Control of Acinetobacter baumannii is a challenge.
MethodsA survey was conducted on the control measures introduced against A baumannii in 30 Spanish hospitals.
ResultsWe found significant differences in the application of contact precautions, active surveillance, hygiene of colonised patients, environmental cleaning, and educational activities. Hospitals with a written control program for A. baumannii had a lower incidence of colonisation/infection due to this organism.
ConclusionA multidisciplinary consensus document for the control of A. baumannii is needed in Spain.
El control de Acinetobacter baumannii es complejo.
MétodosSe realizó una encuesta sobre las medidas de control frente a A. baumannii en 30 hospitales españoles.
ResultadosSe encontraron diferencias en la aplicación de precauciones de contacto, cultivos de cribado, higiene de los pacientes colonizados, limpieza ambiental, y actividades formativas. Los hospitales con un programa escrito de control de A. baumannii tuvieron menor incidencia de este patógeno.
ConclusiónEs necesario elaborar un documento de consenso multidisciplinar para el control de A. baumannii en España.
Acinetobacter baumannii has become a very important nosocomial pathogen due to its ability to develop resistance to multiple antibiotics, to colonise patients and to persist in the hospital environment.1 In the US, A. baumannii is the ninth cause of healthcare-associated infections (HAI), and the third cause of ventilator-associated pneumonia (VAP).2 In Spain, the organism is present in most tertiary centres.3 Once endemic in a hospital, controlling A. baumannii is particularly difficult because the epidemiology is usually complex.1,4,5 Hospital-wide multifaceted interventions are necessary to improve such situations.6
Despite this, there is a lack of consensus documents with evidence-based recommendations specifically aimed at controlling A. baumannii. This leads to significant differences in the way hospitals approach the problem. We performed a survey in Spanish hospitals to investigate whether there were differences in control measures against A. baumannii.
MethodsWe conducted a multicentre survey in 30 Spanish hospitals regarding infection control measures for A. baumannii. The project was launched by the Spanish Study Group for Nosocomial Infections (GEIH) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and the Spanish Network for Research in Infectious Diseases (REIPI). A pilot survey was designed, based on previous experience of a multicentre survey of control measures for methicillin-resistant Staphylococcus aureus,7 that included extracted aspects of the Healthcare Infection Control Practices Advisory Committee recommendations for controlling multi-drug resistant organisms,8 and expert recommendations.1,5,9 The pilot survey was sent to 5 hospitals and modified according to their suggestions. The survey included 30 questions regarding the existence of written control programs for multi-drug-resistant pathogens in general and for A. baumannii in particular, availability of alcohol solutions for hand hygiene, microbiological features of A. baumannii isolates prompting the implementation of control measures, periodic reports on the incidence and susceptibility of A. baumannii, barrier precautions implemented, active screening, patient hygiene, cleaning and disinfection procedures, and follow up of the measures. Questions related to hospital description, and incidence of A. baumannii colonisation/infection and bacteraemia during 2006 were also included. The questionnaire was completed between September and November 2007. Telephone and e-mail communications were used to resolve doubts and to complete questionnaires with missing data. Comparisons of categorical and continuous variables were performed by using chi squared test (or Fisher test, as appropriate) and Mann-Whitney U test, respectively.
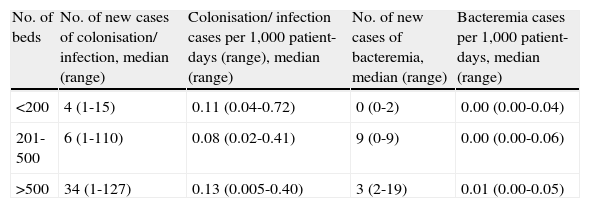
ResultsThirty hospitals from 10 Spanish regions participated in the survey; 8 (37%) had <200 beds, 14 (47%) had 201–500 beds, and 8 (26%) had > 500 beds. Data regarding the incidence of colonisation/infection and bacteraemia due to A. baumannii in participating hospitals during 2006 are shown in Table 1. Twelve hospitals (40%) reported having a written control program for A. baumannii, while 14 (47%) applied a generic program for the control of multi-drug-resistant organisms. Only 12 hospitals (40%) had alcohol solutions available for hand hygiene in every room. Sixteen hospitals (53%) directed control measures at all A. baumannii, regardless of their antibiotic resistance profile (and excluding multi-susceptible isolates); 10 (33%) only carried out measures against multi-drug resistant (including carbapenem-resistant) isolates. The way multi-drug resistance was defined varied among hospitals. In 3 (10%), only carbapenem-resistant isolates were subject to specific control measures, and in 1 (3%), measures were taken only in the event of an outbreak.
Number of cases and incidence of colonisation/infection and bacteremia due to A. baumannii in participating hospitals during 2006
| No. of beds | No. of new cases of colonisation/ infection, median (range) | Colonisation/ infection cases per 1,000 patient-days (range), median (range) | No. of new cases of bacteremia, median (range) | Bacteremia cases per 1,000 patient-days, median (range) |
| <200 | 4 (1-15) | 0.11 (0.04-0.72) | 0 (0-2) | 0.00 (0.00-0.04) |
| 201-500 | 6 (1-110) | 0.08 (0.02-0.41) | 9 (0-9) | 0.00 (0.00-0.06) |
| >500 | 34 (1-127) | 0.13 (0.005-0.40) | 3 (2-19) | 0.01 (0.00-0.05) |
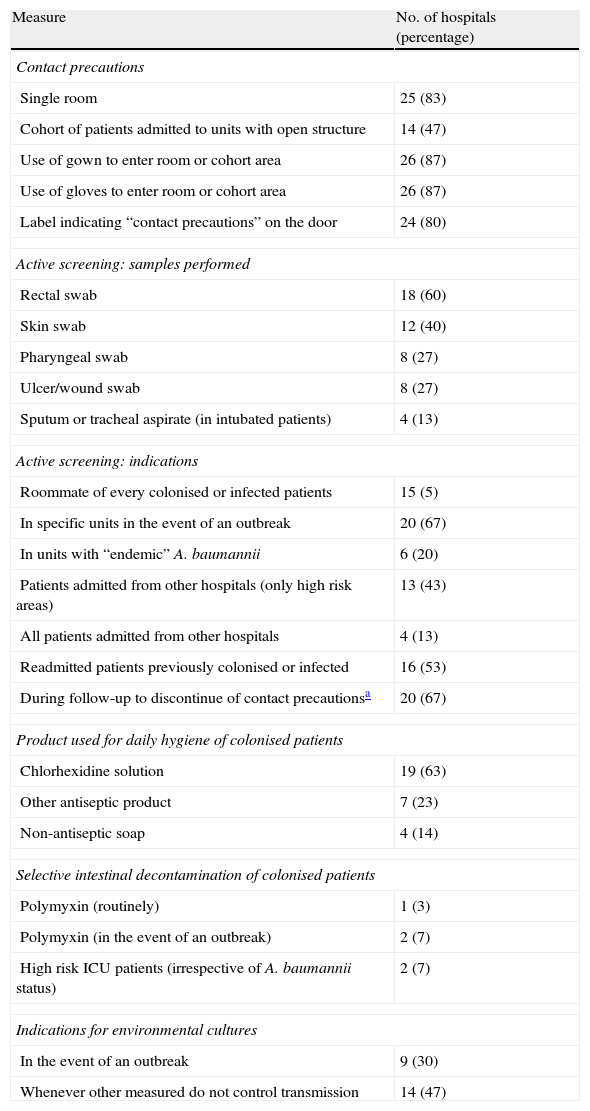
Control measures in all hospitals included contact precautions for patients colonised or infected with A. baumannii (depending on susceptibility as shown above). However, there were some differences in the measures included under the heading of “contact precautions” (Table 2). Contact precautions were implemented during the whole admission period in 10 (33%) hospitals. In 19 centres (63%), droplet precautions were also introduced for patients with respiratory tract colonisation. Active surveillance of colonised patients using screening samples was performed in certain situations in 27 (90%) hospitals. The situations in which samples were taken varied (Table 2). The products used for the daily cleaning of colonised patients, the use of selective intestinal decontamination, and indications for environmental cultures are shown in Table 2.
Selected measures implemented in the participating centres to control A. baumannii
| Measure | No. of hospitals (percentage) |
| Contact precautions | |
| Single room | 25 (83) |
| Cohort of patients admitted to units with open structure | 14 (47) |
| Use of gown to enter room or cohort area | 26 (87) |
| Use of gloves to enter room or cohort area | 26 (87) |
| Label indicating “contact precautions” on the door | 24 (80) |
| Active screening: samples performed | |
| Rectal swab | 18 (60) |
| Skin swab | 12 (40) |
| Pharyngeal swab | 8 (27) |
| Ulcer/wound swab | 8 (27) |
| Sputum or tracheal aspirate (in intubated patients) | 4 (13) |
| Active screening: indications | |
| Roommate of every colonised or infected patients | 15 (5) |
| In specific units in the event of an outbreak | 20 (67) |
| In units with “endemic” A. baumannii | 6 (20) |
| Patients admitted from other hospitals (only high risk areas) | 13 (43) |
| All patients admitted from other hospitals | 4 (13) |
| Readmitted patients previously colonised or infected | 16 (53) |
| During follow-up to discontinue of contact precautionsa | 20 (67) |
| Product used for daily hygiene of colonised patients | |
| Chlorhexidine solution | 19 (63) |
| Other antiseptic product | 7 (23) |
| Non-antiseptic soap | 4 (14) |
| Selective intestinal decontamination of colonised patients | |
| Polymyxin (routinely) | 1 (3) |
| Polymyxin (in the event of an outbreak) | 2 (7) |
| High risk ICU patients (irrespective of A. baumannii status) | 2 (7) |
| Indications for environmental cultures | |
| In the event of an outbreak | 9 (30) |
| Whenever other measured do not control transmission | 14 (47) |
As regards environmental cleaning, complete cleansing of a room or cubicle occupied by a patient colonised or infected with A. baumannii was carried out after the patient was discharged. Also, in 18 (60%) hospitals, a thorough cleansing of rooms occupied by patients with A. baumannii was performed daily. There was a specific protocol for disinfecting devices and mobile elements after patient use in 21 centres (70%).
Specific educational activities concerning measures for controlling A. baumannii were regularly carried out in 4 (13%) hospitals, and occasionally (typically, when an outbreak or increase in incidence was noted) in 20 (67%). In 6 (20%) hospitals, there were no specific educational activities.
Although hospitals with a specific written control program for the control of A. baumannii more frequently implemented several control measures (particularly screening cultures), the differences were not statistically significant (data not shown). The incidence of A. baumannii colonisation/infection was lower in these centres compared to those without specific written control programs (median [range], 0.05 [0.00-0.12] vs. 0.14 [0.03-0.72], P=.02).
DiscussionOur data show that there were substantial differences in the control activities employed to control A. baumannii. Some of these differences affect key elements of control policy. In the first place, only half of the hospitals took measures directed at all A. baumannii isolates (except for those susceptible to most antibiotics), while in most of the rest, only multi-drug-resistant or carbapenem-resistant A. baumannii prompted implementation of control measures. In addition, the definition of multi-drug-resistance varied. Finally, one hospital only implemented measures in the event of an outbreak. Since A. baumannii is able to acquire further resistance very easily, it seems prudent to control the spread of all A. baumannii, except for multi-drug-susceptible isolates.
Also, important differences were found in the policy regarding use of active screening to detect colonised patients. Patients are an important reservoir in endemic situations, and a substantial proportion of colonised patients are not detected by means of clinical samples alone.1,5,6 Active screening for A. baumannii is complex, since it may be necessary to include several samples and suitable microbiological methods in order to detect most colonised patients. The other important reservoir for A. baumannii is the hospital environment.1,5,6,8,9 All surfaces surrounding colonised patients and all mobile devices may be contaminated. The thorough and repeated cleaning and disinfection of all surfaces and mobile devices is key to controlling the spread of organisms. While environmental cleaning was considered an important task in all the hospitals surveyed, their protocols varied. As regards selective intestinal decontamination, it was rarely used, probably reflecting the fact that it is a controversial measure which is not routinely recommended.1,5,6,8,9
We found that hospitals with a written control program against A. baumannii had lower incidences of colonisation/infection caused by this organism, although the limited sample size did not allow us to control for confounders. Our study has other limitations. The participating hospitals, although numerous and diverse in size and incidence of A. baumannii, are not representative of all Spanish hospitals. Although antibiotic use might be an important variable in the spread of A. baumannii, questions related to antibiotic stewardship were not included. Finally, the quality of the evidence supporting some of the control measures recommended in the references used is limited, and their cost-benefit ratio has not been assessed.
In conclusion, infection control measures were carried out in all hospitals, but with significant variations in some key aspects. Specific guidelines for the control of A. baumannii are necessary to help hospitals improve their control activities.
FundingSupported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III - FEDER, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008).
Conflict of interestThe authors declare no conflict of interest.
The study was supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III - FEDER, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008). The authors declare no conflict of interest.
Other participants in the study were: A. Delgado-Iribarren (Hospital de Alcorcón, Madrid); J. M. Tricas, R. Vidal, E. Redon (Hospital de Mollet, Barcelona); C. Ferrer (Hospital Vall d’Hebron, Barcelona); M. Pujol, M.A. Domínguez (Hospital de Bellvitge, Barcelona); G. Mestre, S. González-Falgueras (Centro Médico Delfos, Barcelona); J. P. Horcajada, C. Segura (Hospital del Mar, Barcelona); R. García-Penche (Hospital Sagrat Cor, Barcelona); M. Chavez, J. Delgado, S. Expósito (Hospital S. Juan de Dios, Bormujos, Sevilla); M.C. Gómez-González, M.P. Teno (Hospital San Pedro de Alcantara, Caceres); A. Martínez-Blázquez, G. Lucas (Hospital de Cieza, Murcia); C. Bischofberger (Hospital de El Escorial, Madrid and Hospital de Guadarrama, Madrid); M.D. Navarro, J. Cuquet, C. Martí (Hospital de Granollers, Barcelona); M. García de la Veja (Hospital de Riotinto, Huelva); B. Padilla, E. Cercenado (Hospital Gregorio Marañon, Madrid); E. Matilla, B. Martínez. M.S. Cuétara, C. Gómez (Hospital Severo Ochoa, Madrid); C. Pérez-Canosa, I. Sánchez-Romero (Hospital Puerta de Hierro, Madrid); E. Padilla, C. Capó, J. de Otero (Hospital de Manacor, Mallorca); J. Molina, M. Bolaños, M. Hernández (Hospital de Gran Canaria, Las Palmas de Gran Canaria); E. Calbo, M. Xercavins, N., Freixas (Hospital Mutua de Tarrasa, Barcelona); A. Leturia (Hospital de Parapléjicos, Toledo); A. J. Cruz, A. García (Hospital Sant Boi Sant Joan de Deu, Barcelona); J.A. Jiménez-Alfaro, A. Otamendi (Policlinica Gipuzkoa, San Sebastian); M. J. Martínez, J. Vilaró (Hospital de Vic, Barcelona); A. Vilamala, M. Cusco, D. Orta (Hospital Alt Penedes, Barcelona); A. Yagüe, V. Rodrigo (Hospital La Plana, Castellon); M. Canals, I. Fernández, D. Mariscal (Hospital de Sabadell, Barcelona); M. J. Hernández, J.L. Aribas, C. Lapresta, A. Rezusta (Hospital Miguel Servet, Zaragoza); A. Pascual (Hospital Virgen Macarena, Sevilla); J. J. García-Irupe (Hospital de Navarra, Pamplona); F. Barcenilla, A. Jover, M. Garcia, D. Castellana, R. López (Hospital Arnau de Vilanova, Lleida).
The investigators of Nosocomial Infections group (GEIH-SEIMC) are listed in Appendix 1.