The incidence of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients after an episode of Pneumocystis jirovecii pneumonia (PJP) seems to be lower than with other opportunistic infections. We conducted an observational study in order to determine the incidence, clinical characteristics and outcome of patients diagnosed with PJP-related IRIS.
MethodsWe conducted an observational study of HIV patients diagnosed with PJP-related IRIS from January 2000 to November 2015. We analyzed epidemiological and clinical characteristics as well as laboratory findings. We also carried out a systematic review of published cases.
ResultsSix cases of IRIS out of 123 (4.9%) HIV-infected patients with PJP who started ART were diagnosed. All six cases were men with a median age of 34 (IQR: 8) years. The six patients developed paradoxical IRIS. Subjects younger than 40 years old (p=0.084) and with an HIV-RNA viral load >100000 copies/ml (p=0.081) at diagnosis showed a tendency to develop IRIS. Thirty-seven published cases of PJP-related IRIS were identified. Although 51% of cases involved respiratory failure, no deaths were reported.
ConclusionsPJP-related IRIS is rare condition compared to other opportunistic infections. It can lead to a severe respiratory failure in a significant proportion of cases, although no deaths have been reported.
La incidencia del síndrome inflamatorio de reconstitución inmune (SIRI) en pacientes infectados por el virus de la inmunodeficiencia humana (VIH) después de un episodio de neumonía por Pneumocystis jirovecii (PJP) parece ser menor que con otras infecciones oportunistas. Hemos realizado un estudio observacional con el objetivo de conocer la incidencia, las características clínicas y la evolución de los pacientes diagnosticados de SIRI asociado con la PJP.
MétodosSe ha realizado un estudio observacional de pacientes con VIH diagnosticados de SIRI asociado a PJP desde enero del 2000 hasta noviembre de 2015. Fueron analizadas características epidemiológicas y clínicas, así como hallazgos de laboratorio. Asimismo, se ha llevado a cabo una revisión sistemática de los casos publicados previamente.
ResultadosSe identificaron 6 casos de SIRI en 123 pacientes con VIH (4,9%) con PJP que comenzaron TAR. Los 6 casos eran varones con una edad media de 34 (IQR:8) años. En los 6 casos se trató de una SIRI paradójico. Los sujetos menores de 40 años (p=0,084) y con VIH-ARN al diagnóstico mayor de 100.000 copias/ml (p=0,081) mostraron una tendencia a desarrollar SIRI. Se identificaron 37 casos publicados de SIRI relacionado con PJP en la literatura. Aunque el 51% de los casos presentaron insuficiencia respiratoria, no se reportaron muertes.
ConclusionesEl SIRI asociado con PJP es una entidad infrecuente comparada con el relacionado con otras infecciones oportunistas. Puede provocar insuficiencia respiratoria grave en un porcentaje importante de casos, si bien no se han reportado muertes.
Most opportunistic infections experienced a significant decline after the introduction of highly active antiretroviral therapy (ART). However they continue to be present among patients who are unaware of their HIV-infected status or among those subjects who remain unlinked to care or do not take ART properly due to adherence or tolerability issues.1 In fact, Pneumocystis jirovecii pneumonia (PJP) still represents the most common opportunistic infection in patients with AIDS in developed countries.2,3
After an episode of PJP, initiation of ART is mandatory as it has been shown in clinical trials and cohort studies. Indeed, early initiation of ART in the first 10–14 days reduced AIDS progression and death in patients with PJP compared with those who started later.4 Nevertheless the development of an immune reconstitution inflammatory syndrome (IRIS) is a matter of concern when ART is initiated after an opportunistic infection. IRIS has been well described in patients with cytomegalovirus retinitis, cryptococcal meningitis, tuberculosis or progressive multifocal leukoencephalopathy.5 However the incidence of IRIS remains unclear for other common opportunistic infections such as PJP. Most publications regarding PJP-related IRIS consist in single case-reports or small case series.6,7 A first attempt to address this issue was performed by Mok et al. who reviewed 17 cases previously published, however in this study data regarding the outcome of the patients were not reported.7 In any case, the incidence of IRIS after an episode of PJP seems to be lower than with other opportunistic infections. Moreover cases of acute respiratory failure after early introduction of ART in patients treated for PJP have been described.8
Because of the scarce data published regarding PJP-related IRIS we performed an observational study in order to determine the incidence, clinical characteristics and outcome of patients diagnosed with PJP-related IRIS. We also carried out a comprehensive review of published cases in the literature.
Patients, material and methodsStudy designThis is an observational retrospective study of all HIV adult patients diagnosed with PJP-related IRIS between January 2000 and November 2015 at the University Hospital Vall d’Hebron, in Barcelona, Spain. A cohort study of all cases of PJP in our institution was previously published.3 Our institution is a 1000-bed tertiary hospital where approximately 2100 HIV-infected are regularly followed in the outpatient clinic. All clinical data of HIV-infected patients are routinely included in a database as a part of a continuous observational study. Data analyzed in the present study have been taken from this database. This study was approved by the Ethics Committee of Vall d’Hebron Research Institute.
Study variables and data collectionFor each patient we recorded demographic, clinical, laboratory and radiological data. CD4 lymphocyte count and HIV viral load were recorded at diagnosis of PJP and at diagnosis of IRIS as well as the timing of ART initiation. Development of respiratory failure, need of ICU admission and outcome of PJP and PJP-related IRIS were also recorded.
DefinitionsDiagnosis of PJP was performed in patients with suggestive clinical and radiological findings and demonstration by direct immunofluorescence of P. jirovecii (PJ) in bronchoalveolar lavage (BAL). In those cases in which fibrobronchoscopy could not be performed, a probable diagnosis of PJP was assumed if all the following conditions were fulfilled: CD4 lymphocyte count <200 cells/μL; clinical and radiological findings suggestive of PJP, no prophylaxis against PJP, good response to specific PJP treatment and exclusion of other pulmonary pathogens, mainly bacterial pneumonia and tuberculosis.
Respiratory failure was defined as oxygen saturation <90% using a pulse oximeter or partial pressure of oxygen <60mmHg in arterial blood.
PJP IRIS was diagnosed in patients who fulfilled the following criteria: (1) development of inflammatory symptoms after initiation of ART, (2) significant decrease in HIV-RNA level from baseline and an increase in CD4+ cells from baseline and (3) exclusion of a newly diagnosed opportunistic infection or drug toxicity. These criteria have been previously published by other authors.9
TreatmentAll patients were initially treated with intravenous cotrimoxazol and in those who presented adverse effects treatment was switched to intravenous pentamidine. Metilprednisolone was indicated when the patient developed respiratory failure (pO2<60mmHg) with the following dose: 40mg/12h during five days, 40mg per day during five days and after that a progressive reduction in the following weeks until day 21.
Literature reviewA search in PubMed database was done using the topics “Immune reconstitution inflammatory syndrome”, “Pneumocystis IRIS”, “Pneumocystis jirovecii immune reconstitution”, “Pneumocystis carinii pneumonia immune reconstitution syndrome”, “immune restoration Pneumocystis”, “immune reconstitution Pneumocystis carinii pneumonia” and “Pneumocystis carinii pneumonia worsening HAART” in order to find published cases of P. jirovecii related IRIS. Results were limited to adult patients (above 18 years old) and to Spanish, English or French literature.
From each case we attempt to obtain the following data: demographic data, CD4 lymphocyte count and HIV-RNA viral load at ART initiation, CD4 lymphocyte count and HIV-RNA viral load at diagnosis of IRIS, use of steroids, clinical and radiological characteristics, antiretroviral therapy, outcome and mortality.
Statistical analysisCategorical variables are presented as numbers (proportions) and continuous are expressed as mean and standard deviation or median and interquartile range as appropriate. We carried out an unvaried analysis in order to find differences between patients who developed IRIS and those who did not. The Chi-square test was used to compare categorical variables, and the Mann–Whitney U test for continuous variables. All statistical tests were two-tailed and were performed at a level of statistical significance of 0.05. IBM SPSS statistics software for Windows (Version 21.0; IBM Corp., Armonk, NY, USA) was used for statistical analysis.
ResultsFrom January 2000 to November 2015, 148 HIV infected patients were diagnosed of PJP in our Institution. In 74 (50%) of them the PJP episode was the initial event of HIV infection. Fifteen patients (10.1%) died during the course of PJP before starting ART. Of the 133 subjects who survived, 123 began ART. In this study we focus on these 123 patients with PJP who started ART.
Baseline characteristics of patients at PJP diagnosisThe mean age of the 123 patients was 40.8 (± 9.3) years old. There were 85 (69.1%) men and 38 (30.9%) women. The mean CD4+ lymphocyte count was 48.1/μL (± 59.7). One hundred and eighteen (96.9%) patients had <200 CD4+ lymphocytes/μL at the time of diagnosis and 86 (70.5%) <50 CD4+ lymphocytes/μL. Mean HIV-RNA viral load was 5.78 (± 6.16) log10 copies/mL. Seventy (56.9%) patients had >100000 RNA copies/mL at diagnosis.
Characteristics of patients with PJP-IRISIn the following weeks after ART initiation, 6 out of 123 (4.9%) patients presented a PJP associated IRIS. All 6 cases were men with a median age of 34 (IQR: 8) years old. All of them had <200 CD4+ lymphocytes/μL and >100000 RNA copies/mL at the time of diagnosis of PJP. The 6 patients had been diagnosed by bronchoscopy with microbiological confirmation. The 6 patients developed a paradoxical IRIS. At the moment of IRIS, all 6 patients had completed treatment for PJP and were all receiving secondary prophylaxis with cotrimoxazol. The clinical presentation consisted of reappearance of fever and dyspnea, and worsening of previous bilateral radiological infiltrates in all cases. All but one had been treated with corticosteroids in the previous episode of PJP. Despite CMV was isolated in three of these patients, none of them received specific antiviral therapy with ganciclovir or foscarnet.
ART was begun between days 2 and 35 after the initiation of PJP treatment. Four out of 6 patients developed respiratory failure during IRIS episode and required intensive care unit admission. All 6 patients were treated with steroids and in none of the ART was interrupted. Two of them required noninvasive mechanic ventilation. However, the final outcome was favorable and all 6 patients survived. Tables 1 and 2 show the clinical characteristics and outcome of the patients of the study.
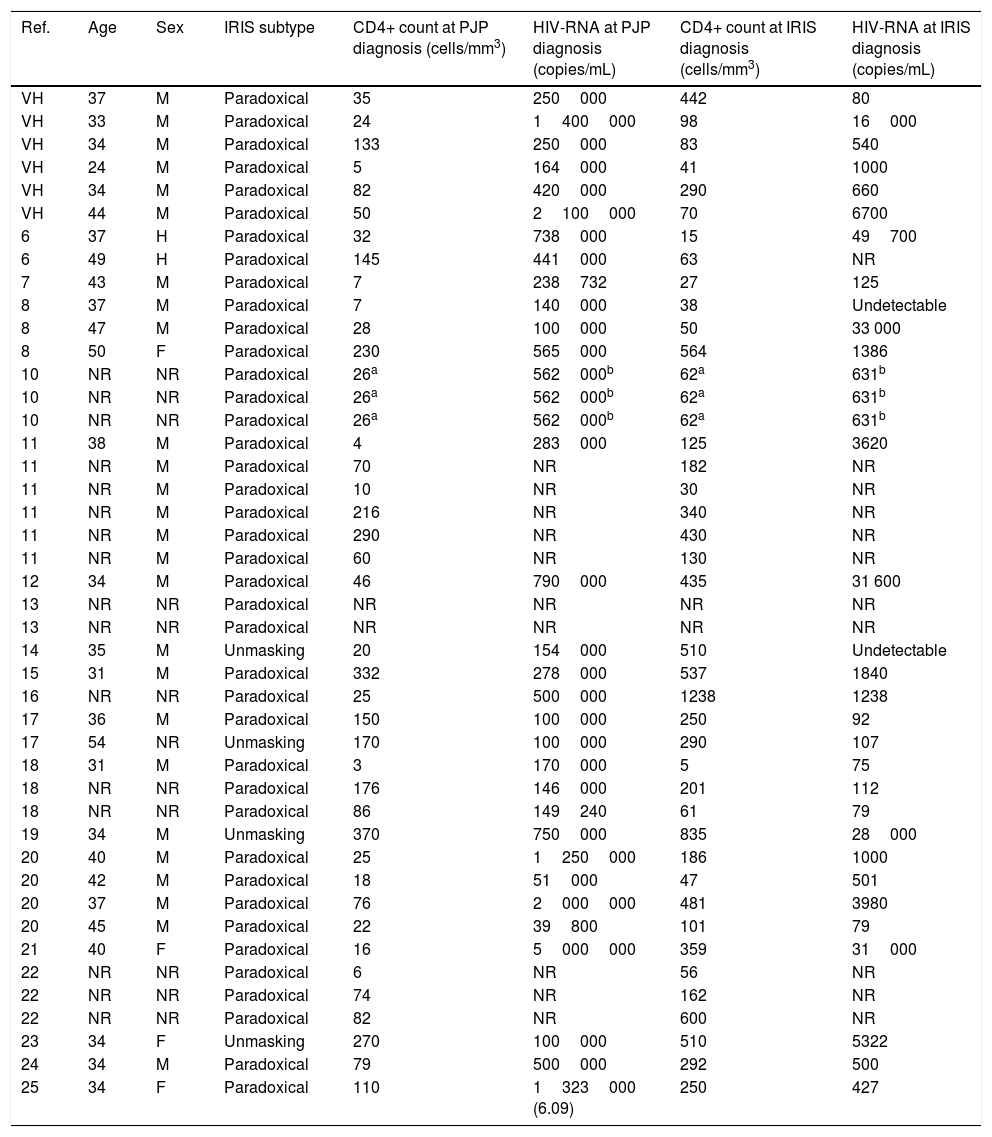
Characteristics of patients of Hospital Vall d’Hebron and cases reported in the literature with PJP IRIS.
| Ref. | Age | Sex | IRIS subtype | CD4+ count at PJP diagnosis (cells/mm3) | HIV-RNA at PJP diagnosis (copies/mL) | CD4+ count at IRIS diagnosis (cells/mm3) | HIV-RNA at IRIS diagnosis (copies/mL) |
|---|---|---|---|---|---|---|---|
| VH | 37 | M | Paradoxical | 35 | 250000 | 442 | 80 |
| VH | 33 | M | Paradoxical | 24 | 1400000 | 98 | 16000 |
| VH | 34 | M | Paradoxical | 133 | 250000 | 83 | 540 |
| VH | 24 | M | Paradoxical | 5 | 164000 | 41 | 1000 |
| VH | 34 | M | Paradoxical | 82 | 420000 | 290 | 660 |
| VH | 44 | M | Paradoxical | 50 | 2100000 | 70 | 6700 |
| 6 | 37 | H | Paradoxical | 32 | 738000 | 15 | 49700 |
| 6 | 49 | H | Paradoxical | 145 | 441000 | 63 | NR |
| 7 | 43 | M | Paradoxical | 7 | 238732 | 27 | 125 |
| 8 | 37 | M | Paradoxical | 7 | 140000 | 38 | Undetectable |
| 8 | 47 | M | Paradoxical | 28 | 100000 | 50 | 33 000 |
| 8 | 50 | F | Paradoxical | 230 | 565000 | 564 | 1386 |
| 10 | NR | NR | Paradoxical | 26a | 562000b | 62a | 631b |
| 10 | NR | NR | Paradoxical | 26a | 562000b | 62a | 631b |
| 10 | NR | NR | Paradoxical | 26a | 562000b | 62a | 631b |
| 11 | 38 | M | Paradoxical | 4 | 283000 | 125 | 3620 |
| 11 | NR | M | Paradoxical | 70 | NR | 182 | NR |
| 11 | NR | M | Paradoxical | 10 | NR | 30 | NR |
| 11 | NR | M | Paradoxical | 216 | NR | 340 | NR |
| 11 | NR | M | Paradoxical | 290 | NR | 430 | NR |
| 11 | NR | M | Paradoxical | 60 | NR | 130 | NR |
| 12 | 34 | M | Paradoxical | 46 | 790000 | 435 | 31 600 |
| 13 | NR | NR | Paradoxical | NR | NR | NR | NR |
| 13 | NR | NR | Paradoxical | NR | NR | NR | NR |
| 14 | 35 | M | Unmasking | 20 | 154000 | 510 | Undetectable |
| 15 | 31 | M | Paradoxical | 332 | 278000 | 537 | 1840 |
| 16 | NR | NR | Paradoxical | 25 | 500000 | 1238 | 1238 |
| 17 | 36 | M | Paradoxical | 150 | 100000 | 250 | 92 |
| 17 | 54 | NR | Unmasking | 170 | 100000 | 290 | 107 |
| 18 | 31 | M | Paradoxical | 3 | 170000 | 5 | 75 |
| 18 | NR | NR | Paradoxical | 176 | 146000 | 201 | 112 |
| 18 | NR | NR | Paradoxical | 86 | 149240 | 61 | 79 |
| 19 | 34 | M | Unmasking | 370 | 750000 | 835 | 28000 |
| 20 | 40 | M | Paradoxical | 25 | 1250000 | 186 | 1000 |
| 20 | 42 | M | Paradoxical | 18 | 51000 | 47 | 501 |
| 20 | 37 | M | Paradoxical | 76 | 2000000 | 481 | 3980 |
| 20 | 45 | M | Paradoxical | 22 | 39800 | 101 | 79 |
| 21 | 40 | F | Paradoxical | 16 | 5000000 | 359 | 31000 |
| 22 | NR | NR | Paradoxical | 6 | NR | 56 | NR |
| 22 | NR | NR | Paradoxical | 74 | NR | 162 | NR |
| 22 | NR | NR | Paradoxical | 82 | NR | 600 | NR |
| 23 | 34 | F | Unmasking | 270 | 100000 | 510 | 5322 |
| 24 | 34 | M | Paradoxical | 79 | 500000 | 292 | 500 |
| 25 | 34 | F | Paradoxical | 110 | 1323000 (6.09) | 250 | 427 |
VH: patients of Hospital Vall d’Hebron; NR: not reported data in the original publication.
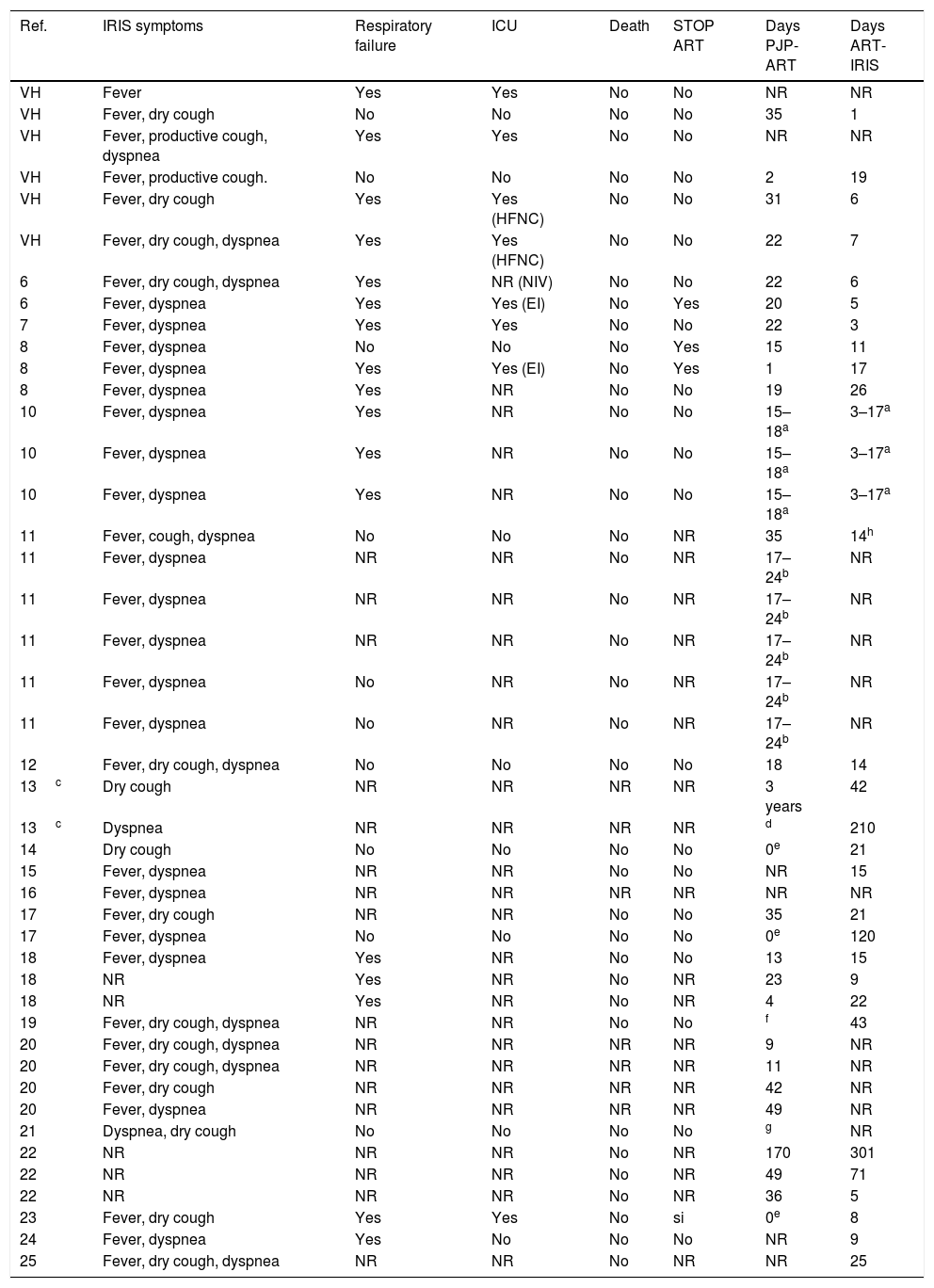
Clinical data and outcome of patients of Hospital Vall d’Hebron and cases reported in the literature with PJP IRIS.
| Ref. | IRIS symptoms | Respiratory failure | ICU | Death | STOP ART | Days PJP-ART | Days ART-IRIS |
|---|---|---|---|---|---|---|---|
| VH | Fever | Yes | Yes | No | No | NR | NR |
| VH | Fever, dry cough | No | No | No | No | 35 | 1 |
| VH | Fever, productive cough, dyspnea | Yes | Yes | No | No | NR | NR |
| VH | Fever, productive cough. | No | No | No | No | 2 | 19 |
| VH | Fever, dry cough | Yes | Yes (HFNC) | No | No | 31 | 6 |
| VH | Fever, dry cough, dyspnea | Yes | Yes (HFNC) | No | No | 22 | 7 |
| 6 | Fever, dry cough, dyspnea | Yes | NR (NIV) | No | No | 22 | 6 |
| 6 | Fever, dyspnea | Yes | Yes (EI) | No | Yes | 20 | 5 |
| 7 | Fever, dyspnea | Yes | Yes | No | No | 22 | 3 |
| 8 | Fever, dyspnea | No | No | No | Yes | 15 | 11 |
| 8 | Fever, dyspnea | Yes | Yes (EI) | No | Yes | 1 | 17 |
| 8 | Fever, dyspnea | Yes | NR | No | No | 19 | 26 |
| 10 | Fever, dyspnea | Yes | NR | No | No | 15–18a | 3–17a |
| 10 | Fever, dyspnea | Yes | NR | No | No | 15–18a | 3–17a |
| 10 | Fever, dyspnea | Yes | NR | No | No | 15–18a | 3–17a |
| 11 | Fever, cough, dyspnea | No | No | No | NR | 35 | 14h |
| 11 | Fever, dyspnea | NR | NR | No | NR | 17–24b | NR |
| 11 | Fever, dyspnea | NR | NR | No | NR | 17–24b | NR |
| 11 | Fever, dyspnea | NR | NR | No | NR | 17–24b | NR |
| 11 | Fever, dyspnea | No | NR | No | NR | 17–24b | NR |
| 11 | Fever, dyspnea | No | NR | No | NR | 17–24b | NR |
| 12 | Fever, dry cough, dyspnea | No | No | No | No | 18 | 14 |
| 13c | Dry cough | NR | NR | NR | NR | 3 years | 42 |
| 13c | Dyspnea | NR | NR | NR | NR | d | 210 |
| 14 | Dry cough | No | No | No | No | 0e | 21 |
| 15 | Fever, dyspnea | NR | NR | No | No | NR | 15 |
| 16 | Fever, dyspnea | NR | NR | NR | NR | NR | NR |
| 17 | Fever, dry cough | NR | NR | No | No | 35 | 21 |
| 17 | Fever, dyspnea | No | No | No | No | 0e | 120 |
| 18 | Fever, dyspnea | Yes | NR | No | No | 13 | 15 |
| 18 | NR | Yes | NR | No | NR | 23 | 9 |
| 18 | NR | Yes | NR | No | NR | 4 | 22 |
| 19 | Fever, dry cough, dyspnea | NR | NR | No | No | f | 43 |
| 20 | Fever, dry cough, dyspnea | NR | NR | NR | NR | 9 | NR |
| 20 | Fever, dry cough, dyspnea | NR | NR | NR | NR | 11 | NR |
| 20 | Fever, dry cough | NR | NR | NR | NR | 42 | NR |
| 20 | Fever, dyspnea | NR | NR | NR | NR | 49 | NR |
| 21 | Dyspnea, dry cough | No | No | No | No | g | NR |
| 22 | NR | NR | NR | No | NR | 170 | 301 |
| 22 | NR | NR | NR | No | NR | 49 | 71 |
| 22 | NR | NR | NR | No | NR | 36 | 5 |
| 23 | Fever, dry cough | Yes | Yes | No | si | 0e | 8 |
| 24 | Fever, dyspnea | Yes | No | No | No | NR | 9 |
| 25 | Fever, dry cough, dyspnea | NR | NR | No | NR | NR | 25 |
“two weeks later” in original publication.
ICU: intensive care unit; EI: endotracheal intubation; HFNC: high flow nasal cannula, NIV: non-invasive ventilation; NR: not reported in original publication; STOP ART: interruption of ART because of IRIS symptoms; Days PJP-ART: days between the beginning of PJP treatment and the beginning of ART. Days ART-IRIS: days between the beginning of ART and the beginning of IRIS symptoms.
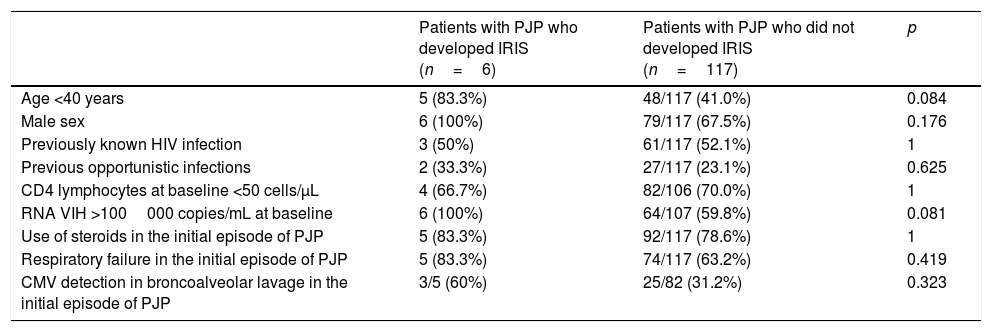
In Table 3 we show a comparison of epidemiological, clinical, and immuno-virological data present at baseline between those patients who developed a PJP associated IRIS and those who did not. In the univariate analysis, we observed a non-significant trend to a higher risk of IRIS in patients <40 years old (p=0.084) and in patients with HIV-RNA viral load >100000 copies/mL at baseline (p=0.081).
Characteristics of patients with PJP depending on the development of IRIS.
| Patients with PJP who developed IRIS (n=6) | Patients with PJP who did not developed IRIS (n=117) | p | |
|---|---|---|---|
| Age <40 years | 5 (83.3%) | 48/117 (41.0%) | 0.084 |
| Male sex | 6 (100%) | 79/117 (67.5%) | 0.176 |
| Previously known HIV infection | 3 (50%) | 61/117 (52.1%) | 1 |
| Previous opportunistic infections | 2 (33.3%) | 27/117 (23.1%) | 0.625 |
| CD4 lymphocytes at baseline <50 cells/μL | 4 (66.7%) | 82/106 (70.0%) | 1 |
| RNA VIH >100000 copies/mL at baseline | 6 (100%) | 64/107 (59.8%) | 0.081 |
| Use of steroids in the initial episode of PJP | 5 (83.3%) | 92/117 (78.6%) | 1 |
| Respiratory failure in the initial episode of PJP | 5 (83.3%) | 74/117 (63.2%) | 0.419 |
| CMV detection in broncoalveolar lavage in the initial episode of PJP | 3/5 (60%) | 25/82 (31.2%) | 0.323 |
CMV: citomegalovirus.
We identified a total of 38 PJP-related IRIS cases reported in 19 publications. Demographic data, clinical characteristics, laboratory parameters and outcome were taken from the original publications. All this information, together with our 6 cases, is summarized in Tables 1 and 2.
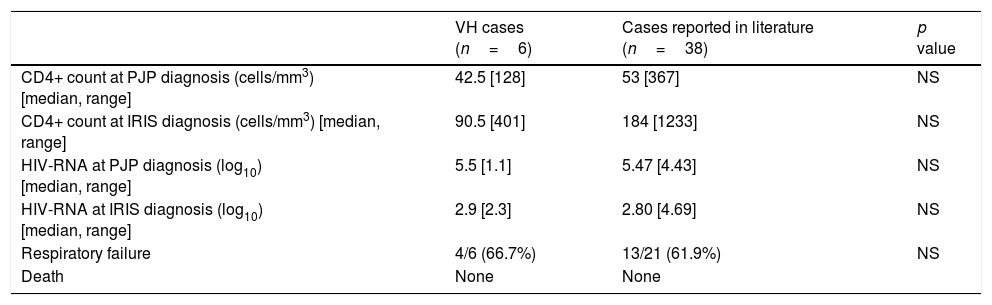
Considering the 38 cases published in the literature and the 6 diagnosed in our hospital, 5 out of 44 (11.4%) were unmasking IRIS. Seventeen out of the 27 (62.9%) patients in which this information was available developed respiratory failure. Need of intensive care admission was documented in 8 out of these 27 (29.6%) cases. None of the 44 patients died due to IRIS episode. We have compared different data between our patients and those reported in the literature (Table 4) and we have not found significant differences regarding CD4 count and viral load at baseline and IRIS diagnosis. The presentation of patients who developed respiratory failure was similar in both groups.
Comparison between characteristics of patients of Hospital Vall d’Hebron and cases reported in the literature with PJP IRIS.
| VH cases (n=6) | Cases reported in literature (n=38) | p value | |
|---|---|---|---|
| CD4+ count at PJP diagnosis (cells/mm3) [median, range] | 42.5 [128] | 53 [367] | NS |
| CD4+ count at IRIS diagnosis (cells/mm3) [median, range] | 90.5 [401] | 184 [1233] | NS |
| HIV-RNA at PJP diagnosis (log10) [median, range] | 5.5 [1.1] | 5.47 [4.43] | NS |
| HIV-RNA at IRIS diagnosis (log10) [median, range] | 2.9 [2.3] | 2.80 [4.69] | NS |
| Respiratory failure | 4/6 (66.7%) | 13/21 (61.9%) | NS |
| Death | None | None |
VH: Vall d’Hebron; NS: non significant.
In our study we report 6 cases of PJP related IRIS out of 123 patients with PJP which represents an incidence of 4.9%. This incidence of IRIS after an episode of PJP is similar to the 4% (3 out of 72 patients) in a previous retrospective study and much lower than the incidence of IRIS associated to other common opportunistic infections such as tuberculosis or criptococcosis.8 In one prospective randomized study that compared early versus deferred antiretroviral therapy after an acute opportunistic infection that included 282 subjects, IRIS was uncommon (7%). Even in those patients with baseline conditions associated with IRIS (low CD4 cell count, ART initiation within first 2 weeks of opportunistic infections diagnosed and good immunovirological response after ART initiation), IRIS incidence remained low.4 In this study most patients (63%) had PJP and probably this is why the incidence of IRIS was lower than that expected.
The reason why IRIS seems to occur less frequently after an episode of PJP than with other opportunistic infections remains unclear. There are some explanations that can be argued. It is possible that the fungal load after institution of therapy against PJ decreases rapidly and the amount of remaining antigens when ART is initiated is too low to trigger an exuberant immunological response as occurs with other cases of IRIS. According to that, infections caused by microorganisms with a slower decay of the bacterial burden once treatment is established, have been associated with higher rates of IRIS. Indeed, rates of IRIS up to 20–26% have been observed in criptococcal meningitis or tuberculosis infection.26–28 Therefore, the slower decay of microorganism burden seems to have a role in IRIS occurrence. Previous corticoid treatment has also been proposed as a possible explanation. However, previous publications have found o relation between corticoid treatment during different opportunistic infections and IRIS development.4,13,22 In our study, only one patient did not received corticoid treatment during previous PJP, so we were not able to asses this issue.
We have made an analysis in order to find factors that could be associated to the development of IRIS. However we have not found any significant variable that could help us to identify those patients with greater risk to develop IRIS, probably because of the small number of subjects with PJP related IRIS reported in our series. The only variables that had a non significant trend to identify these patients were a high viral load at baseline and age <40 years old. The relation between IRIS and age has been described previously.29 In older patients the loss of the thymic function makes CD4 recovery slower and lower.29 Conversely in younger subjects a stronger and faster immunological restoration is expected after the initiation of ART which could also play a role in an overexpressed inflammatory response.11
From a clinical point of view it is worth to note that most of the patients with PJP related IRIS presented with a severe clinical picture. In our cohort 4 out of 6 patients developed respiratory failure that required intensive care unit admission when IRIS was diagnosed. When we have reviewed together our cases and those previously published in the literature up to 50% of patients had respiratory failure at the moment of IRIS diagnosis. Despite clinical severity at presentation the clinical outcome of these patients was satisfactory and no patient died in our series. Similarly none of the 42 patients previously published in the literature died due to the IRIS episode compared to other opportunistic infections, especially in central nervous system opportunistic infections, in which IRIS is a severe and frequently fatal event.
Our study has some limitations due to its observational design. Moreover the low number of patients with IRIS does not allow identifying risk factors for the development of IRIS in patients with PJP who initiate ART.
In conclusion, although PJP-related IRIS is less frequent than other OI-related IRIS, it usually causes respiratory failure and further ICU admission. Despite its severity, clinical outcome is usually favorable.
Conflict of interestsThe authors confirm they don’t have any conflict of interest.
The authors are supported by Research Network RD12/0017/0003. Acción Estratégica en Salud. Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica 2008–2011. Instituto de Salud Carlos III. Fondos FEDER.