Cytomegalovirus (CMV) infection is an important and frequent complication in solid organ transplant (SOT) recipients. This infection causes direct effects by viral inclusion in the cells of various tissues, as well as indirect effects by interaction between low levels of viremia and the host immune response. These indirect effects, such as increased incidence of graft rejection and opportunistic infections or decreased recipient survival, can be as severe as the direct effects, and thus require implementation of preventative practice guidelines. Universal prophylaxis against CMV has shown to be effective for preventing CMV disease and asymptomatic CMV replication, and can be considered the treatment of choice for preventing the indirect effects of this infection.
La infección por citomegalovirus (CMV) es una complicación importante y frecuente en los receptores de trasplante de órgano sólido (TOS). La infección por este virus da lugar a unos efectos directos, debido a su inclusión en las células de los diferentes tejidos, y a una serie de efectos indirectos, provocados por la interacción de niveles bajos de viremia con la respuesta inmune del huésped. Estos efectos indirectos, entre los que se encuentran un aumento en la incidencia de rechazo del injerto, un mayor número de infecciones oportunistas, así como un descenso de la supervivencia del receptor, son tan graves como los efectos directos, por lo que es necesaria una pauta de actuación para poder prevenirlos. La profilaxis universal frente a CMV ha demostrado ser efectiva en la prevención tanto de la nfermedad como de la replicación asintomática del CMV, por lo que podría considerarse como alternativa de elección en la prevención de los efectos indirectos.
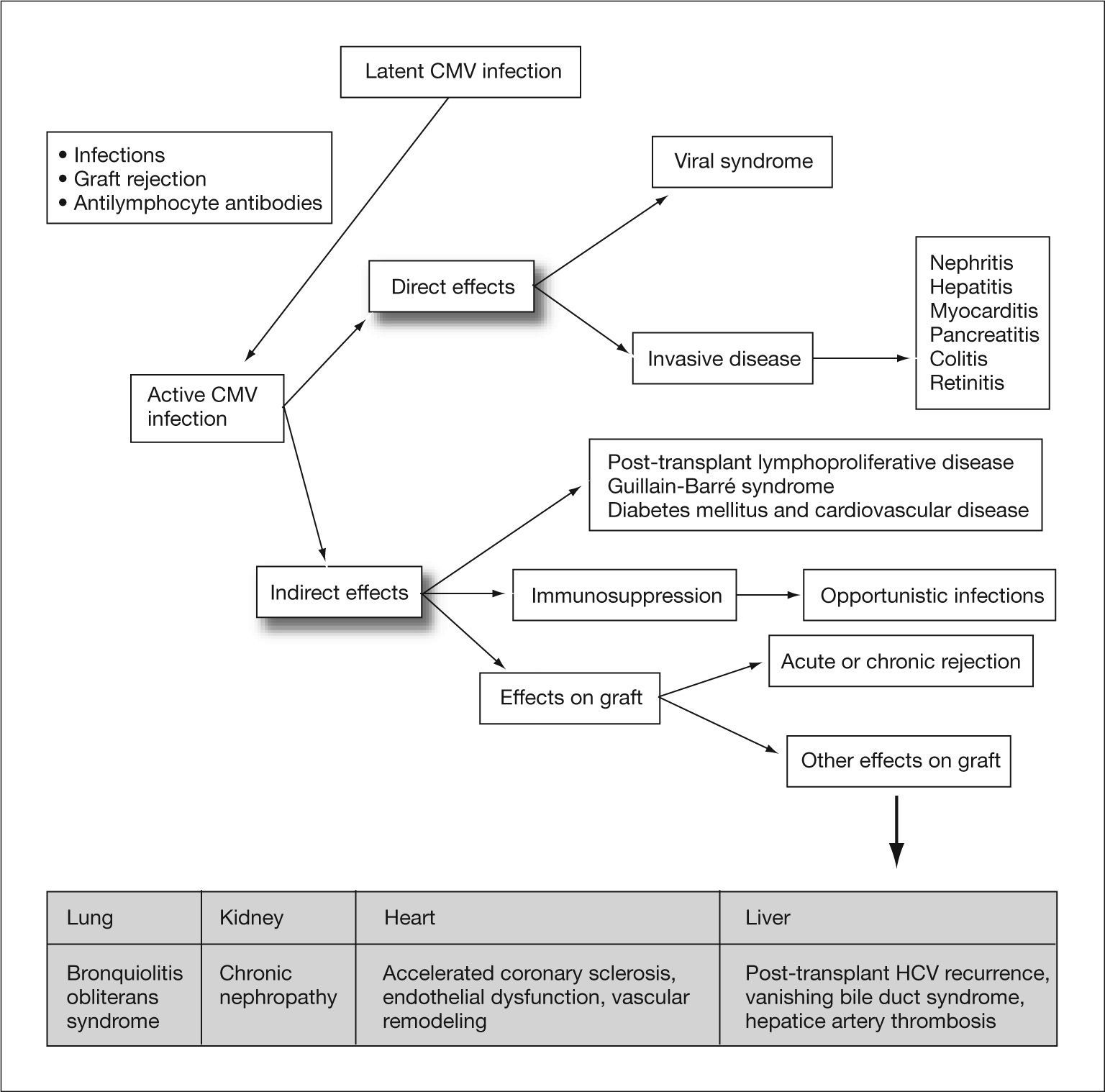
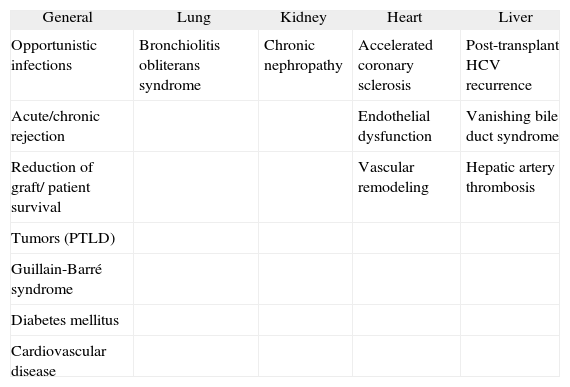
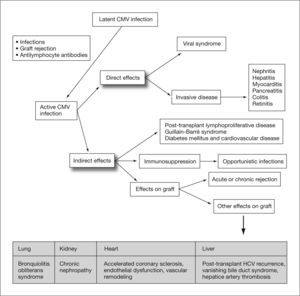
Human cytomegalovirus (CMV) is a DNA virus belonging to the Hesperviridae family. In the immunocompetent host, infection by this virus can manifest as a heterophil-negative mononucleosis-like syndrome and persist indefinitely in latent form in host tissues after primary infection. CMV infection in solid organ transplant (SOT) recipients can result in direct effects or CMV disease, related to the presence of high rates of viral replication, and indirect effects caused by virus interaction with the host immune response1 (fig. 1). These indirect effects result not so much from high levels of viremia as from the presence of low rates of viral replication over prolonged periods of time, and have been associated with an increased risk of rejection and graft dysfunction, accelerated atherosclerosis, opportunistic infections, malignancies, post-transplant diabetes mellitus and Guillain-Barré syndrome (table 1).2–10
Direct and indirect effects of cytomegalovirus. CMV: cytomegalovirus; HCV: hepatitis C virus, Adapted from Fishman et al1.
Indirect effects of cytomegalovirus by type of transplant
| General | Lung | Kidney | Heart | Liver |
| Opportunistic infections | Bronchiolitis obliterans syndrome | Chronic nephropathy | Accelerated coronary sclerosis | Post-transplant HCV recurrence |
| Acute/chronic rejection | Endothelial dysfunction | Vanishing bile duct syndrome | ||
| Reduction of graft/ patient survival | Vascular remodeling | Hepatic artery thrombosis | ||
| Tumors (PTLD) | ||||
| Guillain-Barré syndrome | ||||
| Diabetes mellitus | ||||
| Cardiovascular disease |
HCV: hepatitis C virus; PTLD: post-transplant lymphoproliferative disease.
To understand the pathogenesis of the indirect effects, we need to recall some basic CMV virology. This virus, which is also known as human herpesvirus 5, belongs to the Betaherpesvirinae subfamily of the Herpesviridae family. Human CMV infection is specific to humans, although it bears a certain resemblance to infection by CMV in other mammalian species. The virus has a structure similar to other members of the group and is composed (from the inside out) of a nucleus containing the viral genome (formed by linear double-stranded DNA) and proteins, an icosahedral protein capsid, a protein matrix, and an outer envelope derived from the host cell nuclear membrane.
Replication of CMV is similar to other members of the family, although it has a longer replication cycle. The CMV genome contains 3 types of genes, called α, β and γ, which encode the so-called immediate early, early, and late proteins, respectively. The immediate early proteins are regulatory, while the late proteins are structural, and many of them have important antigenic properties for diagnosis, such as phosphoprotein pp65.
The most important biological property of CMV is its ability to establish latency, which, unlike other herpesviruses, is carried out in very different types of cells. Reactivation of viral replication is related to host immune status, particularly to cellular immunity, and these properties determine the pathogenesis of the virus.11
The natural history of CMV infection can develop in three different ways: primary infection, latent infection, and superinfection or reinfection by an external strain in a patient with previous latent infection.
When a primary infection takes place, a B cell-mediated immune response is activated, producing antibodies of immunoglobulin (Ig) classes IgM, IgG and IgA. These antibodies do not confer protection against CMV, however, and it is the cellular immune response mediated by natural killer (NK) and cytotoxic T lymphocytes which controls the infection. As a result, CMV remains in a latent state in circulating cells, polymorphonuclear cells and T cells, as well as in tissue cells of the vascular endothelium and renal epithelium.
T cells and NK cells12–14 play a central role in the control of CMV infection, so that the virus remains in latent form and only occasionally reactivates in immunocompetent individuals. Detection of anti-CMV antibodies and CMV-specific CD8(+) T cells allows the adaptive immune response to CMV to be evaluated.15,16
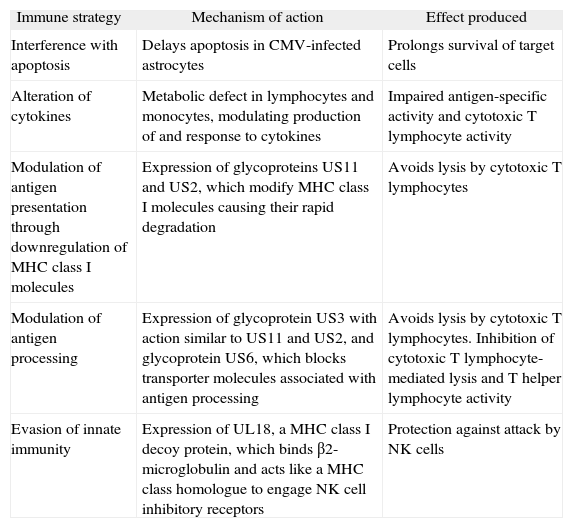
Cytomegalovirus has developed various escape mechanisms to avoid the destruction of infected cells by CD8+ T cells (table 2). CMV possesses a series of genes grouped in a characteristic region of the genome called US (unique short), which encodes glycoproteins (US2, US3, US6, US10 and US11) that inhibit expression of HLA class I molecules and interfere with antigen presentation in the infected cell, thereby preventing its recognition by CD8+ T cells.17–19 Because decreased expression of HLA class I antigens makes infected cells more susceptible to lysis by NK cells,20 CMV has also developed mechanisms to escape from NK cells.21 One of the most important of these mechanisms is expression of proteins encoded in the UL region, such as UL16 or UL141, which interfere with expression of NK activating receptor ligands NKG2D22 and DNAM-1,23 respectively. Other viral proteins such as UL18 and UL40 can also induce activation of NK receptors that inhibit their cytotoxic function.24,25
Immune evasion strategies employed by cytomegalovirus
| Immune strategy | Mechanism of action | Effect produced |
| Interference with apoptosis | Delays apoptosis in CMV-infected astrocytes | Prolongs survival of target cells |
| Alteration of cytokines | Metabolic defect in lymphocytes and monocytes, modulating production of and response to cytokines | Impaired antigen-specific activity and cytotoxic T lymphocyte activity |
| Modulation of antigen presentation through downregulation of MHC class I molecules | Expression of glycoproteins US11 and US2, which modify MHC class I molecules causing their rapid degradation | Avoids lysis by cytotoxic T lymphocytes |
| Modulation of antigen processing | Expression of glycoprotein US3 with action similar to US11 and US2, and glycoprotein US6, which blocks transporter molecules associated with antigen processing | Avoids lysis by cytotoxic T lymphocytes. Inhibition of cytotoxic T lymphocyte-mediated lysis and T helper lymphocyte activity |
| Evasion of innate immunity | Expression of UL18, a MHC class I decoy protein, which binds β2-microglobulin and acts like a MHC class homologue to engage NK cell inhibitory receptors | Protection against attack by NK cells |
CMV: cytomegalovirus; MHC: major histocompatibility complex; NK: natural killer.
Despite these escape mechanisms, which contribute to the inability of the immune system to completely eliminate the virus and to maintenance of latent infection, CD8+ T cells in immunocompetent individuals show a strong response against peptides derived from CMV protein IE1. This response acts as a control mechanism on the process of virus reactivation, as these IE1-specific CD8 T cells recognize and terminate CMV reactivation at the first opportunity.26
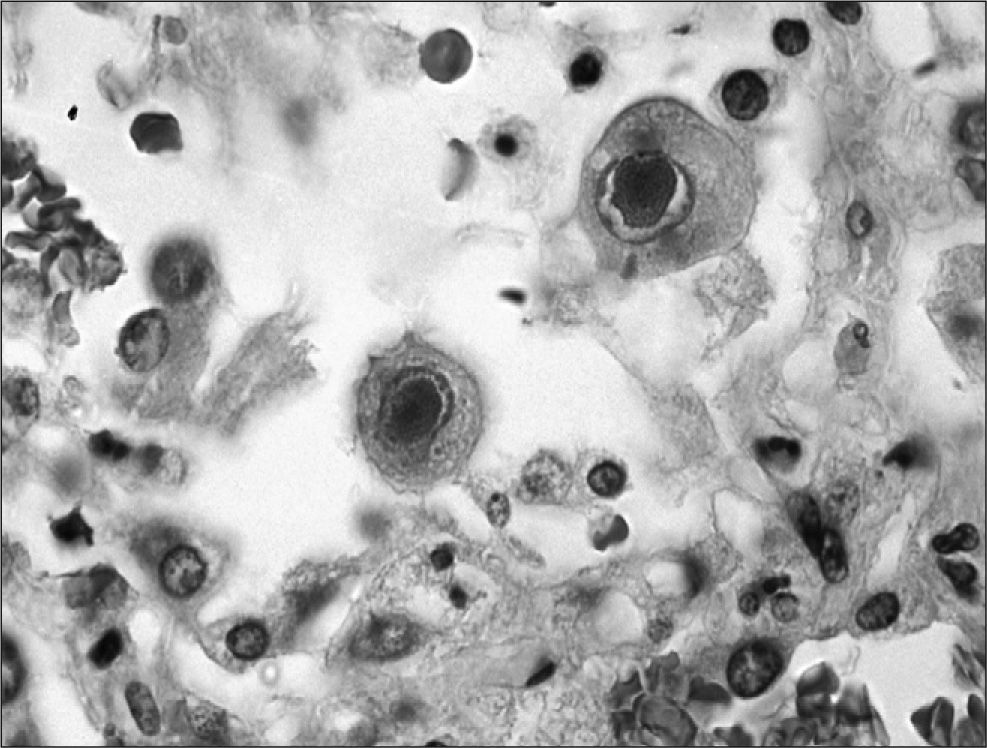
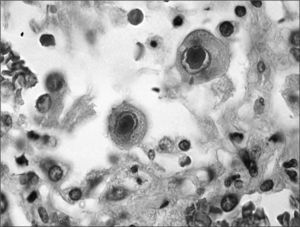
Reactivation of the latent virus takes places when there is a reduction in the host immune response either because immunosuppressive therapy is administered, as occurs in transplant recipients, or other causes such as human immunodeficiency virus (HIV) infection. The primary mediator during reactivation is the proinflammatory cytokine TNF-a, which induces activation of protein kinase C and nuclear factor κB, which in turn results in expression of immediate early proteins, commencing the process of viral replication. This replication occurs in the nucleus of the infected cell, imparting the characteristic “owl's eye” appearance to the cell, which is the key finding for histologic diagnosis of CMV disease (fig. 2).
Although it is generally accepted that CMV is harmless in immunocompetent individuals, recent studies suggest that CMV is responsible for many age-related changes in the immune system. In fact, longitudinal studies in the elderly have shown that CMV is the principal agent responsible for many of the T cell changes found in elderly individuals, such as the appearance of oligoclonal T cell expansions, reductions in the T cell repertoire, and accumulation of T cell subpopulations with altered functional activity. These changes are associated with CMV seropositivity and an increased risk of death in 2 or 4 years in these individuals.27–30
Cytomegalovirus replication also causes a series of indirect effects resulting from its interaction with the host immune system, which will depend on the peculiarities of each individual. For example, in transplant patients the effect of immunosuppressive proteins produced by CMV may be added to the effect of therapy to control rejection, while immunoactivation in response to viral antigens may contribute to the development of rejection.
As previously indicated and summarized in table 2, immunosuppression induced by CMV replication results from functional alterations in lymphocytes and monocytes, which alter their ability to respond and to produce cytokines. CMV replication also favors suppression of cytotoxic T cell-specific antigen recognition, as well as an inversion in the CD4/CD8 lymphocyte ratio31 (table 2). These alterations in the immune response would explain the frequent association of CMV infection with other nosocomial infections (bacteria, Candida spp.) or opportunistic infections, such as Pneumocystis jiroveci pneumonia and invasive Aspergillus spp. infection (fig. 3). CMV infection has also been associated with activation of herpesviruses such as herpes simplex and varicella-zoster virus, Epstein-Barr virus11,32 associated with post-transplant lymphoproliferative disease, or herpesvirus 8, the agent responsible for Kaposi's sarcoma.33
In contrast to the increased frequency of opportunistic infections caused by the immunosuppressive effects of CMV replication, other indirect effects of CMV, such as graft rejection, may be explained by the immunoactivation produced by the virus in the host. The virus replicates in a large number of cells, activating cellular DNA, mRNA and protein synthesis and resulting in the production of Ig receptors, cellular oncogenes (myc and fos), intracellular adhesion molecules and glycoproteins similar to major histocompatibility complex (MHC) class I antigens. It also produces a series of inflammatory cytokines that enhance the expression of MHC class I antigens in endothelial cells of the allograft. This immunological cascade leads to activation of the host immune system, thus facilitating the appearance of indirect effects such as graft rejection. However, the relationship between CMV infection and graft rejection appears to be bidirectional, with CMV leading to rejection and rejection increasing CMV replication, which in the absence of an adequate response by CMV-specific CD8+ T cells will progress uncontrollably. Thus, both acute rejection and other forms of chronic rejection in the transplanted organ (bronchiolitis obliterans syndrome, accelerated atherosclerosis or bile duct loss) are associated with CMV infection, even if they are not caused solely by it.34
There is debate, however, regarding whether the intensity of CMV replication is associated with the development of indirect effects. Periods of asymptomatic viremia with low viral loads may predispose to these effects because they may be dependent on cytokines, chemokines and growth factors produced by the host in response to any level of viral replication or tissue invasion by CMV.35–37
General indirect effectsA number of the indirect effects caused by CMV appear in transplant patients in general, such as the increased incidence of both acute and chronic rejection and the development of cardiovascular disease. These effects cause a reduction in both graft and patient survival. Each of these indirect effects will be analyzed in detail in the section corresponding to each specific organ.
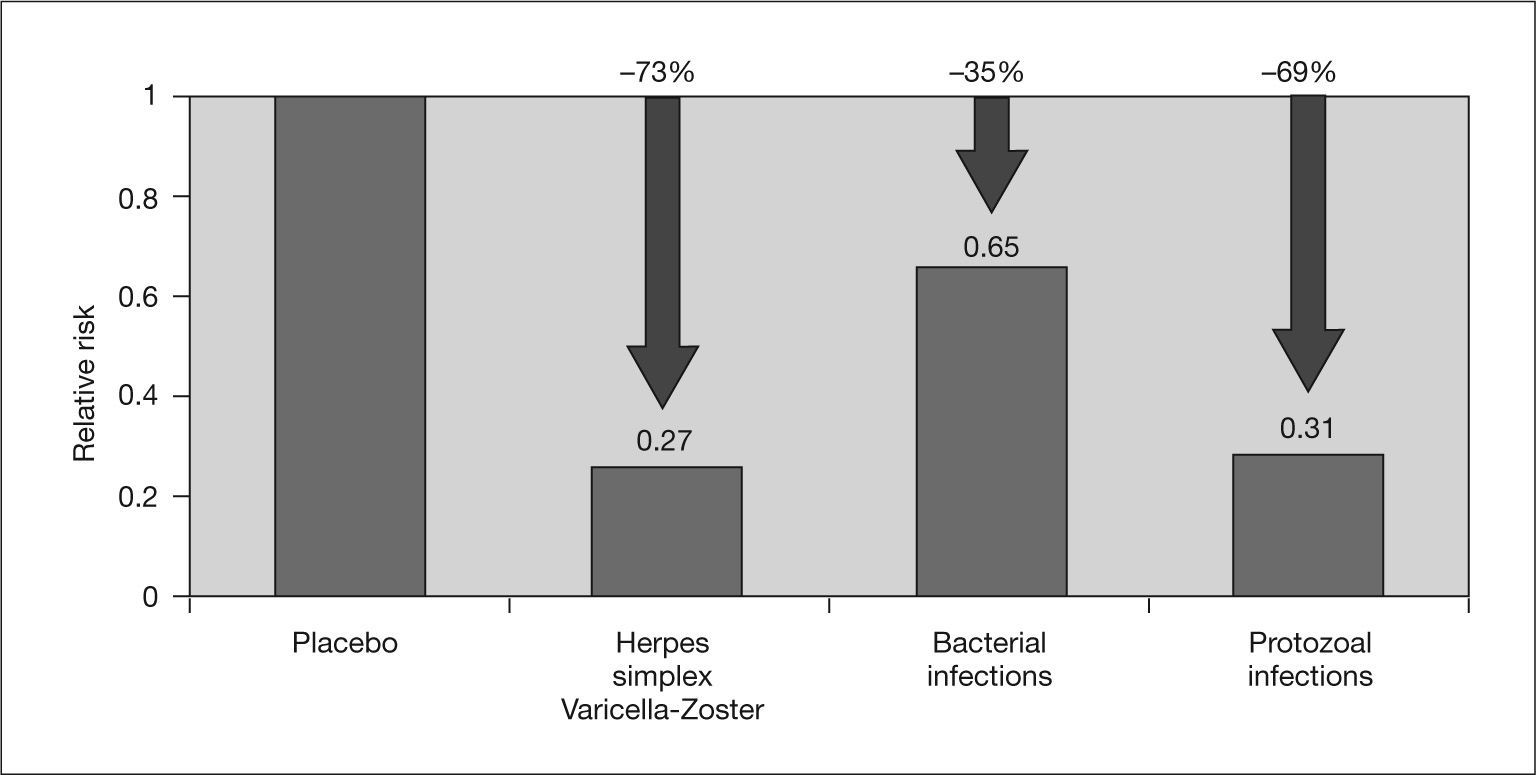
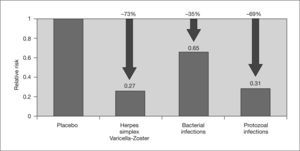
On the one hand, the immunomodulatory effect of CMV may favor the development of opportunistic infections and malignancies, such as post-transplant lymphoproliferative disease associated with Epstein-Barr virus, in SOT recipients.2,3,7 In this regard, a recent meta-analysis by Hodson et al3 found a reduction in bacterial and protozoal infections in transplant patients who received CMV prophylaxis (fig. 4).
Effect of cytomegalovirus prophylaxis on concomitant infections. Adapted from Hodson et al3.
Other indirect effects that may be caused by CMV infection are Guillain-Barré syndrome and diabetes mellitus. With respect to Guillain-Barré syndrome, studies in experimental models suggest that this entity has an immunological basis and that infectious agents such as CMV and Campylobacter jejuni are responsible for initiating the immune response that causes neural damage. In this regard, El-Sabrout et al9 reported five cases of Guillain-Barré syndrome after SOT associated with CMV infection, which clearly illustrate the possible relationship between this neurological disease and viral replication in SOT.
Diabetes mellitus is a common complication in SOT recipients, with an incidence ranging from 3% to 45%. The wide range of this estimated incidence reflects differences in the immunosuppressive regimens used, different definitions of post-transplant diabetes mellitus, and, in some cases, errors in the determination of its incidence in the transplant population. Although the most important cause of post-transplant diabetes mellitus is the effect of immunosuppressive drugs on glycemia control, CMV infection has also been identified as a risk factor for this entity.10
The specific indirect effects that have been reported for each type of transplant are described below.
Organ-specific indirect effectsLung transplantDespite prophylactic regimens, the incidences of CMV infection and disease in this type of transplant are very high, with percentages close to 60% and 40%, respectively.38 Bronchiolitis obliterans syndrome (BOS) is the main cause of long-term morbidity and mortality in patients undergoing lung transplant. In addition to rejection and lymphocytic bronchiolitis, active CMV infection has been identified as one of the main risk factors for this syndrome, and a statistically significant association has been shown between the development of BOS and at least one positive DNAemia at both 3 and 6 months post-transplant,39 which raises the need to implement preemptive treatment protocols until at least 1 year after transplantation. There are conflicting results in the literature regarding the impact of donor and recipient CMV serological status on the development of BOS.40 However, because transplantation of a seropositive organ into a seronegative recipient is the main risk factor for CMV disease, we can conclude that the best way to prevent CMV infection is to ensure that both recipient and donor are seronegative for this virus41 (table 3).
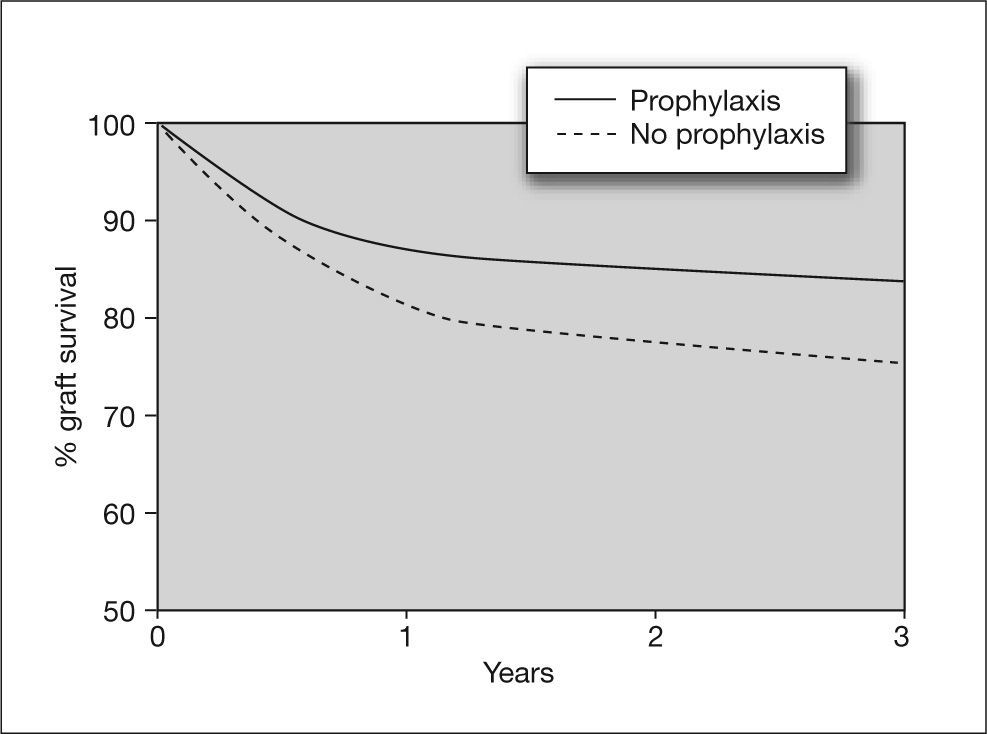
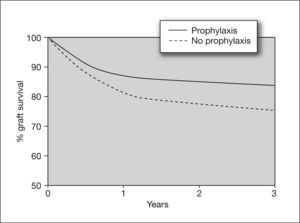
Kidney transplantAlthough the incidence of CMV infection and disease in kidney transplants is lower than in other solid organ transplants such as lung or heart, there is evidence that CMV infection is an independent risk factor for kidney graft loss.42 Moreover, there are studies showing that CMV seromismatched renal transplant patients (D+/R-) have a significantly higher risk of acute rejection (odds ratio [OR] = 2.28), which implies that CMV infection is a risk factor for this event.43 In these high-risk patients, universal prophylaxis not only reduces the risk of CMV disease, but has been associated with a 50% reduction in the incidence of acute graft rejection compared to placebo44,45 (fig. 5). This may be due to the fact that the use of universal prophylaxis, in contrast to preemptive therapy, results in marked inhibition of viral replication and impedes the presence of low levels of viremia during long periods of time, which have been implicated in the pathogenesis of the indirect effects of CMV. Nevertheless, in contrast to high-risk cases, patients with an intermediate risk of CMV disease (R+), in whom preemptive therapy is commonly used, may be at greater risk of long-term indirect effects that would not be prevented by this therapy.
Effect of cytomegalovirus prophylaxis on kidney graft survival in D+/R− patients. D+: CMV-seropositive donor; R-: CMV-seropositive recipient Taken from Opelz et al87.
In addition to short-term effects such as acute rejection, CMV disease has been associated with the two most common causes of late kidney graft loss: cardiovascular disease and chronic graft rejection.46
Cardiovascular disease is currently the leading cause of death in a transplant patient with a functioning kidney graft. In addition to age greater than 45 years and the presence of diabetes mellitus, CMV infection was shown to be an independent risk factor for death from a cardiovascular cause in these patients, especially those with high viral replication.47,48 Similarly, activation of the arteriosclerosis process in small renal arterioles has been associated with the development of chronic allograft nephropathy, which may be explained by the fact that intimal thickening is more intense in CMV-infected patients than in non-infected patients.49 These arteriosclerotic changes may be triggered by a CMV-induced intragraft inflammatory response that results in continuous immunological injury, leading to vascular changes in response to the injury and the accelerated changes of chronic allograft nephropathy.50
The appearance of indirect effects of long-term CMV infection, such as cardiovascular disease and chronic rejection, which will determine both patient survival and graft functionality, raise the need to investigate the possible benefit of prolonged CMV prophylaxis strategies for more than 100 days post-transplantation.
In addition to the effects caused by CMV on the graft itself, infection by this virus can modify the prognosis of these patients, even in the presence of a functioning kidney graft. In this regard, it has been shown that both asymptomatic CMV infection and overt CMV disease in the first 100 days after transplantation were associated with reduced patient survival beyond 100 days.51 Although these results have not been confirmed in other studies,52 the fact that asymptomatic CMV infection has been associated with a reduction in the survival of kidney transplant recipients may show the importance of preventing asymptomatic viremia even in the presence of low viral replication by administration of universal prophylaxis to all at-risk patients (table 4).
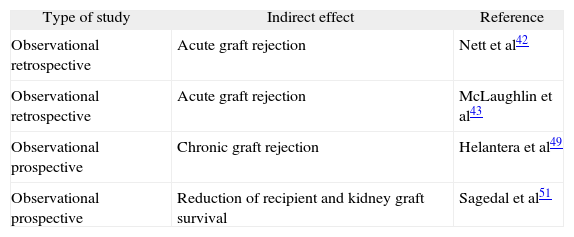
Indirect effects of cytomegalovirus in kidney transplant recipients
| Type of study | Indirect effect | Reference |
| Observational retrospective | Acute graft rejection | Nett et al42 |
| Observational retrospective | Acute graft rejection | McLaughlin et al43 |
| Observational prospective | Chronic graft rejection | Helantera et al49 |
| Observational prospective | Reduction of recipient and kidney graft survival | Sagedal et al51 |
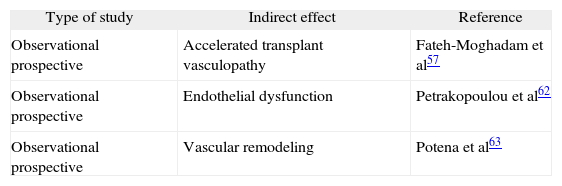
Cytomegalovirus infection has been associated with acute rejection and graft vascular remodeling in heart transplant recipients, as well as with accelerated coronary atherosclerosis and endothelial dysfunction.53–55
There are studies showing the role of CMV infection in accelerated vasculopathy in both the general population and heart graft recipients. With regard to native atherosclerosis, it has been shown that a high titer of anti-CMV antibodies (≥ 8 U/mL) is an independent risk factor for coronary artery disease, along with smoking, male gender and high levels of C-reactive protein (CRP).56
As to accelerated transplant vasculopathy, CMV infection in heart transplant recipients is an independent predictor of plaque progression in the first year, with a high frequency of calcified lesions and greater plaque thickening in the CMV-positive group than the CMV-negative group,57 which also resulted in lower 5-year survival.58 However, other studies have shown that CMV seropositivity alone is not an independent risk factor for coronary artery disease, but that the combination of CMV seropositivity and elevated CRP levels is predictive of coronary artery disease (OR = 4.3; P = 0.01).59
Cytomegalovirus has been implicated in the development of atherosclerosis in heart grafts because of its ability to activate inflammatory mechanisms that cause the formation of atherosclerotic lesions. Other factors that have been implicated in the development of atherosclerosis are expression of viral chemokine US28, a stimulator of smooth muscle cell migration,60 and the ability of CMV to induce a procoagulant environment in the endothelial surface.61 More recently, it has been suggested that CMV might accelerate transplant atherosclerosis by reducing the vascular effects of endothelium-derived nitric oxide. This leads to endothelial dysfunction, which would imply a loss of the normal vasodilatory response and generation of a proatherogenic environment. In this regard, it has been reported that CMV infection is an independent predictor of distal epicardial/endocardial dysfunction in D+/R− patients after adjusting for transplant indication, statin therapy and risk factors such as ischemia time, acute rejection episodes, hyperlipidemia, arterial hypertension, smoking and diabetes mellitus. Furthermore, CMV infection in this group of D+/R− patients caused an increased incidence of cardiovascular events and death during follow-up as compared to the other serological groups.62
In addition to atherosclerosis and accelerated transplant vasculopathy, CMV infection has been associated with other important complications that may have an impact on morbidity and mortality in heart transplant patients, such as impaired vascular remodeling and left ventricular dysfunction associated with graft rejection.
To study the role of impaired vascular remodeling, a clinical trial was conducted in patients with and without CMV infection who had a similar intimal volume at the start of the study and developed a similar increase in intimal hyperplasia during the 12 months of observation. However, the authors found a significant coronary lumen loss in the patients with CMV infection but not in the other patients, and concluded that the ability of the vascular wall to compensate for intimal hyperplasia was impaired in patients who presented CMV infection.63
The pathophysiological mechanism causing vascular impairment is not known, but it has been observed that CMV may exert an inflammatory effect on the vascular wall as a result of both a direct cytotoxic action on endothelial cells and indirect induction of graft antigens, causing fibrosis of the vascular wall and altering the response to endothelial injury. Lack of knowledge of the mechanisms by which CMV causes vascular remodeling and endothelial dysfunction and may reduce survival of these patients reveals the need for further studies to investigate whether universal prophylaxis should be administered in heart transplant patients to decrease the incidence of the asymptomatic viremias that cause these indirect effects of CMV (table 5).
Liver transplantCytomegalovirus infection has been associated with accelerated chronic allograft rejection or vanishing bile duct syndrome (VBDS) in liver transplant recipients, which significantly increases the risk of fibrosis and graft dysfunction after transplantation. The possibility that the virus directly destroys infected structures or triggers an immune response against ductal cells has been suggested as a mechanism by which CMV causes VBDS.64 As in kidney transplantation, CMV prophylaxis has been shown to reduce the risk of graft rejection and improve patient survival.65
Hepatic artery thrombosis with consequent bile duct ischemia and VBDS are also found more frequently in D+/R− liver transplant recipients than other serological groups. Persistence of CMV DNA in bile ducts and in endothelial cells of the vascular structures of the liver graft leads to chronic rejection and also reduces long-term survival in these patients.66,67 Based on this evidence, it can be hypothesized that universal prophylaxis against CMV would reduce the incidence of these indirect effect by completely halting viral replication, a concept that needs to be confirmed.
Cytomegalovirus infection influences the natural history of hepatitis C virus (HCV) infection after transplantation, increasing the risk of graft failure and mortality.68,69 Some studies have shown an increased risk of HCV recurrence in patients who developed post-transplant CMV infection65,70–72 as well as an increased risk of graft cirrhosis (50% in CMV-positive patients versus 11% in CMV-negative patients, P = 0.027),73 with more rapid and aggressive progression to fibrosis than in CMV-negative patients.68,74 Although the reasons for these findings are not well understood, it is thought that CMV-induced immunosuppression may accelerate the effects of HCV and influence post-transplant recurrence, graft failure and mortality.
An association has also been described between CMV reactivation and herpesvirus 6 infection, a virus that has a strong capacity to induce the release of cytokines related to the pathogenesis of hepatitis C, such as tumor necrosis factor. It is likely that these immune system alterations favor HCV replication and cause more aggressive disease.74 Although conclusive data are not available, these results suggest that patients transplanted for HCV liver disease (currently the main indication for liver transplantation) require profound inhibition of CMV viral replication, since, as with other viruses such as Epstein-Barr virus, CMV replication could activate HCV replication. In this regard, it was observed that the use of universal CMV prophylaxis with ganciclovir for 3 months significantly reduced the incidence of biopsy proven rejection in liver transplant recipients (hazard ratio [HR] = 0.51; 95% confidence interval [95% CI] = 0.33-0.79; P = 0.003), and these results were independent of other known factors associated with rejection.75 Although this was a retrospective study and the results need to be confirmed in prospective studies in larger patient samples, it should be taken into account when choosing the best strategy to avoid both the direct and indirect effects of CMV infection.
In addition to these adverse effects, CMV infection has been associated with an increased risk of bacterial and invasive fungal infections. In fact, infection by this virus has been shown to be an independent risk factor for late invasive aspergillosis in patients who received prolonged prophylaxis with ganciclovir (OR = 6.7; 95% CI = 1.0-42.5),76 and therefore we believe that close monitoring is required in these patients beyond the periods of maximum immunosuppression in the first months after transplantation.
As we have seen, the indirect effects of CMV infection have large impact on graft survival in liver transplant recipients. Although available data are insufficient, it appears that the use of preemptive therapy in low-risk patients (R +) could result in prolonged maintenance of low levels of viremia and thus facilitate the development of severe complications in the graft and patients. Prospective studies are needed to evaluate the possible benefit of universal prophylaxis versus preemptive therapy in these situations.
Some authors have expressed doubts about an increased risk of indirect effects of CMV in patients receiving pre-emptive therapy. This is based on the results obtained in comparative studies of preemptive therapy versus deferred treatment (antivirals are not administered until CMV disease develops) or versus patients without CMV infection, in which no differences were found in the incidence of opportunistic infections or rejection.77,78 These findings are not conclusive, however, because the design and the number of patients included in the studies was inadequate to observe these differences, since the risk of development of indirect effects was not the primary endpoint of the studies (table 6).
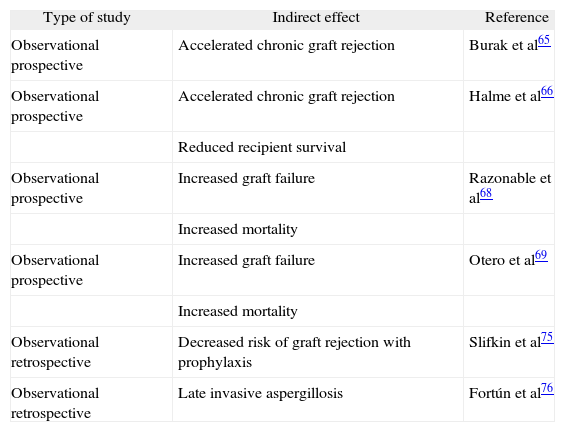
Indirect effects of cytomegalovirus in liver transplant recipients
| Type of study | Indirect effect | Reference |
| Observational prospective | Accelerated chronic graft rejection | Burak et al65 |
| Observational prospective | Accelerated chronic graft rejection | Halme et al66 |
| Reduced recipient survival | ||
| Observational prospective | Increased graft failure | Razonable et al68 |
| Increased mortality | ||
| Observational prospective | Increased graft failure | Otero et al69 |
| Increased mortality | ||
| Observational retrospective | Decreased risk of graft rejection with prophylaxis | Slifkin et al75 |
| Observational retrospective | Late invasive aspergillosis | Fortún et al76 |
The knowledge acquired over the past few years on both the consequences and risk factors for CMV disease has al-lowed well-defined prophylaxis and treatment regimens to be adopted in these patients.79 Nevertheless, many questions still remain about the importance of the indirect effects of CMV infection in transplant patients because they have been little evaluated in the studies conducted to date. One of the most important questions that remains to be answered is the impact of the different prophylaxis regimens on the morbidity and mortality associated with these effects. Current options for CMV prophylaxis include preemptive therapy (monitoring and administration of antivirals when significant levels of viremia are detected) and universal prophylaxis (administration of antivirals to all patients).
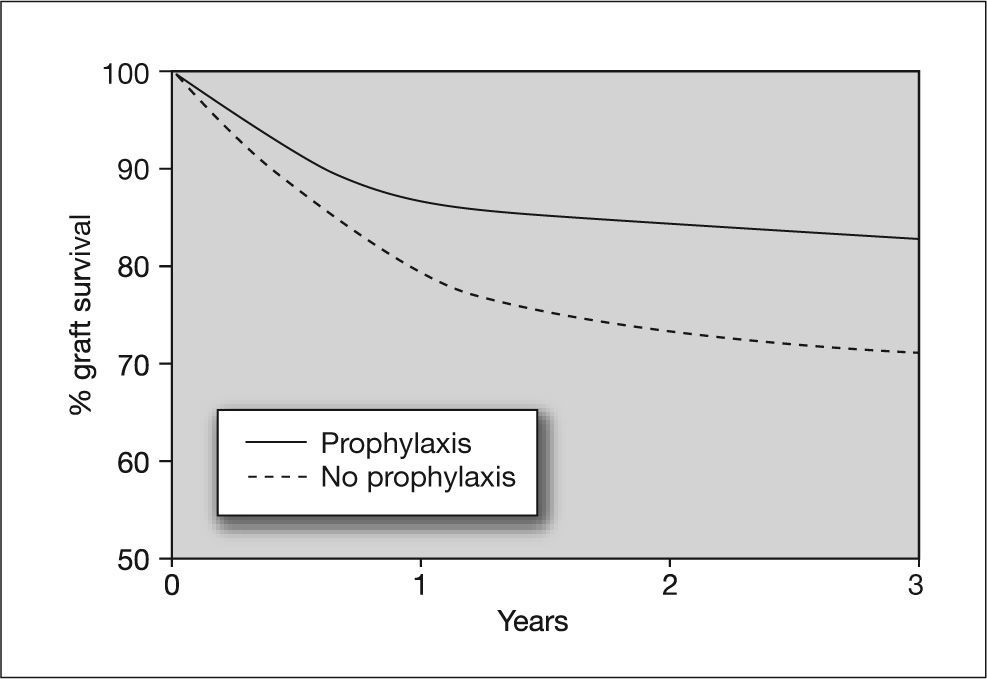
In a study by Khoury and Storch80 it was shown that preemptive therapy does not prevent asymptomatic CMV replication. Because preemptive therapy allows persistence of low levels of viremia, one might expect it to be ineffective for preventing the indirect effects of CMV infection.81,82 In contrast, universal prophylactic regimens completely suppress viral replication, preventing both the direct and indirect effects of CMV infection, which is important considering that the indirect effects include serious situations such as acute rejection or an increased risk of opportunistic infections (figs. 5 and 6). However, universal prophylaxis poses the problem of an increased risk of late-onset CMV disease, which may be due to inhibition of immune reconstitution. Furthermore, some antiviral agents, such as ganciclovir, affect cellular DNA synthesis and have an inhibitory effect on T cell proliferation. This could result in greater inhibition of the immune response against CMV in patients receiving universal prophylaxis with ganciclovir.8,83–85 The answer to whether universal prophylaxis is a better strategy than preemptive therapy for preventing the indirect effects of CMV should be confirmed in the future. This hypothesis has greater importance if we consider that preemptive therapy is used in patients at intermediate risk of CMV disease (R + transplant), who would, however, be at high risk of other serious complications such as graft dysfunction or rejection and opportunistic infections.
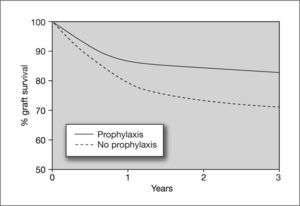
Effect of cytomegalovirus prophylaxis on heart graft survival in D+/R− patients. D+: CMV-seropositive donor; R-: CMV-seropositive recipient. Taken from Opelz et al87.
Few data are currently available regarding the possible benefits of prophylaxis on the indirect effects of CMV infection, because most studies were designed to evaluate the impact of prophylaxis on the development of CMV disease. In one of these studies, conducted in seronegative recipients of a kidney transplant from seropositive donors (D+/R-) and placebo-controlled, a 50% reduction in the rate of graft rejection was observed in patients who received CMV prophylaxis.86 A study designed to determine the relationship between donor and recipient CMV pairing and CMV prophylaxis found a strong association between CMV prophylaxis and graft survival only in the D+/R− combination. The study also found a significant reduction in the risk of graft failure in patients who received CMV prophylaxis (relative risk [RR] = 0.80; 95% CI = 0.73-0.89; P < 0.0001), as well as an improvement in graft survival (RR = 0.86; 95% CI = 0.78-0.95; P = 0.0017) and patient survival (RR = 0.71; 95% CI = 0.61-0.83; p < 0.0001). In addition, an inhibitory effect of CMV prophylaxis on acute rejection was observed in patients who received CMV prophylaxis.87
In contrast, two meta-analyses conducted by Hodson et al3 and Strippoli et al88 concluded that CMV prophylaxis did not significantly reduce the risk of acute rejection or graft loss in solid organ transplants, although the meta-analysis by Hodson et al showed that the risk of bacterial and protozoan infections was significantly lowered by prophylaxis3 (fig. 4). Although more in-depth studies are needed on this subject, these data suggest a possible beneficial effect of universal prophylaxis versus preemptive therapy for preventing the indirect effects of CMV infection.
ConclusionCytomegalovirus infection in SOT recipients not only causes direct effects but also indirect effects associated with the interaction between low levels of viremia and the host immune response. Although we currently have insufficient data on their immunological basis, incidence, morbidity and mortality, these effects are associated with serious complications such as opportunistic infections, graft rejection and dysfunction and an increased risk of malignant disease; therefore, their prevention is as important as preventing CMV disease. Universal prophylaxis, which has been shown to be effective for preventing both CMV infection and disease, could be the option of choice for avoiding these indirect effects. However, this hypothesis is not supported by sufficient scientific evidence. Furthermore, prolonged use of ganciclovir can inhibit the CMV-specific immune response that facilitates the development of late-onset CMV disease when prophylaxis is discontinued. This hypothesis urgently requires testing in prospective studies specifically designed with this objective.
Financial support: Supported by Ministerio de Sanidad y Consumo. Instituto de Salud Carlos III. Spanish Network of Infection in Transplantation (RESITRA G03/075) and Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008).