisms of these treatments. In addition, AA levels in healthy controls were missing. The comparison of AA levels in patients with SZ before and after antipsychotic treatment with healthy controls can more accurately demonstrate the implication of AAs in the pathophysiology of SZ.
ConclusionsAntipsychotic treatment has differential effects on the plasma levels of AAs in patients with first-episode SZ. The serum AAs pattern might be an interesting tool to monitor metabolic side effects. The evidence provides valuable insight of the potential underlying involvement of AAs related pathway in the pathogenesis of SZ.