In the present update of the guidelines, starting antiretroviral treatment is recommended in symptomatic patients, in pregnant women, in sero-discordant couples with high transmission risk, in patients co-infected with hepatitis B requiring treatment and in patients with HIV-related nephropathy. Guidelines on combined antiretroviral treatment (cART) are included in the event of concurrent HIV infection diagnosis with an AIDS-defining event. In asymptomatic naïve patients, cART will be based on CD4 lymphocyte count, plasma viral load (VL), patient age and patient comorbidity: (i) cART is recommended if CD4 count is lower than 350cells/μL; (ii) cART is equally recommended if CD4 count is between 350 and 500cells/μL and may only be deferred in the event of patient refusal with stable CD4 count and low VL; (iii) if CD4 count is higher than 500cells/μL cART can be delayed, but it may be considered in patients with liver cirrhosis, chronic virus C hepatitis, high cardiovascular risk, VL >105copies/mL, CD4 proportion lower than 14% and age over 55 years. cART in naïve patients requires a combination of three drugs and its aim is to achieve undetectable VL. Treatment adherence plays a basic role in sustaining good response. cART could and should be changed if virologic failure occurs in order to achieve undetectable VL again. Approaches to cART in HIV acute infection, in women and pregnancy and post exposure prophylaxis are also commented on.
En la presente actualización se recomienda iniciar el tratamiento en los pacientes sintomáticos, en las embarazadas, en las parejas serodiscordantes con alto riesgo de transmisión, en la hepatitis B que requiera tratamiento y en la nefropatía relacionada con el VIH. En caso de diagnóstico simultáneo de infección VIH y evento definitorio de sida, se incluyen directrices sobre el inicio del tratamiento antirretroviral (TAR). En los pacientes asintomáticos el inicio de TAR se basará en la cifra de linfocitos CD4, la carga viral plasmática, la edad y las comorbilidades del paciente: 1) Si los linfocitos CD4 son inferiores a 350 células/μL se recomienda TAR; 2) Igualmente se recomienda si la cifra de linfocitos CD4 se encuentra entre 350 y 500 células/μL y sólo podría diferirse en caso de poca disposición del paciente cuando los CD4 se mantienen estables y la CVP es baja; 3) Si los linfocitos CD4 son superiores a 500 células/μL se puede diferir el tratamiento, pero puede considerarse en los pacientes con cirrosis hepática, hepatitis crónica por virus C, riesgo cardiovascular elevado, CVP >105 copias/mL, proporción de CD4 inferior a 14% y edad superior a 55 años. El TAR inicial requiere tres fármacos y el objetivo es conseguir CVP indetectable. La adherencia juega un papel fundamental en la duración de la respuesta. En caso de fracaso virológico se debe y puede conseguir de nuevo CVP indetectable. Se comentan los criterios de TAR en la infección aguda, en la mujer, el embarazo y la profilaxis postexposición.
Combined antiretroviral treatment (cART) is evolving so quickly that GESIDA and the Spanish National Plan on AIDS update their Treatment Guidelines annually. A Panel of experts has revised the advances published or reported to conferences in the last year and updated them. This is an executive summary of current guidelines.1
After more than 20 years of cART experience, a series of general principles have been defined on which this treatment is based. Decisions on treatment are taken taking into account the patient's clinical situation, CD4 lymphocyte count and plasma viral load. The goal of cART is to achieve VL <50copies/mL or under the detection threshold. In order to achieve this goal we need three different drugs and a high level of patient adherence to treatment, this being essential to sustain good virologic response. By inhibiting viral replication it is possible to achieve a variable restoration of the immune system depending on previous damage level. Several negative aspects of cART have also been defined, such as adverse effects, frequent drug-drug interactions and the presence of resistant mutations when therapeutic drug levels are not achieved. By taking these principles into account and selecting available drugs, it is possible to design multiple treatment combinations. Clinicians should know patient characteristics in order to offer him/her the most suitable treatment and instruct on HIV transmission prevention measures.
How to monitor cARTA limited number of parameters are needed to monitor cART.
CD4 T-cell count. CD4 count is the main risk marker of opportunistic events and sets out when to start cART.
Recommendation:
- 1.
CD4 count should be frequently monitored as it is the most important parameter in determining when to start cART (A-I).
Plasma HIV RNA (viral load) testing. Viral load is a secondary determinant of starting cART and its fast and sustained suppression is a marker of cART effectiveness.
Recommendations:
- 1.
Viral load should be measured jointly with CD4 T-cell count (A-II).
- 2.
Viral load is the most important indicator of response to antiretroviral therapy and is the main parameter to assess, change and define failures in cART (B-I).
- 3.
Viral load should be measured with a technique with a detection threshold of at least 50copies/mL. It is convenient not to change the method (A-I).
- 4.
If a decision is to be taken after a plasma viral load testing result, this result should be confirmed before (with another test) (A-II).
Drug-resistance testing. Viral genome mutations are the consequence of rapid HIV-1 turnover and the error prone reverse transcriptase. There is a relationship between the appearance of resistant mutations and virologic failure. Resistance mutations can be either primary or secondary to virologic failure.
Recommendations:
- 1.
Genotypic resistance assay is recommended in clinical practice at diagnosis, when starting cART (if more than 1 year from previous determination), in pregnant women with detectable viral load, in virologic failure, and in post-exposition prophylaxis (to the source) (B-II).
- 2.
HIV subtype should be determined in immigrant patients or if there is quick clinical progression (CIII).
Plasma drug levels. Plasma concentrations of antiretroviral drugs correlate with effectiveness or toxicity; therefore ascertaining drug levels may be useful to optimize drug doses.
Recommendation:
- 1.
Measuring plasma drug levels of antiretroviral agents may be helpful in the management of specific clinical situations such as drug-drug interactions, in transplant recipients, severe underweight or obesity, and kidney or liver failure (C-III).
HLA B*5701 screening. The presence of the HLA-B*5701 allele is related to a hypersensitivity reaction (HSR) to abacavir (ABC), a life threatening multiorgan clinical syndrome seen during the first 6 weeks of treatment.
Recommendations:
- 1.
Screening for HLA-B*5701 should be performed at diagnosis or before starting an ABC-containing regimen (A-I).
- 2.
If HLA B*5701 is positive ABC should not be prescribed (A-I).
- 3.
Negative tests do not rule out completely a future HSR in the future, so the patient should be informed about this possibility (A-I).
HIV-1 co-receptor tropism assays. Tropism assay is useful when prescribing maraviroc.
Recommendations:
- 1.
A tropism assay should be performed whenever the use of a CCR5 inhibitor is being considered (A-I).
- 2.
Tropism assay is also recommended to patients on virologic failure when salvage therapy is to be been considered (A-III).
Acute HIV infection is symptomatic in more than half of the cases, but this is rarely recognized because symptoms are similar to a common viral infection.
It has been observed that progression to AIDS is faster in patients with certain characteristics such as symptom type, viral load, CD4 count and viral tropism. Taking these parameters into account we should choose whether or not to start cART. At the present time, starting cART during acute infection is controversial as the long-term potential benefit is not known.
Recommendations:
- 1.
cART is recommended in acute HIV-infection if: (i) there is neurological involvement (meningitis, encephalitis, Guillain-Barré syndrome, etc.) or any other organ or system is affected (hepatitis, myocarditis, thrombocytopenia, etc.); (ii) the acute symptoms last for more than 7 days; (iii) an immunosuppression related event is diagnosed; or (iv) in case of severe immunosuppression (CD4 T-cell <350/μL) (B-II).
- 2.
Starting treatment should be considered when there is a high risk HIV-1 transmission (A-II).
- 3.
Drug-resistance testing and co-receptor tropism assay should be performed at diagnosis (of an acute or recent HIV infection), regardless of starting cART or not (B-II).
- 4.
If cART is to be started, the same drug-regimens recommended in chronic infection should be used. Raltegravir (RAL) and 2 nucleoside reverse transcriptase inhibitors (NRTI) (tenofovir/emtricitabine [TDF/FTC] of preference) would be of choice, due to the faster reduction in plasma viral load and higher concentration in genital secretions which may help in reducing HIV transmission (B-III).
- 5.
If drug-resistance testing result is not available, a boosted protease inhibitor (PI)-based regimen is preferred until this result is available (A-II).
- 6.
Antiretroviral therapy is a lifelong therapy (A-I).
- 7.
If acute infection is not treated, patients should be evaluated in a 4-6 months schedule as a chronic infection (A-I).
The objectives of antiretroviral treatment are reduction of related morbidity and mortality, improving the quality of life, restoring and preserving the immune system, suppressing viral load maximally and persistently and preventing HIV transmission.
Recommendations:
- 1.
cART should be initiated depending on the clinical manifestations, CD4 T-cell count, plasma viral load and comorbidities (A-II).
- 2.
cART should be initiated if a symptomatic infection (clinical B or C event, CDC 2003 classification) is diagnosed (A-I).
- 3.
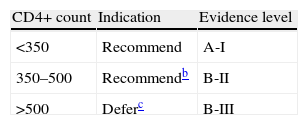
In asymptomatic patients, starting cART is determined by CD4 count, viral load and certain comorbidities and characteristics (Table 1):
Table 1.cART indication for asymptomatic patients with chronic HIV infection.a
acART will be always recommended, regardless CD4 cell count, in pregnant women, in serodiscordant couples with high transmission risk, in patients co-infected with hepatitis B requiring treatment and in patients with HIV-related nephropathy.
- -
cART is recommended if CD4 counts are <350cells/μL (A-I).
- -
Patients with CD4 counts between 350 and 500cells/μL should start cART. This may be deferred in weak commitment patients if CD4 counts are stable and viral load is low (B-II).
- -
In patients whose CD4 are >500cells/μL the benefit of starting or deferring cART is still unknown, but cART should be recommended for patients with liver cirrhosis, chronic hepatitis C, plasma viral load >105copies/mL, CD4 proportion <14%, age over 55 years, high cardiovascular risk and neurocognitive disorders (C-III).
4. Nevertheless, starting cART is recommended regardless of CD4 counts:
- -
In sero-discordant couples if there is a high risk of sexual transmission, in order to reduce it (A-I). cART should not substitute in any case other preventive measures to avoid HIV-1 transmission (A-II).
- -
In pregnant women (A-I).
- -
In HIV-associated nephropathy (A-II).
- -
In patients with hepatitis B co-infection requiring treatment (A-II).
In spite of the above recommendations, commencing cART should always be assessed on a case-by-case basis. Before starting cART, plasma viral load and CD4 counts should be confirmed and clinicians should prepare patients to accept the treatment, adjusting cART to patient lifestyle, comorbidities, drug–drug interactions and assessing risk of poor adherence (A-III).
What to start with?The preferred cART for naïve patients consists of a combination of at least three drugs including 2 NRTIs plus a boosted PI, or a non-nucleoside reverse transcriptase inhibitor (NNRTI) or an integrase inhibitor (IntIn) (Table 2). Considerations regarding the choice of a NNRTI, a boosted PI or an IntIn:
- -
As initial treatment, a combination of 2 NRTIs+1 NNRTI, or 2 NRTIs+1 boosted PI or 2 NRTIs+1 IntIn can be used (preferred agents are described further on) (A-I).
Preferred treatment combinations for treatment-naïve patients.a.
| 3rd drug | Regimenb |
| NRTIs | TDF/FTC/EFV1,2,3 |
| ABC/3TC+EFV1,2,4,5c | |
| TDF/FTC+NVP2,3,6c | |
| PI/r | TDF/FTC+ATV/r3,7 |
| TDF/FTC+DRV/r3 | |
| TDF/FTC+LPV/r3,8c | |
| ABC/3TC+ATV/r4,5,7c | |
| ABC/3TC+LPV/r5,8c | |
| IntIn | TDF/FTC+RAL3 |
aOrdered by 3rd drug and preference according to a structured and objective method of evaluation designed by GESIDA. The use of co-formulated drugs is recommended. There are not enough data regarding the therapeutic equivalence of FTC and 3TC, so the use of one or the other in the selected regimens depends basically on the available experience of use combined with the other drugs.
bComments show different issues with respect to the regimen, but they do not intend to be exhaustive guidelines on the precautions to be taken when using the drugs. To know more it is recommended to revise the text of the document as well as drug fact sheets.
cThese regimens have not been supported as preferred by all members of the panel.
TDF, tenofovir; FTC, emtricitabine; EFV, efavirenz; ABC, abacavir; 3TC, lamivudine; NVP, nevirapine; ARV/r, boosted atazanavir; DRV/r, boosted darunavir; LPV/r, boosted lopinavir; RAL, raltegravir
- 1.
To be avoided in women wanting to be mothers and in patients with unstable neuropsychiatric impairments. To be used with precaution in patients performing dangerous activities if they present somnolence, dizziness and/or impaired concentration.
- 2.
It is mandatory to perform a drug-resistance testing to discard resistance mutations to NNRTIs.
- 3.
To be used with precaution in patients with risk factors of kidney failure. Not indicated if GFR <30mL/min).
- 4.
It is mandatory to perform a HLA-B*7501 screening. Do not use if the screening is positive.
- 5.
Higher risk of virologic failure than using TDF/FTC/EFV in patients with VL >100,000copies/mL.
- 6.
Do not start in women with CD4 count >250cell/μL nor in men with CD4 cell count >400cell/μL.
- 7.
To be avoided when using proton pump inhibitors.
- 8.
To be used with precaution in patients with hyperlipidemia and/or high cardiovascular risk.
Nucleoside reverse transcriptase inhibitors
Preferred combinations:
- 1.
NRTI preferred drugs for initial treatment are TDF/FTC or abacavir/lamivudine (ABC/3TC) (A-I).
- 2.
If possible a co-formulated fixed-dose combination of NRTIs is recommended (A-I).
- 3.
TDF/FTC should be used with caution in patients with kidney dysfunction (B-II).
- 4.
ABC/3TC may be used with caution in patients with viral load >100,000copies/mL, especially when the third drug is a NNRTI (A-I).
Non-nucleoside reverse transcriptase inhibitors
Only nevirapine and efavirenz are authorized for treatment in naïve patients.
Recommendations:
- 1.
Efavirenz (EFV) is generally preferred to nevirapine (NVP). EFV has been studied in more trials and more experience has been accumulated (C-III).
- 2.
EFV is contraindicated during the first trimester of pregnancy, so other options should be recommended in women who do not use effective contraception. It is also to be avoided in patients performing dangerous tasks or with somnolence, dizziness and/or impaired concentration (B-III).
- 3.
NVP is contraindicated in women with CD4 >250cells/μL and in men with CD4 >400cell/μL (A-II).
Protease inhibitors
Different studies have shown that boosted PI (lopinavir [LPV/r], saquinavir [SQV/r], fosamprenavir [FPV/r], atazanavir ATV/r and darunavir [DRV/r]) offers some advantages concerning efficacy and genetic barrier when compared to a non-boosted PI. Side effects are also more frequent with boosted PI.
Recommendation:
- 1.
ATV/r QD, DRV/r QD, LPV/r BID or QD are the preferred PIs for naïve patients (A-I).
CCR5 co-receptor antagonists
These drugs prevent the entrance of HIV into the cell blocking the CCR5 co-receptor. They are exclusively active on R5-tropic virus.
Recommendation:
- 1.
Maraviroc (MVC) should only be used when a regimen containing a NNRTI, or PI or IntIn is not possible. HIV should be R5-tropic. This recommendation is based on the outcomes of the MERIT study (C-I).
Integrase inhibitors
IntIn prevents viral integrase from binding the reactive edges of viral DNA to the cell DNA.
Recommendation:
- 1.
RAL can be used in naïve patients twice a day, combined with TDF/FTC (A-I).
Initial cART in patients with an AIDS-defining condition
Several clinical trials have studied the appropriate moment of starting cART in patients with an AIDS-defining opportunistic infection.
Recommendations:
- 1.
Patients with a concurrent diagnosis of HIV infection and an AIDS-defining opportunistic infection should start cART as soon as possible (within the first month or, ideally, in the first two weeks) (A-I).
- 2.
Patients with cryptococcal (and tuberculous) meningitis on treatment should undergo close monitoring as they are difficult-to-treat opportunistic infections that may be worsened by the possible harmful consequences of immune restoration (B-II).
We define cART regimen simplification as the switch from a suppressive cART regimen that achieved viral suppression to another easier (“lighter”) regimen (reducing pill burden or dose frequency) that remains suppressive. The main goal of simplification is to improve quality of life, improve adherence and prevent or reverse some adverse effects.
Recommendations:
- 1.
Simplification should never mean a loss of optimal virologic control, and can only be offered to patients without previous virologic failure (A-I).
- 2.
The best candidates to undergo simplification are patients under prolonged virologic suppression (≥6 months) and good adherence (>90%) (B-II).
- 3.
Patients without previous failure to a PI-based cART, with undetectable viral load for ≥6 months, excellent adherence and with signs or symptoms of NRTI-related toxicity can simplify to a monotherapy regimen with LPV/r (BID) or DRV/r (QD) (B-I).
- 4.
A regimen containing a boosted PI plus 2 NRTIs without a previous virologic failure can substitute the PI for EFV, or NVP, or ABC. This switch to a single pill regimen can improve adherence (A-I).
- 5.
It is not recommended to simplify a PI to ABC if the patient has received suboptimal NRTI-based regimens (A-I).
- 6.
Simplification to ABC plus TDF+3TC or TDF+ddI (didanosine) is contraindicated (A-II).
- 7.
The simplification to NVP or RAL may add some metabolic advantages to patients with high cardiovascular risk (B-I).
- 8.
Patients on their Initial boosted PI regimens with undetectable HIV viral load can be simplified to a QD regimen such as EFV+TDF+3TC (or FTC), or EFV+ddI+3TC (o FTC), or ATV/r+TDF/FTC or ATV+ABC/3TC (B-I).
- 9.
Switching from enfuvirtide to RAL in virologically suppressed patients has proved to be safe and effective (A-I).
- 10.
Other possible simplifications should be performed in the context of a clinical trial and not in clinical practice (A-III).
Virologic failure is defined as detection of viral load >50copies/mL in two consecutive blood samples (or if it is detectable 24 weeks after starting therapy).
The factors related to virologic failure may depend of the patient, the therapeutic regimen and the virus. Adherence is the most important predictor of virologic response. Drug-related factors are the potency of the drug or insufficient plasma drug levels due to absorption problems or drug–drug interactions. And among the virus-related factors resistance is the most determining.
When considering a new antiretroviral treatment after virologic failure it is important to know the resistance mutation pattern and previous treatment history. Two scenarios are possible: early virologic failure (first or second failure and few resistance mutations) or advanced failure (there are more mutations to three or more drug families).
Changing cART in early virologic failure:
- 1.
A cART virologic failing regimen requires prompt switch to avoid cumulative mutations that enables the subsequent cART to get undetectable viral load (A-III).
- 2.
New cART regimen should contain 3 fully active agents according to the drug-resistance testing or their equivalence if only partially active drugs are being used (A-I).
- 3.
The goal of new cART is to achieve undetectable viral load (<50copies/mL) (A-I).
- 4.
Drug-resistance testing and a tropism assay should be done in order to tailor the best alternative regimen (A-II).
- 5.
Drug-resistance testing should be performed while the patient is still taking the failing regimen or as soon as he/she stops taking it. We have to take into account the previous mutations detected if there are previous genotypic tests (“cumulative genotype”) (A-II).
- 6.
When choosing a new cART regimen we should analyze the causes of virologic failure inadequate adherence, drug–drug interactions, the previous drug history and previous toxicities and resistance mutations (B-II).
- 7.
A failure on a first generation NNRTI-based regimen should be switched to a PI-based cART because a regimen containing etravirine is less effective and not a preferred choice (A-I).
- 8.
DRV/r (600/100mg BID) has proved to be superior to LPV/r BID as preferred boosted PI in a salvage regimen and is the preferred agent. This superiority can be shown statistically when protease has ≥1 primary mutations or when the fold-change of LPV/r is >10 (A-I). DRV/r 800/100mg QD dose can be used in patients who have not previously taken PI and/or do not present DRV specific mutations (A-I).
- 9.
Thymidine analogs should be avoided for salvage regimens if there are other choices (A-I).
Changing cART in advanced virologic failure (besides previous recommendations):
- 1.
The new cART regimen should be convenient, well-tolerated and as low toxic as possible (C-II).
- 2.
The use of TPV/r is restricted to cases in which the estimated residual activity is clearly superior to that of DRV/r BID and the use of etravirine is not required (combined use of etravirine and TPV/r is contraindicated) (A-II).
- 3.
cART structured treatment interruptions aiming to increase salvage regimen efficacy should not be performed (A-I).
- 4.
Patients with virologic failure and without any other treatment options should not stop cART; even when they are receiving a regimen with proved resistance. Once at this point we should seek a regimen based on drugs which reduce viral fitness without adding mutations (e.g. 3TC or FTC or TDF). CD4 count and viral load should be closely monitored (B-II).
- 5.
Dealing with patients with advanced virologic failure is complex. A clinician or virologist with experience in resistance mutations and cART salvage regimens should be consulted. He/she can have access to restricted drugs (in clinical assays) in order to offer a cART regimen with a chance of success (C-III).
Adherence is the patient's ability to commit to the choice, beginning and carrying out cART in order to achieve a suitable suppression of viral replication.
Recommendations:
- 1.
Patient should be prepared and clinicians should identify and correct causes that limit adherence before starting cART. If the patient is not ready, deferring cART is a better option (A-III).
- 2.
A first control should be done within the first 2–4 weeks after starting cART so inherent factors limiting adherence can be corrected (B-III).
- 3.
A suitable adherence should be monitored and reinforced at every patient visit (A-III).
- 4.
Adherence assessment should be performed by a multidisciplinary team involving not only clinicians and nursing staff but also psychologists and pharmacy staff (B-III).
- 5.
Each clinic setting should carry out a periodic follow-up of adherence in order to detect individual lack of treatment adherence to identify its magnitude. Analysis of these data could help to determine the causes of the detected problems (treatment abandonment or holidays, lost to follow-up, etc.) and create specific strategies for the patient and for the smooth running of the clinical team (B-III).
- 6.
In patients with an irregular adherence it is preferable to use a PI-based regimen rather than a NNRTI-based regimen to avoid resistance mutations selection (B-III).
- 7.
Co-formulated and QD fixed-dose make cART and long-term adherence easier. Using a single pill regimen is the most efficient strategy to prevent selective drug adherence (A-III).
Adverse effects of antiretroviral agents may appear either at the beginning of the treatment or in the mid-long term.
Recommendations:
- 1.
cART acute adverse events should be monitored during the first 2–4 weeks, especially in those patients showing comorbidities or taking other drugs that may interact with cART and have clinical consequences. There should be close patient-to-clinician contact on these weeks. cART (or any other treatment) should be modified according to the drug interactions or severe adverse effects (A-III).
- 2.
Agents that might worsen preexisting diseases should be avoided (A-III).
- 3.
Fasting glycaemia and plasma lipid levels (total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides) should be monitored at each control visit (A-II).
- 4.
Assessment of cardiovascular risk is recommended at least once a year (B-III).
- 5.
We recommend performing a basic urine analysis with proteinuria and renal glomerular filtration rate (calculated on MDRD equation or Cokcroft-Gault) at the first visit and then annually (if there is no nephrotoxicity risk factors) or every six months (if there are risk factors at baseline) (B-III). In patients on cART this study should be performed at every visit (A-III), particularly if they are taking TDF (A-II). If glomerular filtration rate is <50mL/min or there is proteinuria, TDF and IDV should be avoided and every NRTI dose should be adjusted, except ABC (A-III). TDF is not recommended in patients with acute renal failure or if the renal failure is TDF-related (A-III).
- 6.
In patients at risk of osteoporosis (post-menopausal women, smokers, low BMI, age over 50, lack of vitamin D, hepatitis C, renal failure, diabetes, CD4 count <250cell/μL or chronic steroid intake) a bone mineral density DXA scan is recommended at treatment start and then periodically (B-III).
- 7.
A bone metabolic balance is recommended (including 25-OH vitamin D) in patients with bone density loss, osteoporosis or frequent stress fractures (B-III).
Drug–drug interactions between cART agents and other drugs may incur serious clinical consequences. The most relevant consequences are PK interactions, especially those related to drug metabolism. The most important metabolic system is cytochrome P450 (CYP), its principal isoenzyme being CYP3A4. PIs, NNRTIs and some other drugs are inhibitors or inductors of different CYP isoenzymes.
Recommendations:
- 1.
All concomitant medication or natural and alternative medicine product should be registered, in order to assess potential interactions (B-III).
- 2.
Contraindications should be taken into account and dose adjustments should be made when necessary (B-III).
- 3.
Monitoring drug plasma levels should be considered when two or more drugs with potential relevant interactions are administered in order to avoid toxicity or inefficacy (B-II).
Chronic liver disease due to viral infection is the main relevant comorbidity among HIV-infected patients in Spain, given its frequency, the progression to end-stage liver disease and the increase in cART liver toxicity.
cART and natural progression of chronic hepatitis C and B
Recommendations:
- 1.
In hepatitis C virus (HCV) co-infected patients we recommend starting cART, regardless of CD4 T-cell count. This decision should be based on a case-by-case basis taking into account virological and histological factors and patient commitment (B-II).
- 2.
In hepatitis B virus (HBV) co-infected patients fulfilling hepatitis treatment criteria, cART regimen containing TDF should be started regardless of CD4 T-cell count (B-II).
- 3.
In HBV co-infected patients not requiring hepatitis treatment, a regimen including TDF should be preferred when starting cART (B-II).
cART liver toxicity in patients with previous liver disease
Liver toxicity has been described with every cART family drug, but incidence and pathogenesis mechanisms are different. The real incidence of liver toxicity is difficult to assess.
Recommendations:
- 1.
No cART agent is contraindicated with co-infection with HCV or HBV if liver function is preserved (B-II), but drugs with the less potential liver toxicity should be prioritized (C-III).
- 2.
cART should be discontinued in the event of symptomatic hepatitis. In asymptomatic cases it should be withdrawn if hepatitis is related to mitochondrial toxicity, hypersensitivity reaction or when transaminase elevation >10-fold upper normal limits (B-III).
- 3.
cART change should be considered in asymptomatic hepatitis with transaminase elevation 5–10-fold upper normal limits, taking into account the clinical, virological and immunological situation of the patient and exposure to cART drugs (B-III).
Management of cART in patients with liver disease
Chronic liver disease can alter absorption and metabolism of cART agents increasing their toxicity or modifying antiviral potency.
Recommendations:
- 1.
Every co-infected patient with hepatotropic viruses should be assessed for liver function and fibrosis as these can determine the cART chosen and dosed, and the scheduled monitoring for efficacy and toxicity (C-III).
- 2.
In chronic hepatitis without hepatic failure or mild hepatocellular damage (Child A) cART agents can be used at the usual doses but patients should be closely monitored if there is risk of toxicity (B-II).
- 3.
In chronic liver disease with signs of hepatocellular failure, cART agent doses should be adjusted. If possible plasma drugs levels should be determined (B-III).
- 4.
cART should be discontinued in the event of severe acute hepatitis, and reintroduced once the problem is solved (B-III).
cART in HCV treatment
The recommended treatment for chronic virus C hepatitis is pegylated interferon and ribavirin. HCV protease inhibitors can be added for patients infected by HCV genotype 1.
Recommendations:
cART and HCV treatment should not be initiated simultaneously (B-III).
- 1.
When treating both diseases (HIV-1 and HCV) patient should be closely monitored for the early detection of adverse effects (B-III).
- 2.
Ribavirin should not be combined with ddI or ZDV (A-I).
- 3.
If pegylated interferon and efavirenz are administered simultaneously, close monitoring of central nervous system adverse effects should be carried out (B-III).
- 4.
Telaprevir can be administered to a patient requiring cART without significant interactions to a regimen containing TDF, 3TC, FTC, ATV/r, RAL or EFV, although dose of telaprevir should be increased to 1125mg/8h when co-administered with EFV (B-I).
- 5.
Boceprevir co-administration is not recommended with boosted IPs or EFV (B-III).
Treatment of chronic hepatitis B in co-infected patients
Recommendations:
- 1.
Patients co-infected with HIV and HBV requiring treatment for whatever infection should start a cART regimen containing TDF+FTC (or 3TC) as NRTI (C-III).
- 2.
If we decide to treat HBV and defer the HIV-1 treatment, drugs that do not induce resistance mutations to HIV-1 are recommended (C-III).
- 3.
Entecavir should be avoided in co-infected patients except when HIV-1 replication is suppressed by other drugs (B-III).
- 4.
In VIH-1 and HBV co-infected patients in which 3TC, FTC or TDF are going to be discontinued no matter the reason, another agent with activity against HBV should be included (C-III).
HIV-1 infected patients have a greater risk of suffering from chronic kidney disease than the general population and the causes are the HIV-1 itself, HIV-related diseases and cART treatment or other nephrotoxic drugs.
Recommendations:
- 1.
In HIV-1 infected patients with chronic kidney disease it is mandatory to adjust cART drugs doses. Otherwise severe complications and potentially drug-drug interactions may appear (B-I).
- 2.
IPs, NNRTIs and RAL do not need dose adjustment (A-I).
Standard tuberculosis treatment in HIV-1 infected patients is as equally effective as in the general population, so recommendations are equally applicable to HIV-1 infected patients.
Recommendations:
- 1.
In HIV-1 infected patients with tuberculosis treatment, cART should be started during tuberculosis therapy as there is an increase in survival (A-I).
- 2.
In HIV-1 infected patients with tuberculosis and CD4 counts <50cell/μL, cART should be initiated within the first weeks after initiating tuberculous therapy in order to reduce the risk of death (A-I).
- 3.
In HIV-1 infected patients with tuberculosis and CD4 counts >50cell/μL, cART can be deferred until the induction phase of tuberculous therapy is completed in order to reduce adverse effects and IRIS without compromising survival (A-I).
- 4.
Tuberculous standard therapy in HIV-1 infected patients has the same efficacy as in the general population, so treatment guidelines for general population are equally applicable to HIV-1 infected patients (A-I).
- 5.
Rifampin-based regimens are preferred for the treatment of tuberculosis (A-I).
- 6.
Efavirenz 600mg/day-based cART regimen is the preferred cART for patients with tuberculosis (A-I).
- 7.
If it is not possible to use efavirenz, a nevirapine-based regimen is the alternative proposed (A-I).
- 8.
If it is not possible to use neither NVP nor EFV, there are no data to recommend an alternative cART. Other cART regimens based on RAL or MRV can be used, or a different strategy, such as using rifabutin (instead of rifampin) plus a boosted PI, may be considered (C-III).
HIV-2 contains a similar genomic organization as HIV-1, but displays some structural differences that significantly influence pathogenesis and sensitivity to antiretroviral drugs.
Recommendations:
- 1.
Preferred initial cART in HIV-2 infection is a combination of 2 NRTIs plus a boosted PI (C-III).
- 2.
cART regimens based on NNRTIs, MRV or ENF are contraindicated in HIV-2 infection (B-III).
More than 50% of HIV-1 infected people are women. In Europe there is an increase in sexually transmitted new infections among women. In Spain this pattern regarding new infections in women, specially affecting immigrant population has been confirmed.
Recommendations:
- 1.
Women cART initiation and objectives are the same as men's (A-III).
- 2.
NVP use in naïve women is different (can be used if CD4 <250cell/μL) due to the higher incidence of rash and liver toxicity (A-II).
- 3.
Contraception, current or future, should be taken into consideration when prescribing a cART agent. Hormonal contraception may interact with many antiretroviral drugs, so other contraception methods should be required (A-III).
cART in pregnancy and prevention of mother-to-child transmission (MTCT)
MTCT is the cause of HIV-1 infection in children. The first gestational weeks are the most vulnerable fetal period to drug toxicity during pregnancy. Therefore every potentially pregnant woman should receive information about drug teratogenicity and tailor her cART to her pregnancy desire.
Recommendations:
- 1.
HIV-1 testing should be performed on every pregnant woman (A-III). If risk practices exist the test should be repeated in the third trimester (C-III).
- 2.
In women who reach delivery without knowing her status concerning HIV-1, a rapid HIV-1 test should be performed because an elective Caesarean section reduce the transmission risk by 50% (A-II).
- 3.
cART objective is to achieve undetectable viral load (A-II). ZDV should be included in the cART regimen, except when there is resistance, previous severe toxicity, or reasonable doubts concerning the woman's adherence (when switching from an easier regimen). ZDV should be given during pregnancy, during labor (intravenous infusion) and to the newborn (A-I).
- 4.
A resistance-mutation test should be performed to every pregnant cART naïve woman or with detectable viral load (A-III).
- 5.
Teratogenic drugs (as EFV) should not be administered (B-III) and those whose risks are not well established should be avoided (fosamprenavir, darunavir, tipranavir, tenofovir, enfuvirtide, maraviroc, etravirine and raltegravir) (C-III).
- 6.
Combining ddI and d4T is contraindicated as there is risk of lactic acidosis (B-III).
- 7.
It is essential to plan getting undetectable viral load before labor (week 32–34). If an adequate low viral load is not achieved (<1000copies/mL) a Caesarean section should be programmed at weeks 37–38 (A-II).
HIV-1 epidemic continues to expand. Over the past decades several medical interventions have been launched to reduce the transmission. Some goals have been reached with programs, such as risk reduction practices among illicit drug users, men circumcision, condom use, control of STDs, reducing MTCT and between members of sero-discordant couples.
Pre-exposure prophylaxis
Pre-exposure prophylaxis can be an adequate strategy, but final evaluation would be needed after finishing the ongoing studies. At the present state of the art no recommendation has been offered in Europe.
Occupational post-exposure prophylaxis
cART initiation after an occupational exposure to HIV-1 reduces the risk of transmission. Globally, HIV-1 transmission risk after a percutaneous exposure to infected blood ranges from 0.24% to 0.65%.
Recommendations:
- 1.
Hospitals should have a written guideline for managing HIV-1 exposure, occupational or not, have a quick serological diagnosis kit available and quick, and fulltime access to cART for post-exposure prophylaxis (A-III).
- 2.
In order to prescribe post-exposure prophylaxis, the source patient (suspected or HIV positive), the serologic status of exposed health worker and characteristics of exposure episode should be considered before indicating PEP (A-III).
- 3.
Administration of post-exposure prophylaxis should be initiated as soon as possible (the best within 4h) and within 72h. Prophylaxis will be extended for 4 weeks (A-II).
- 4.
Starting post-exposure prophylaxis is not recommended after 72h post-exposure (A-III).
- 5.
Regimens for prophylaxis should contain 3 antiretroviral agents. Fixed-dose NRTI combinations are preferred (TDF+FTC or ZDV (zidovudine)+3TC) plus a boosted PI. If PI is contraindicated, EFV can substitute it. A 3 NRTI-based regimen: ZDV/3TC+TDF or TDF/FTC+ZDV can also be offered (A-III).
- 6.
Should a resistant virus be suspected in source patient, prophylaxis should include drugs without cross resistance (A-III).
- 7.
If there is any doubt as to the convenience of post-exposure prophylaxis, immediate first dose is recommended and reassessing its continuation within the next 24h with a HIV-1 expert (A-III).
- 8.
Follow-up should include an indication reassessment to PEP and monitoring adherence and tolerability within 24–72h. Screening for HIV-1, HBV, HCV (in infected source or highly suspicion) should be performed in months 1, 3 and 6 after exposure (B-III).
Non-occupational post-exposure prophylaxis
Non-occupational post-exposure prophylaxis is based on observational studies carried out in women who suffered sexual assault and in men who have sex with men, and on information given by other prophylaxis types and data from animal experiments.
Recommendations:
- 1.
Non-occupational post-exposure prophylaxis should be given on a case-by-case basis and within an integral medical approach (A-III).
- 2.
Non-occupational post-exposure prophylaxis should be recommended in those situations in which there are an “appreciable risk” of transmission and under these circumstances: (i) early initiation (similar to occupational post-exposure prophylaxis), (ii) lack of contraindication to cART agents, (iii) exceptional exposure, and (iv) when there is a guarantee of patient clinical and laboratory monitoring (B-III).
- 3.
Antiretroviral agents, length of prophylaxis and patient's follow-up will be equal to those of occupational post-exposure prophylaxis (A-III).
- 4.
In the event of a sexual exposure, other STD and pregnancy status should be evaluated (A-III).
Koldo Aguirrebengoa has carried out consultancy work in Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare; he has received grants for clinical research from Abbot Laboratories, GlaxoSmith Kline, and Merck; he has received fees for giving talks from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Roche, and ViiV Healthcare; and has received payments for developing educational presentations for Abbot Laboratories, Gilead Sciences, and GlaxoSmith Kline.
Antonio Antela has carried out consultancy work for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, and Janssen-Cilag; he has received fees for giving talks from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare, as well as payments for developing educational material for Boehringer Ingelheim, Gilead Sciences, and ViiV Healthcare.
José R Arribas has carried out consultancy work in Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare; he has received grants for clinical research from Janssen; he has received payments for giving talks from Abbot Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare, and has received payment for developing educational presentations for Janssen.
Víctor Asensi has carried out consultancy work for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Boehringer-Ingelheim, GlaxoSmith Kline, and ViiV Healthcare; he has received payments for giving talks from Abbot Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Boehringer-Ingelheim, GlaxoSmith Kline, and ViiV Healthcare; and has received payment for developing educational presentations for Bristol-Myers Squibb.
Juan Berenguer has carried out consultancy work in Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith-Kline, Janssen, Merck, and ViiV Healthcare; he has received grants for clinical research from Bristol-Myers Squibb; Glaxo Smith-Kline, and ViiV Healthcare; he has received payments for giving talks from Abbot Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith-Kline, Janssen, Merck, Roche, and ViiV Healthcare.
José Ramón Blanco has carried out consultancy work in Abbott Laboratories, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, and ViiV Healthcare; he has received payments for giving talks from Abbot Laboratories, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith Kline, Janssen, Merck, and ViiV Healthcare, as well as payments for developing educational presentations for Gilead Sciences and Bristol-Myers Squibb.
Vicente Boix has carried out consultancy work in Abbott Laboratories, Boehringer Ingelheim, GlaxoSmith Kline, Janssen, Merck, Pfizer, and ViiV Healthcare; he has received grants for clinical research from Gilead Sciences, GlaxoSmith Kline, Janssen, and Merck; he has received payments for giving talks from Janssen, Bristol-Myers Squibb, Merck, Pfizer, and ViiV Healthcare; and has received payment for developing educational presentations for Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmith Kline, Janssen, and Merck.
Pere Domingo has carried out consultancy work in Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare; he has received grants for clinical research from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare; and he has received payments for giving talks from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare.
Vicente Estrada has carried out consultancy work in Abbott Laboratories, Gilead Sciences, and Janssen; he has received grants for clinical research from Abbot Laboratories and Janssen; he has received payments for giving talks from Abbot Laboratories, Bristol-Myers Squibb, Gilead Sciences, Merck, and ViiV Healthcare; as well as payments for developing educational presentations for Abbott Laboratories.
Federico Garcia has carried out consultancy work in GlaxoSmith Kline, Merck, and ViiV Healthcare; he has received grants for clinical research from Merck and ViiV Healthcare; he has received payments for giving talks from Abbot Laboratories, Bristol-Myers Squibb, Merck, and ViiV Healthcare.
José M Gatell has carried out consultancy work in Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmith Kline, Merck, and ViiV Healthcare; he has received grants for clinical research from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmith Kline, Merck, and ViiV Healthcare; he has received payments for giving talks from Abbot Laboratories, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmith Kline, Merck, and ViiV Healthcare.
Félix Gutiérrez has carried out consultancy work in Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmith Kline, Merck, and ViiV Healthcare; he has received grants for clinical research from Merck; he has received payments for giving talks from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmith Kline, Merck, and ViiV Healthcare; as well as payments for developing educational presentations for Gilead Sciences.
Hernando Knobel has received payments for giving talks from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare.
Josep Maria Llibre has carried out consultancy work in Abbott Laboratories, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Pfizer, Roche, and ViiV Healthcare, and he has received payments for giving talks from Abbot Laboratories, Boehringer-Ingelheim, Gilead Sciences, GlaxoSmith-Kline, Jansen-Cilag, Merck Sharp & Dohme, Pfizer, Roche, and ViiV Healthcare; as well as payments for developing educational presentations for Boehringer-Ingelheim, Merck, and ViiV Healthcare.
José López Aldeguer has received financial reward from GESIDA for his work as editor, has carried out consultancy work in Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare; he has received grants for clinical research from ViiV Healthcare and Merck, he has received payments for giving talks from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare.
Josep Mallolas has carried out consultancy work in Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck, Janssen, Roche, and ViiV Healthcare; he has received grants for clinical research from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck, and ViiV Healthcare; he has received payments for giving talks from Abbot Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Roche, Merck, and ViiV Healthcare.
Esteban Martínez has carried out consultancy work in Abbott Laboratories, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec, and ViiV Healthcare; he has received payments for giving talks from Abbot Laboratories, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec, and ViiV Healthcare, as well as payments for developing educational presentations for Abbott Laboratories, Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, and ViiV Healthcare.
Celia Miralles has carried out consultancy work in Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare; she has received payments for giving talks from Abbot Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare; she has received payments for writing articles from Abbot Laboratories, Bristol-Myers Squibb, Gilead Sciences, and ViiV Healthcare; as well as payments for developing educational presentations for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare.
Jose M. Miro has carried out consultancy work in Abbott Laboratories, Bristol-Myers Squibb, Cubist, Gilead Sciences, Merck, Novartis, Pfizer, and Theravance, he has received grants for clinical research from Cubist, Novartis, the Health Research Fund (Fondo de Investigaciones Sanitarias (FIS)) and the Carlos III Health Institute (Instituto de Salud Carlos III (Madrid)), Foundation for Research and Prevention of AIDS in Spain (Fundación para la Investigación y Prevención del Sida en España) (FIPSE, Madrid)), Ministry of Health, Social Policy and Equality (Ministerio de Sanidad, Politica Social e Igualdad (MSPSI), Madrid)), National Institutes of Health (NIH, Bethesda, MA, USA); he has received payments for giving talks from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Cubist, Glaxo Smith Kline, Gilead Sciences, Janssen, Merck, Novartis, Pfizer, Roche, Schering-Plough, Theravance, and ViiV Healthcare. Dr. In 2011, JM Miró received a grant (INT 10/219) from the Intensification of Research Activity (Intensificación de la Actividad Investigadora) from the National Health System and from the Health Department of the Catalan Regional Government (Programs I3 SNS and PRICS).
Santiago Moreno has carried out consultancy work in Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and Roche; he has received grants for clinical research from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and Roche; and he has received payments for giving talks from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and Roche.
Rosario Palacios, has carried out consultancy work in Boehringer Ingelheim, and has received payments for giving talks from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare, and Roche.
Maria Jesús Pérez Elías has carried out consultancy work in Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare; she has received grants for clinical research from Abbot Laboratories, Gilead Sciences, ViiV Healthcare, and Janssen; he has received payments for giving talks from Abbot Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare, as well as payments for developing educational presentations for Abbott Laboratories, Bristol-Myers Squibb, Janssen, Merck, and ViiV Healthcare.
Juan A. Pineda has carried out consultancy work in Abbott Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim, Glaxo Smith Kline, Gilead Sciences, Janssen, Merck, Pfizer, Schering-Plough, and ViiV Healthcare; he has received grants for clinical research from Abbot Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim, Glaxo Smith Kline, Gilead Sciences, Janssen, Merck, Pfizer, Roche, Schering-Plough, and ViiV Healthcare, and he has received payments for giving talks from Abbot Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Glaxo Smith Kline, Gilead Sciences, Janssen, Merck, Roche, Schering-Plough, and ViiV Healthcare.
Rosa Polo states to have no conflict of interests.
Antonio Rivero has carried out consultancy work in Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare; he has received grants for clinical research from Abbot Laboratories, Gilead Sciences, Merck, and ViiV Healthcare; he has received payments for giving talks from Abbot Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare, as well as payments for developing educational presentations for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare.
Jesús Santos has carried out consultancy work in Boehringer Ingelheim y Janssen; he has received payments for giving talks from Bristol-Myers Squibb and Gilead Sciences, as well as payments for developing educational presentations for Bristol-Myers Squibb.
Montse Tuset has received grants for clinical research from Bristol-Myers Squibb, Gilead Sciences, and Merck; and she has received payments for giving talks from Janssen, Merck, and ViiV Healthcare.
Francesc Vidal states to have no conflict of interests.
Coordinators:
Juan Berenguer1, Pere Domingo Pedrol2, Rosa Polo3
Editors:
Koldo Aguirrebengoa4, Vicente Estrada5, Félix Gutiérrez Rodero6, Hernando Knobel7, Josep M. Llibre8, Celia Miralles9, Jose M. Miro10; Antonio Rivero11, Jesús Santos12, Montserrat Tuset10;
Reviewers:
Antonio Antela13, Victor Asensi14, Jose R. Arribas15, José Ramón Blanco16, Vicente Boix17, Esteban Martínez10, Federico García18, José Mª Gatell10, Josep Mallolas10, Santiago Moreno19, Rosario Palacios12, Maria Jesús Pérez Elías19, Juan Antonio Pineda20, Francesc Vidal21
General editor:
José López Aldeguer22
1Hospital Gregorio Marañón, Madrid; 2Hospital de la Santa Creu i Sant Pau, Barcelona, Spain; 3Secretaría Plan Nacional sobre el Sida, Ministerio de Sanidad, Servicios Sociales e Igualdad, Madrid, Spain; 4Hospital de Cruces, Bilbao, Spain; 5Hospital Clínico, Madrid, Spain; 6Hospital General Universitario, Elche, Spain; 7Hospital del Mar, Barcelona, Spain; 8Hospital Universitari Germans Trias i Pujol, Badalona, Spain; 9Hospital Xeral, Vigo, Spain; 10Hospital Clínic, Barcelona, Spain; 11Hospital Reina Sofía, Córdoba, Spain; 12Hospital Universitario Virgen de la Victoria, Málaga, Spain; 13Hospital Clínico Universitario, Santiago; 14Hospital Universitario Central de Asturias, Oviedo, Spain; 15Hospital La Paz, IdiPAZ, Madrid, Spain; 16Hospital San Pedro, Logroño, Spain; 17Hospital General Universitario, Alicante, Spain; 18Hospital Universitario San Cecilio, Granada, Spain; 19Hospital Ramón y Cajal, Madrid, Spain; 20Hospital Virgen de Valme, Sevilla, Spain; 21Hospital Universitari Joan XXIII, Tarragona, Spain; 22Hospital Universitari La Fe, Valencia, Spain.
Correspondence: José López Aldeguer, jlopezal@medynet.com.
The list of authors and members of the GESIDA and National AIDS Plan Expert Committee is presented in Appendix 1.