Metallo-β-lactamase (MBL)-producing gram-negative bacteria are an increasing public health concern worldwide. Screening tests for the rapid and specific identification of these pathogens are essential, and should be included among routine diagnostics in laboratories. This study aimed to determine the MBL frequency among carbapenem-resistant Pseudomonas aeruginosa isolates, and to evaluate the accuracy of different tests in screening for MBL production. From January 2001 to December 2008, a total of 142 imipenem-non-susceptible P. aeruginosa strains were isolated from distinct clinical samples from hospitalized patients. These isolates were examined by PCR, MBL E-test, double-disk synergy test (DDST), and combined disk (CD) test. The minimal inhibitory concentration (MIC; μg/mL) was determined by agar dilution, and pulsed field gel electrophoresis (PFGE) was performed on all samples. Sequencing was performed to confirm and define the MBL variant and subtype. Using PCR and DNA sequence analysis, 93 strains were confirmed positive for MBLs, 91 strains for the blaSPM-1 gene, 1 strain for the blaIMP-1 gene, and 1 strain for the blaIMP-16 gene. PFGE displayed a clonal pattern. The sensitivities, specificities, positive and negative predictive values were evaluated for all tests. The DDST assay (CAZ-MPA) was the optimal method for screening MBL production in P. aeruginosa strains. However, the results of the CD assay (IMP/EDTA) showed close agreement with those of the DDST. In addition, the CD assay allowed a more objective interpretation and did not require the use of a toxic substance.
Las metalo-β-lactamasas (MBL) que producen las bacterias gram-negativas son un creciente problema de salud pública en todo el mundo. Las pruebas de detección para la identificación rápida y específica de estos patógenos son esenciales y deben ser incluídas entre los diagnósticos de rutina de los laboratorios. Este estudio tiene como objetivo determinar la frecuencia de MBL en aislamientos de Pseudomona aeruginosa resistentes a carbapenem y evaluar la precisión de diferentes pruebas en la detección de la producción de MBL. Entre enero de 2001 y diciembre de 2008 un total de 142 cepas de P. aeruginosa no susceptibles a imipenem fueron aisladas de muestras clínicas provenientes de pacientes hospitalizados. Estas cepas fueron examinadas por PCR, prueba de MBL-E, prueba de sinergia de doble disco (DDS), y prueba de disco combinado (DC). La concentración inhibitoria mínima (CIM; g/ml) se determinó mediante dilución en agar. Se realizó electroforesis en gel de campo pulsado (PFGE) a todas las muestras. La secuenciación se realizó para confirmar y definir la variante de MBL y subtipo. Por PCR y análisis de secuencia de ADN, 93 cepas fueron confirmadas como positivas para MBL. A su vez, 91 cepas fueron confirmadas para el gen blaSPM-1, 1 cepa para el gen bla IMP-1, y 1 cepa para el gen bla IMP-16. La prueba de PFGE muestra un patrón clonal. Se evaluó la sensibilidad, especificidad, valores predictivos positivos y negativos para todas las pruebas. El ensayo DDS (CAZ-MPA) fue el método óptimo para la detección de la producción de MBL en las cepas de P. aeruginosa. Sin embargo, los resultados del ensayo de DC (IMP/EDTA) mostraron una estrecha concordancia con los de la DDS. Adicionalmente, el ensayo de DC permitió una interpretación más objetiva de los resultados, no requiriendo el uso de una sustancia tóxica.
Metallo-β-lactamases (MBLs) are resistance determinants of increasing clinical relevance in gram-negative bacteria, especially in P. aeruginosa, Acinetobacter spp., and members of the Enterobacteriaceae family.1,2 The worldwide dissemination of acquired metallo-β-lactamases genes and the emergence of new variants are becoming an emerging threat to public health because they usually are carried by mobile genetic elements that disseminate rapidly.3–5 Increased mortality rates have been documented for patients infected with MBL-producing P. aeruginosa, rates that have been exacerbated by inadequate empirical therapy.6 Therefore, early detection and identification of MBL-producing organisms is of crucial importance for the prevention of nosocomial dissemination through appropriate treatment, as well as the implementation of infection control measures.2,7
Several phenotypic methods used to detect microorganisms carrying MBL have been reported.8–14 Currently, the most frequently used tests are the double-disk synergy test (DDST), the combined disk (CD) assay, and the MBL E-test. However, these tests have shown discordant results depending on the employed methodology, β-lactam substrates used, presence of MBL inhibitors (IMBL), bacterial genus tested and local prevalence of MBL types. Although there are numerous studies evaluating screening tests with IMP and VIM producing P. aeruginosa, there is no inclusion of SPM producing isolates, the most prevalent in our country.15 Therefore, hospital microbiology laboratories should evaluate a variety of assays and identify the most appropriate one for local routine application. The aim of this study was to determine the MBL frequency among carbapenem-resistant (CR) P. aeruginosa isolates and evaluate the accuracy of different tests in screening for MBL production.
Materials and methodsThis study was performed at the Hospital de Clínicas da Universidade Federal do Paraná (HC-UFPR), a 640-bed tertiary care academic hospital in Curitiba, Brazil. The study was approved by The HC-UFPR Institutional Review Board (IRB#0248.0.208.000-09).
Bacterial isolatesFrom January 2001 to December 2008, a total of 142 non-duplicate imipenem (IP)-nonsusceptible P. aeruginosa (MIC≥8μg/mL) isolates were collected from different units of the hospital. All of these samples were isolated from different patients and were identified by conventional biochemical tests in accordance with published recommendations.15P. aeruginosa ATCC 27853 was used as an MBL-negative control. P. aeruginosa strain P1088 producing SPM, A. baumanii strain 17–4 producing IMP-1 and P. aeruginosa producing VIM were used as MBL-positive controls.
Susceptibility testingThe agar dilution method was used to determine the minimal inhibitory concentrations (MICs) of the following drugs: imipenem (IP), meropenem (MEM), piperacillin/tazobactam (PTZ), ceftazidime (CAZ), ciprofloxacin (CIP), gentamicin (GEN), amikacin (AMI), aztreonam (ATM), cefepime (CPM), and B polymyxin (POL). The results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI; 2009). P. aeruginosa ATCC 27853 was used as a control strain for susceptibility testing.
Phenotypic detection of MBLDDST: The DDST phenotypic tests were performed by following the CLSI recommendations for the disk diffusion method (CLSI 2011, M100-S21). Briefly, a 0.5 McFarland bacterial suspension was inoculated on a Mueller–Hinton (MH) agar plate. One ceftazidime (CAZ) disk was placed into the agar, aligned 20mm apart a blank filter disk (edge-to-edge) containing 5μL of 1.4mM (1:8) mercaptopropionic acid (MPA; Sigma; St. Louis, MO, USA) solution.14 Each agar plate was incubated at 35°C±1°C overnight. Enhancement of the zone of inhibition in the area between the MPA and CAZ disk was interpreted as a positive test result.
CD: Two IP (10μg) disks (Becton Dickinson, Franklin Lakes, NJ, USA) were placed on an agar MH plate containing the bacterial suspension (0.5 McFarland), and 5μL of a 0.5M EDTA solution (pH 8.0)11 was added to one of the IP disks. After incubation overnight at 35°C±1°C, the inhibition zones of the IP disks in the presence and absence of EDTA were compared.
E-test MBL: The MBL E-test (AB Biodisk, Solna, Sweden) was performed according to the manufacturer's recommendations.
MBL gene PCR amplification and sequencingPCR assays were performed to amplify the sequences of the blaIMP, blaVIM, blaGIM, blaSPM-1, blaSIM and blaKPC genes, as previously described.16–20 The PCR products were purified and sequenced (MegaBACE; ABI PerkinElmer, Waltham, MA, USA) to confirm and define MBL variant and subtype. These tests were used as gold standard in the evaluation of screening testes.
Genetic similarityGenetic relatedness among the IP-nonsusceptible P. aeruginosa isolates was evaluated by pulsed-field gel electrophoresis (PFGE) using the restriction enzyme SpeI (Invitrogen, Carlsbad, CA, USA) at 37°C. Electrophoresis was performed on a CHEF-DRIII (Bio-Rad Laboratories, Hercules, CA, USA) for 23h at 6V/cm, at 12°C, and pulse times from 5 to 60s. The gels were analyzed with Gel-Pro Analyzer 4.0 (Media Cybernetics, Bethesda, MD, USA) and NTSYS 2.02 software (Exeter Software, Setauket, NY, USA). Clusters of potentially related isolates were identified using the Dice similarity coefficient and unweighted pair-group method with arithmetic averages (UPGMA).
MBL enzymatic analysesThe MBL enzymatic test was performed on samples with positive results in all phenotypic assays, but without a positive result of PCR for the MBL gene. Cellular extracts possessed hydrolytic activity for meropenem and IP. In addition, extracts displaying β-lactamase activity were pre-incubated for 20min with EDTA (20mM) or a serine-β-lactamase inhibitor (BRL42715, 5mM). The assays were performed by measuring the breakdown of the substrate at a specific wavelength (299nm) and the β-lactam-specific activity was measured in nanomoles of substrate hydrolyzed/min/mg of protein.4
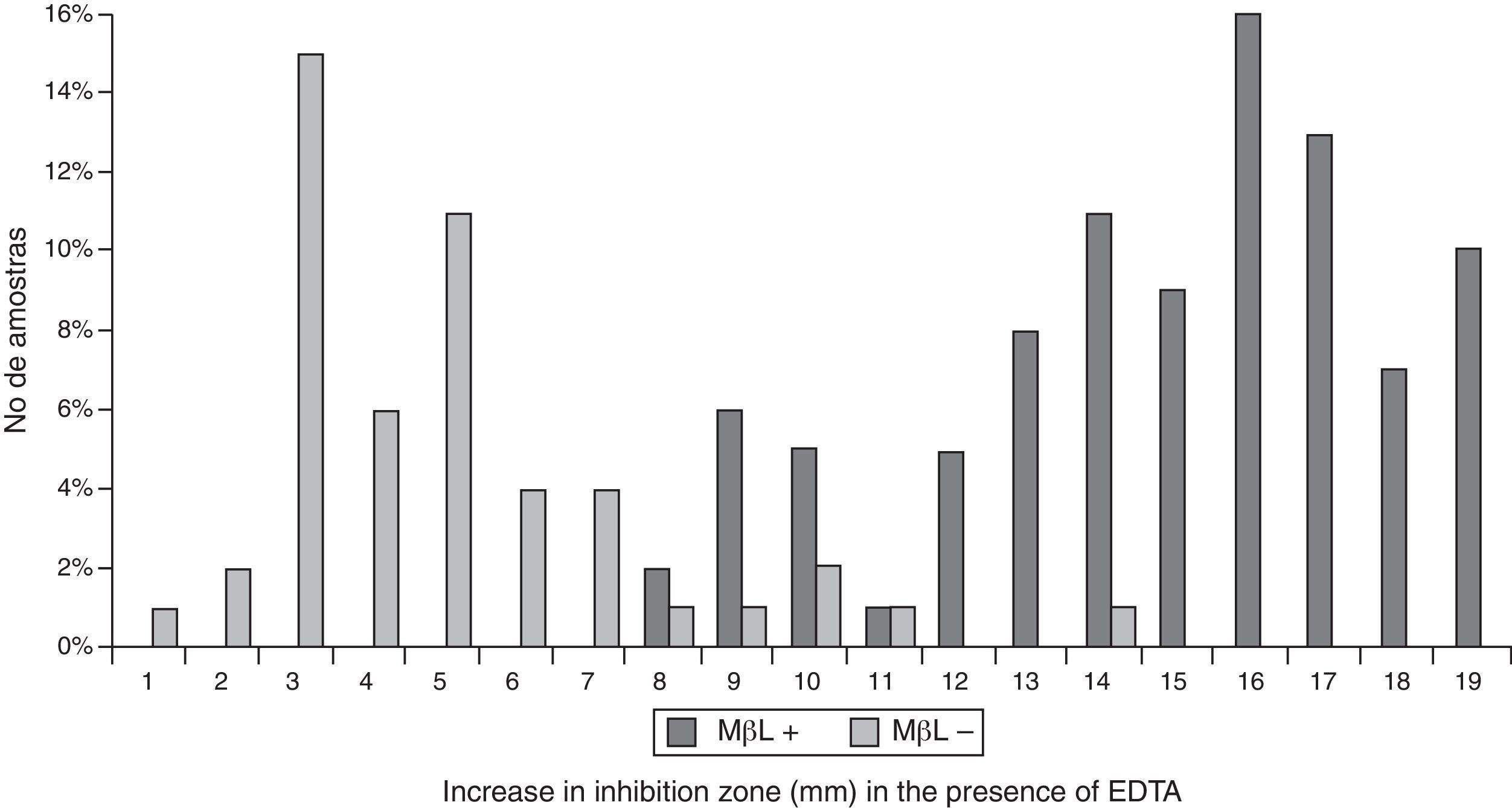
Statistical analysisSensitivity (SN), specificity (SP), positive predictive value (PPV), and negative predictive value (NPV) were calculated for the MBL E-test (IP-EDTA), DDST (CAZ/MPA), and CD (IP/EDTA) assay. The concordance between tests was estimated by the Kappa index. Receiver operating characteristic (ROC) curves were used to determine the best cutoff values for indicating MBL production based on the results of the CD phenotypic method. SN and SP were calculated successively according to the variation of inhibition zones of MBL-producing and MBL-nonproducing isolates.
ResultsTo assess the resistance profile of P. aeruginosa, we conducted a search in the database of the Hospital Information System for the period 2001–2008. The results have shown that P. aeruginosa was isolated from 2158 patients in our Hospital. Overall, the prevalence of CR P. aeruginosa was 23%, of these a total of 142 CR-PA were included in the study, once positive surveillance samples were excluded. The samples were isolated from patients of the following wards: intensive care units (ICU) 107 (75%), surgical wards 21 (15%), and clinical wards 14 (10%). The sources of isolates were: blood samples 24 (17%), respiratory samples 36 (25%), urine samples 32 (22.5%), cerebral spinal fluid 4 (3%), sterile liquids 5 (3.5%), catheter tip 14 (10%) and other secretions 27 (19%).
Ninety-three CR P. aeruginosa were confirmed to be positive for MBLs, 91 strains for the blaSPM-1 gene, 1 strain for the blaIMP-1 gene, and 1 strain for the blaIMP-16 gene by PCR and DNA sequence analysis. Among the SPM P. aeruginosa isolates, 3 samples were submitted to DNA sequencing, along with the IMP-positive isolates. No KPC positive isolate was found.
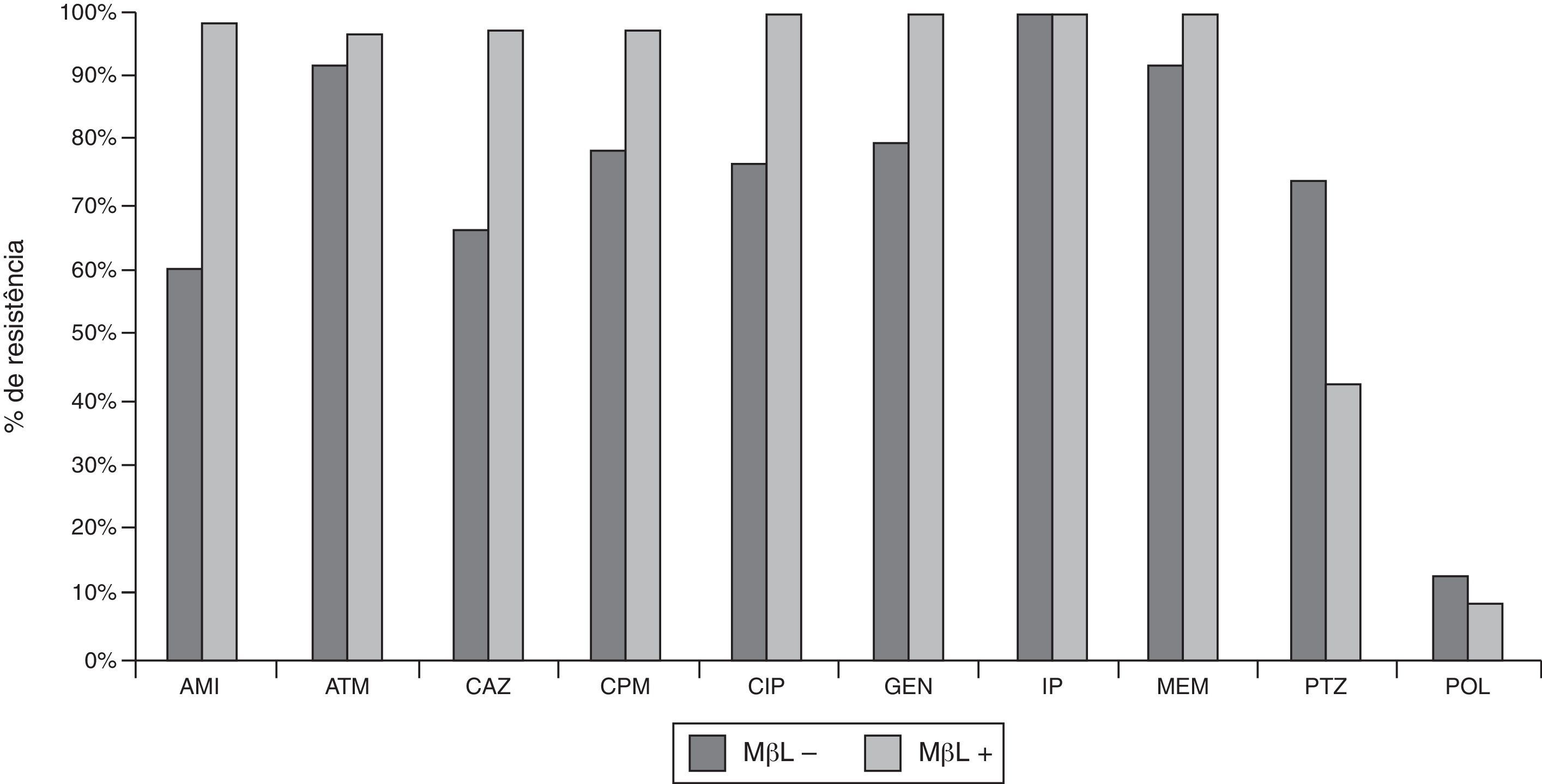
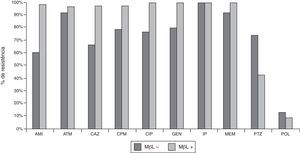
Out of 142 P. aeruginosa clinical isolates, 77 (54%) were resistant to PTZ, 123 (87%) to CAZ, 129 (91%) to CPM, 135 (95%) to ATM, 121 (85%) to AMI, 132 (93%) to GEN, 130 (92%) to CIP, and 15 (11%) to POL. MIC (μg/mL) determinations confirmed 100% imipenem-resistance, an observation that was initially suggested by disk diffusion. IP-nonsusceptible P. aeruginosa producing MBLs were more resistant to CAZ, CPM, ATM, AMI, GEN, and CIP, and less resistant to PTZ and POL than IP-nonsusceptible P. aeruginosa isolates that did not produce MBLs (Fig. 1 and Table 1).
Susceptibilities of clinical strains of CR P. aeruginosa isolated in the Hospital de Clinicas – UFPR from 2001 to 2008. MBL+ (N=93), isolates producing metallo-β-lactamases; MBL− (N=49), isolates not producing metallo-β-lactamases. AMI, amicacin; ATM, aztreonam; CAZ, ceftazidime; CPM, cefepime; CIP, ciprofloxacin; GEN, gentamicin; PTZ, piperacillin/tazobactam; POL, B polymyxin.
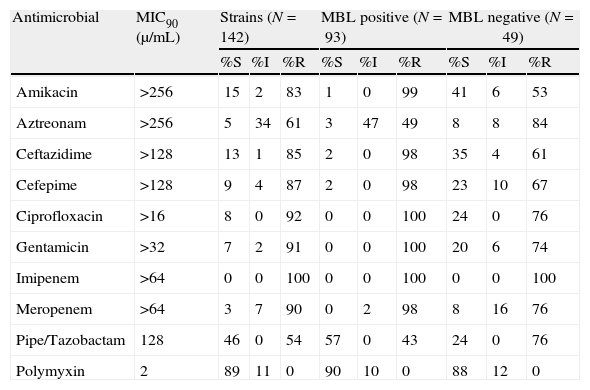
Susceptibility profile and MIC90 of 142 IP-nonsusceptible P. aeruginosa isolates and analysis of MBL production.
| Antimicrobial | MIC90 (μ/mL) | Strains (N=142) | MBL positive (N=93) | MBL negative (N=49) | ||||||
| %S | %I | %R | %S | %I | %R | %S | %I | %R | ||
| Amikacin | >256 | 15 | 2 | 83 | 1 | 0 | 99 | 41 | 6 | 53 |
| Aztreonam | >256 | 5 | 34 | 61 | 3 | 47 | 49 | 8 | 8 | 84 |
| Ceftazidime | >128 | 13 | 1 | 85 | 2 | 0 | 98 | 35 | 4 | 61 |
| Cefepime | >128 | 9 | 4 | 87 | 2 | 0 | 98 | 23 | 10 | 67 |
| Ciprofloxacin | >16 | 8 | 0 | 92 | 0 | 0 | 100 | 24 | 0 | 76 |
| Gentamicin | >32 | 7 | 2 | 91 | 0 | 0 | 100 | 20 | 6 | 74 |
| Imipenem | >64 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 |
| Meropenem | >64 | 3 | 7 | 90 | 0 | 2 | 98 | 8 | 16 | 76 |
| Pipe/Tazobactam | 128 | 46 | 0 | 54 | 57 | 0 | 43 | 24 | 0 | 76 |
| Polymyxin | 2 | 89 | 11 | 0 | 90 | 10 | 0 | 88 | 12 | 0 |
MIC, minimal inhibitory concentration; MBL, metallo-β-lactamases; S, susceptible; R, resistant; I, intermediate.
PFGE analysis performed with all P. aeruginosa isolates identified an A cluster consisting of 99 samples, including 87 SPM-1-producing isolates. This single genotype (corresponding to the genotype of the Brazilian epidemic clone) was classified into 25 subtypes (A1–A25).
Operational characteristics of phenotypic testsOverall results from the different tests are presented in Table 2. DDST assay correctly identified all 93 PCR-confirmed MBL-positive isolates (SN=100%) and presented 1 false-positive result (SP=97%). CD assay detected all MBL-positive isolates (SN=100%) but displayed 6 false positive results (SP=88%), including the false-positive sample detected in the DDST assay. E-test assay failed to identify one MBL-positive sample (SN=99%) and identified 11 false-positive samples (including 5 detected in the CD assay; SP=77%).
Comparison of DDST, DC and E-test for the detection of MBL in 142 clinical isolates of IP-nonsusceptible P. aeruginosa.
| No. of isolatesa | DDST CAZ/MPA | DC IP/EDTA | E-test MBL | |||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| MBL+ (93) | 93 | 0 | 93 | 0 | 92 | 1 |
| MBL− (49) | 1 | 48 | 6 | 43 | 11 | 38 |
| Total (142) | 94 | 48 | 99 | 43 | 103 | 39 |
| SN | 100% | 100% | 99% | |||
| SP | 97% | 88% | 77% | |||
| PPV | 99% | 94% | 89% | |||
| NVP | 100% | 100% | 97% | |||
MBL, metallo-β-lactamases; DDST, double-disk synergy test; CAZ, ceftazidime; MPA, mercaptopropionic acid; CD, combined disk; IP, imipenem; SN, sensitivity; SP, specificity; PPV, positive predictive value; NVP, negative predictive value.
The MBL enzymatic analysis was performed in the single positive sample for the three phenotypic tests (DDST, CD and E-test), but negative for PCR, and results negative.
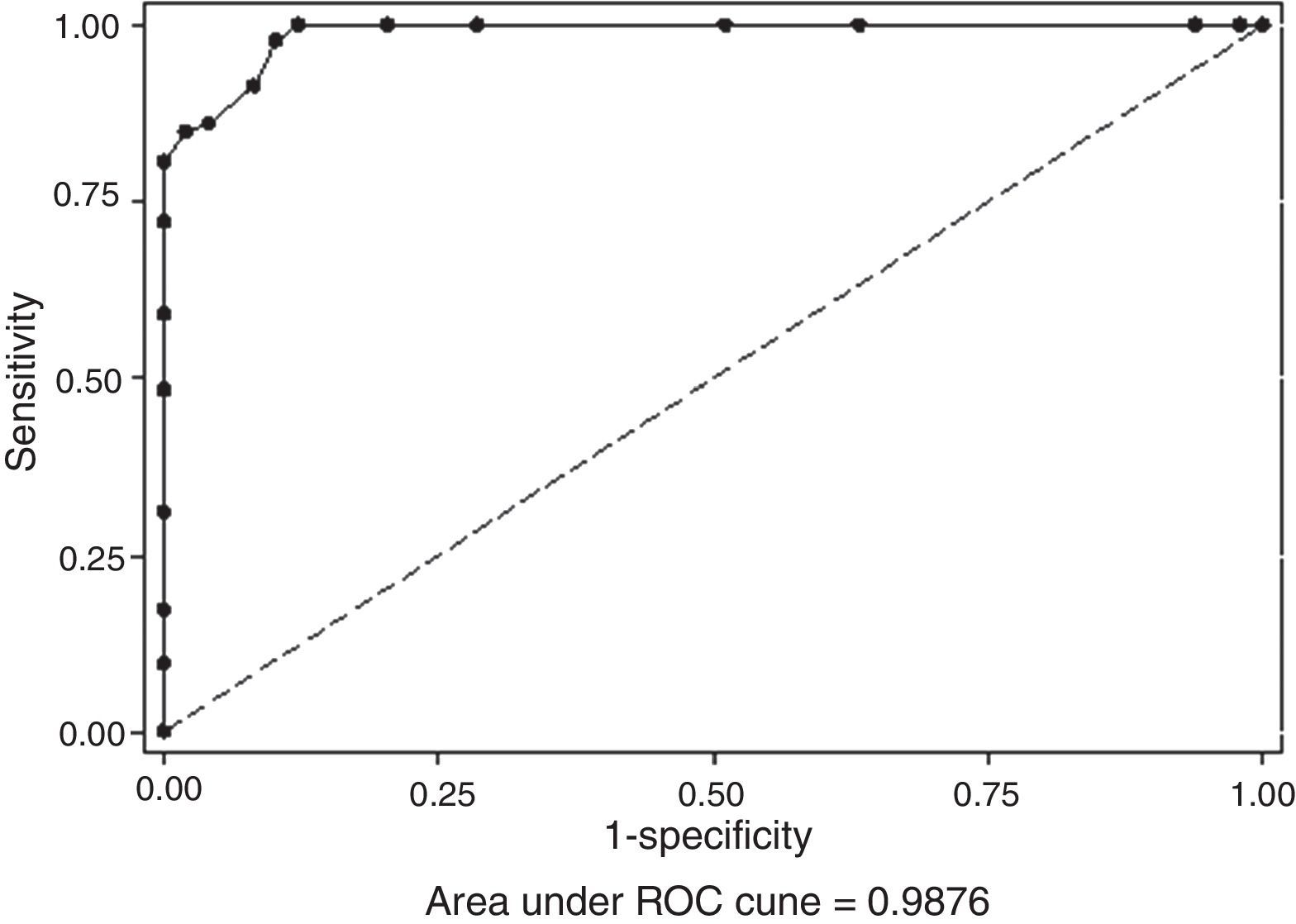
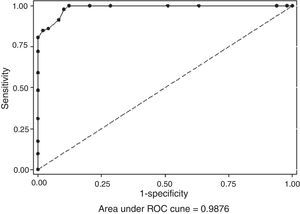
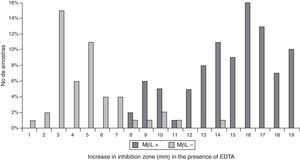
All the results obtained in the CD assay (IP/EDTA, 0.5M) were plotted on an ROC curve to establish the ideal breakpoint (increase in mm) for MBL detection. A cutoff value of ≥8mm was selected because it presented the best results for SN (100%) and SP (88%), with an area under the curve (AUC) of 0.987 (p<0.001; Figs. 2 and 3).
To evaluate the correlation between the tests, the Kappa index was estimated for the testing pairs. IP/EDTA and CAZ/MPA results were concordant in 96.5% of cases, and the Kappa index was 0.92 (95% CI, 0.85–0.99). IP/EDTA and E-test results were concordant in 94.4% of cases, and the Kappa index was 0.86 (95% CI, 0.77–0.96). The results of E-test and CAZ/MPA were concordant in 92.2% of cases, and the Kappa index was 0.82 (95% CI, 0.72–0.92).
DiscussionCR P. aeruginosa constitutes a great public health concern, particularly because of the limited therapeutic options available for this pathogen. MBL has been detected with increasing frequency in P. aeruginosa worldwide and has been frequently implicated in serious nosocomial infections and outbreaks.21 Recently, New Delhi metallo-beta-lactamase (NDM)-producing organisms have been detected for the first time in Brazil, where five individuals were infected/colonized, between September 2012 and April 2013.22 MBL displays a mobile nature and often co-exists with other resistance determinants, resulting in multidrug resistance (MDR) or a pan-resistance profile. Furthermore, the detection of these carbapenemases is difficult, which together with the clinical unavailability of MBL inhibitors makes the MBL resistance a major therapeutic and public health problem. In the present study, 66% of the IP-nonsusceptible P. aeruginosa isolates were MBL positive, with 98% positive for SPM-1 and 2% positive for IMP. SPM-1, first described in 2002,4 is currently prevalent in Brazilian hospitals.22 The blaIMP gene has previously been detected, albeit to a lesser extent.1,5,23–25 The prevalence of MBL-PA in other Brazilian hospitals is highly variable (7–44%).26–31
The IP-nonsusceptible P. aeruginosa strains studied displayed resistance against the majority of available antibiotics, indication of a multidrug-resistance phenotype. Polymyxin and piperacillin/tazobactam were the most active antimicrobial agents.29,30,32 In fact if we use the current CLSI cutoff for piperacillin/tazobactam, the sensitivity to this drug would fall from 46% to 3% (CLSI; 2013). The frequency of drug resistance was higher between MBL-producing than MBL-non-producing negative isolates. Except for piperacillin/tazobactam and carbapenems, there was no change in the cutoff points of the studied drugs (CLSI, 2013). Polymyxin has shown high rates of intermediate resistance (11%). The high prevalence of many severe nosocomial infections and consequent selective pressure applied by polymyxin in the hospital may explain this phenomenon. Aztreonam likewise displayed high resistance, suggesting a probable association with other resistance mechanisms in P. aeruginosa.15
PFGE analysis showed a clonal-predominant genotype among the SPM-1 P. aeruginosa isolates. This high clonality (70%) suggests cross-transmission as an important mechanism of dissemination. This finding may explain the high levels of resistance among P. aeruginosa isolated in the hospital, especially in ICUs where there are critically ill patients, who underwent invasive procedures, using multiples devices and broader spectrum antibiotics. The high clonality of the isolates is one of the limitations of this study, once the samples are very similar. However, it is important to note that in this study there was a varied pattern in carbapenems resistance with the presence of different MICs. Moreover, unlike samples producing of VIM and IMP, (usually polyclonal), SPM producing P. aeruginosa specimens are characteristically monoclonal.
These findings highlight the need for continuous surveillance and improved strategies for infection control to reduce cross-infection, in particular, when the majority of MBL-encoding genes are in high-mobility genetic elements.
There exist no standard national or international guidelines for screening for MBL production in P. aeruginosa, although several criteria have been suggested for detection of these enzymes. It is desirable that selection of the appropriate MBL test is based on studies using local pathogens.
To set the test and the inhibitor that would be used in this study, we carried out some preliminary experiments and found that EDTA was most appropriate for testing CD-type; however, the interpretation of result of the DDST-type test was difficult to analyze, independent of the EDTA concentration. In contrast, MPA-2 inhibitor was best suited to the DDST format (unpublished data)”. DDST (CAZ/MPA) provided the best results in SN (100%), SP (97%), VPP (99%), and VPN (100%). Surprisingly, there was no difficulty in distinguishing positive from negative results in this test. This could probably be due to the dilution of MPA (1:8), which might have resulted in low bacterial growth inhibition. By contrast, preliminary tests were performed using an undiluted solution of MPA,8 resulting in a large number of false-positive results (unpublished data). Similarly, other studies showed unsatisfactory results with the Arakawa test,33,34 including at locations where there is a high prevalence of SPM-1–producing isolates.28,30,35 In addition, this test uses a toxic substance (2-MPA), which requires the use of specialized equipment. Another inconvenient aspect of DDST is that it depends on the users’ technical skills in discriminating true synergism from the intersection of inhibition zones.
In the CD assay using IP/EDTA, the best breakpoint for MBL detection (measured as an increase in length) was 8mm, which easily discriminated between positive and negative results. This assay is practical test to perform as part of a laboratory routine and provides results that are based solely on the differences in the enlargement of the inhibition zones obtained in the presence and absence of MBL inhibitor. Therefore, interpretation of the CD assay results may be considered more objective than that of DDST assay results. However, the zone diameters of IP among 6 MBL-negative isolates markedly varied between 8 and 14mm in diameter. These false-positive results can be explained by the EDTA concentration used in this study (950μg); several investigators have observed lower error rates when lower concentrations of EDTA were used.14,36 We previously tested the EDTA concentration suggested by Picão and colleagues (10μL, 0.1M EDTA).14 However, the small enlargement of the inhibition zone obtained made it difficult to discriminate between the presence or absence of MBL production. Furthermore, EDTA may also affect membrane permeability, thereby increasing the susceptibility of P. aeruginosa to IP, leading to false interpretations of the results of MBL synergy tests.37,38 Danel and colleagues reported that oxacillinase (OXA) enzymes, which function similar to carbapenemases and which are often present in P. aeruginosa, may also be inhibited by EDTA.39
The MBL E-test displayed the least satisfactory results among the tests examined. The poor specificity might be due to the use of EDTA. Furthermore, the test failed to identify one positive sample.
ConclusionIn this study, we found a high prevalence of P. aeruginosa producing MBL in HC-UFPR during the years 2001–2008. As expected, SPM was the predominant MBL subtype. The optimal method for MBL screening in P. aeruginosa strains was the DDST assay using MPA (1:8)/CAZ. However, MPA is known to be toxic, and the use of this substance in the laboratory routine is a major drawback of this test. Therefore, we suggest, as an alternative test, the CD assay IP/EDTA, which showed excellent agreement with the DDST assay (Kappa index=0.92). This method can therefore be used as a simple, inexpensive, and accurate functional screen for MBL-producing P. aeruginosa strains.
Conflict of interestThe authors declare no conflict of interest.