The first pneumococcal conjugate vaccine introduced in 2006 in the paediatric immunization calendar of the Madrid region was the 7-valent (PCV7), which was substituted in 2010 by the 13-valent pneumococcal conjugate vaccine (PCV13). In 2012, PCV13 was withdrawn from the Madrid paediatric public immunization programme, being prescribed privately. In 2015, the PCV13 was re-introduced at the Spanish national paediatric public vaccine scheme.1 The Quellung reaction is the gold standard for pneumococcal serotyping. This method requires strains isolated in culture. However, in samples as parapneumonic effusion (PPE) and pleural empyema (PE), commonly grouped as PPE/PE,2 the bacterial growing is fastidious and the availability of colonies may be difficult. The objective of this work was to identify the not growing Streptococcus pneumoniae serotypes (SPNGST) causing pleural infection in children, in which the standard microbiological culture shows negative results.
Thirty-five PFS from paediatric patients (aged six months to 8 years; mean 3.4 years, standard deviation 2.1) with negative culture results, obtained between September 2018 and December 2022, were processed for the detection of the α-fucosidase gene using a real time PCR method (Streptococcus pneumoniae alpha-fucosidase gene Genesig® Advanced Kit; Primerdesign Ltd, United Kingdom). Positive α-fucosidase samples were subsequently tested by a PCR reverse-hybridization strip-based assay (S. PneumoStrip test; Operon S.A., Zaragoza, Spain) that allows serotype identification.
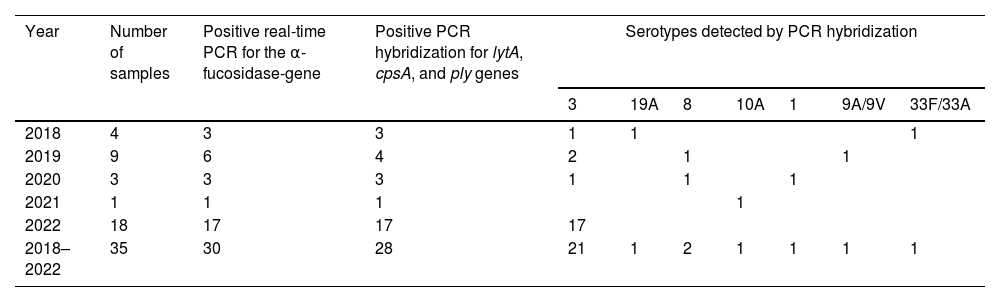
The α-fucosidase real-time PCR method was positive in 30 of the 35 samples studied (85.7%). The PCR reverse-hybridization assay showed positive results for the lytA, cpsA and ply genes in 28 of the 30 samples (93.3%). The serotypes detected by this technique in these 28 samples were twenty-one serotype 3 (75%), two serotype 8 (7.1%), one serotype 19A (3.6%), one serotype 1 (3.6%), one serotype 10A (3.6%), one 9A/9V (3.6%) and one 33F/33A (3.6%). Table 1 shows the distribution of PCR SPNGST detected in PFS according to time. Most serotype 3 cases occurred in 2022 (17/21). The only one PCV13 covered serotype 19A was identified in 2018. The only one PCV13 serotype 1 case was detected in 2020. The number of serotype 3 cases detected in PFS after 2020 was significantly higher (Fisher exact test p<0.01) than the rest of the serotypes. Among the twenty-one children infected by serotype 3, one had received one dose of PCV13, another one two doses, fifteen children received three doses, one child four doses, and in three this data was unknown. The two cases caused by serotype 19A received two PCV13 doses and the case due to serotype 1 received four.
Distribution of not-growing Streptococcus pneumoniae serotypes detected by PCR in pleural fluid samples according to the year the sample was obtained.
| Year | Number of samples | Positive real-time PCR for the α-fucosidase-gene | Positive PCR hybridization for lytA, cpsA, and ply genes | Serotypes detected by PCR hybridization | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 19A | 8 | 10A | 1 | 9A/9V | 33F/33A | ||||
| 2018 | 4 | 3 | 3 | 1 | 1 | 1 | ||||
| 2019 | 9 | 6 | 4 | 2 | 1 | 1 | ||||
| 2020 | 3 | 3 | 3 | 1 | 1 | 1 | ||||
| 2021 | 1 | 1 | 1 | 1 | ||||||
| 2022 | 18 | 17 | 17 | 17 | ||||||
| 2018–2022 | 35 | 30 | 28 | 21 | 1 | 2 | 1 | 1 | 1 | 1 |
Although the number of cases included is small and this is not a systematic epidemiological study, the elevate frequency of detected SPNGST 3 in PFS is worrying. Serotype 3 is a well-identified cause of pleural PPE/PE in children and these cases are increasing in the PCV13 era.2–4 We do not have a definitive explanation for the high number of cases of serotype 3 in 2022. The synergism between Influenza virus and S.pneumoniae5 would to have influenced in this fact. During 2022, the number of influenza cases in the region of Madrid was higher than in the previous season and affected children to a great extent.6
Despite the fact that the use of PCV13 in children has led to a substantial reduction of IPD in Spanish children7 and pneumococcal PPE/PE (especially associated to the drop of serotype 1),8 the immunogenicity and the effectiveness against each of the 13 serotypes of the vaccine seems to be non-homogeneous.3,4 The immunogenic activity has been described lower for serotype 3 than for other PCV13 serotypes,9 and the individual vaccine protection against this serotype is also lower.10 Moreover, the sensitivity of culture seems to be particularly very low for this serotype. Thus, serotype 3 prevalence data in pleural infection may be underestimated when surveillance is only based in culture and conventional phenotypic serotyping techniques. The routine implementation of PCR procedures in order to identify the serotype involved in culture-negative specimens provides additional information and can improve substantially the microbiological investigation of Streptococcus pneumoniae pleural infections. This approach can support epidemiological surveillance and monitoring of immunization plans.4
To the Microbiological Laboratory Services of the Hospital Universitario 12 de Octubre, Hospital Clínico San Carlos, Hospital Universitario de Getafe, Hospital General Universitario Gregorio Marañón and Hospital Infantil Universitario Niño Jesús from Madrid for sending the pleural fluid samples to Laboratorio Regional de Salud Pública de la Comunidad de Madrid during the study period.