Statin therapy has a very important role in decreasing cardiovascular risk, and treatment non-compliance may therefore be a concern in high cardiovascular risk patients. Myotoxicity is a frequent side effect of statin therapy and one of the main causes of statin discontinuation, which limits effective treatment of patients at risk of or with cardiovascular disease. Because of the high proportion of patients on statin treatment and the frequency of statin-related myotoxicity, this is a subject of concern in clinical practice. However, statin-related myotoxicity is probably underestimated because there is not a gold standard definition, and its diagnosis is challenging. Moreover, information about pathophysiology and optimal therapeutic options is scarce. Therefore, this paper reviews the knowledge about the definition, pathophysiology and predisposing conditions, diagnosis and management of statin-related myotoxicity, and provides a practical scheme for its management in clinical practice.
El tratamiento con estatinas tiene un papel fundamental en la reducción del riesgo cardiovascular, y la falta de adherencia al mismo es motivo de preocupación en los pacientes con alto riesgo. La miotoxicidad es un efecto secundario frecuente y una de las principales causas de interrupción de las estatinas, lo que limita el tratamiento eficaz de los pacientes con riesgo de enfermedad cardiovascular. Considerando la elevada proporción de pacientes en tratamiento con estatinas, y la frecuencia de miotoxicidad asociada, este es un tema relevante en la práctica clínica. Sin embargo, la miotoxicidad por las estatinas está probablemente subestimada debido a que no hay una definición bien establecida y su diagnóstico resulta difícil. Además, la información sobre la fisiopatología y las opciones terapéuticas óptimas es escasa. En este artículo revisamos la definición, fisiopatología y condiciones predisponentes, diagnóstico y tratamiento de la miotoxicidad por las estatinas, y proponemos un esquema práctico para su manejo.
Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are the most widely used lipid-lowering drugs. Their value in reducing the risk of atherosclerotic diseases and their good patient acceptance make them one of the most widely prescribed drug classes.1,2 Despite their safety, a significant proportion of patients report adverse effects. Muscular toxicity is a well-recognized side effect of statins, and 7–29% of patients experience statin-associated muscle symptoms according to registries.3,4 Moreover, statin-associated muscle symptoms are one of the main reasons for statin therapy non-adherence or discontinuation.4,5 In a retrospective cohort study, 59.2% of patients with documented statin-associated events discontinued statins at least temporarily, which may have a marked impact on the cardiovascular benefits of statin therapy.6–8 Thus, considering the high number of patients on statin therapy and the possibility that side effects cause treatment discontinuation in high cardiovascular risk patients, statin-related myotoxicity is a relevant problem in daily clinical practice. However, muscle toxicity of statins has been largely neglected until recently and there is no gold standard diagnostic test, its pathophysiology remains unclear, and information about the optimal therapeutic option is scarce.9,10 This article reviews and discusses the information available on the pathophysiology, diagnosis, and management of statin-related myotoxicity, and provides a practical scheme for its management in clinical practice.
Definition and epidemiologyThere is not a gold standard definition of statin-related myotoxicity, but multiple definitions have been provided by different organizations such as the American College of Cardiology (ACC)/American Heart association (AHA), the National Heart, Lung and Blood Institute (NHLBI), the Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the European Atherosclerosis Society (EAS). Traditionally, statin-related myotoxicity was referred to as statin-associated myopathy, but this term included several distinct conditions with different outcomes and particular requirements for management. The 2014 National Lipid Association Statin Muscle Safety Task Force proposed that muscle-related adverse effects of statins consisted of a range of 5 categories9:
- •
Myalgia: muscle discomfort similar to a viral syndrome (“flue-like” symptoms) including muscle aches, soreness, stiffness, tenderness, or cramps (with or soon after exercise, not nocturnal), with a normal creatine kinase (CK) level.
- •
Myopathy: muscle weakness (not related with pain), without association with CK level.
- •
Myositis: muscle inflammation.
- •
Myonecrosis: elevation of muscle enzymes compared with baseline CK levels or upper limit of normal adjusted for age, sex and race. Myonecrosis may be divided into 3 different degrees according to CK elevation: mild (if there is a three-fold to ten-fold CK elevation), moderate (ten-fold to fifty-fold elevation) and severe (fifty-fold or greater elevation).
- •
Clinical rhabdomyolysis: myonecrosis with myoglobinuria or acute renal failure (an increase in serum creatinine of at least 0.5mg/dL).
This spectrum does not represent a continuum of increasingly severe myopathic manifestations. Different definitions have been used by different societies and studies, which should be taken into account when results from different reports are compared.11
Data on the incidence of statin-related myotoxicity has been collected from randomized clinical trials (RCTs) and observational studies. Large clinical trials have reported statin-induced muscular side effects in up to 12.7% of patients (usually <1.5–5%), while observational studies have shown a significantly higher incidence (7.9–30%). Despite these differences, both types of studies have shown that muscle side effects are common, with myalgia being most prevalent, while rhabdomyolysis is a rare occurrence.3,4,12–18 The Effects of Statins on Skeletal Muscle Function and Performance (STOMP) study, specifically designed to assess the impact of high-dose statin therapy for 6 months in healthy, statin-naive subjects, revealed that atorvastatin 80mg significantly increased the frequency of myalgia as compared to placebo (9.4% versus 4.6%).13 The reported differences may be due to scarce assessment of muscle complaints in RCTs, exclusion of patients with risk factors or previous family or personal history of muscular side effects induced by lipid-lowering drugs, and exclusion of patients with muscle complaints during the pre-randomization, unblinded run-in phase.9,11 All these factors can contribute to a lower incidence rate of muscle side effects in RCTs, and may be assumed to result in an underestimation of the actual incidence in clinical practice. On other hand, both RCTs and observational studies may overestimate this incidence by attributing to statins muscle complaints due to other causes.9 Accordingly, 90% of patients reporting statin-associated muscle symptoms to one statin were able to tolerate an alternative statin.6
Rhabdomyolysis, the most severe side effect related to statin therapy, is rarely seen in RCTs and is more common in patients with other risk factors.19 Its estimated incidence is 1 in 100,000 per year.20 A large meta-analysis of 135,243 patients participating in 35 RCTs found no significant association between statin therapy and rhabdomyolysis (176 [0.25%] with statins versus 168 [0.25%] in controls: odds ratio (OR) 1.04, 95% confidence interval (CI) 0.82–1.30, p=0.73).21 It has been suggested that these findings may be due to small sample sizes and to short duration of the RCT.1,11
Although severe statin-induced myopathy is rare, muscle side effects could be expected in a high number of patients because of the widespread use of this drug class and the high incidence of muscle complaints. This could lead to treatment discontinuation, which is a concern in high cardiovascular risk patients. In the PRIMO study, 10.5% of patients had muscle complaints, and in 38% muscle pain prevented even mild physical activities, which emphasizes the relevance of this side effect.3 Indeed, previous reports have shown that a significant number of patients on statin therapy discontinue treatment due to muscle side effects. In a retrospective cohort study from Israel, up to 75% of the 229,918 patients enrolled discontinued statin therapy within 2 years of its start.5 In another report, the main reason for discontinuation of statin therapy was the onset of side effects.18
Pathophysiology and predisposing conditionsThe exact cellular mechanism by which statins cause muscle toxicity is poorly understood, and different mechanisms have been suggested. To understand these mechanisms, it is important to remember that statins block hydroxyl-methyl-glutaryl CoA reductase, thus inhibiting conversion of HMG CoA to mevalonic acid. Although this is an important step in cholesterol synthesis, the entire mevalonate pathway will be blocked, leading to a decrease in the synthesis of coenzyme Q10 (CoQ10), also known as ubiquinone. CoQ10 is a member of the mitochondrial electron transport pathway with a very important role in mitochondrial function.10 According to the impaired cellular energy utilization and mitochondrial function hypothesis, it has been speculated that: (1) decreased CoQ10 levels could lead to mitochondrial dysfunction and thus to skeletal muscle dysfunction with myotoxicity development; (2) mevalonate pathway blockade would lead to increased muscle protein catabolism; (3) changes in calcium homeostasis, namely an increase in sarcoplasmic calcium, could contribute to statin-related myotoxicity; (4) as cholesterol is an important component of the cell membrane, including the sarcolemma, decreased cholesterol levels could lead to cell membrane lysis and myocyte damage.2,10,11 All of the above mentioned mechanisms are possible causes of statin-related myotoxicity that require confirmation.
There are many factors predisposing to statin-related myotoxicity, either related to the individual patient or to external factors.
Individual factorsSome patient characteristics are risk factors for statin-related myotoxicity, including older age (>75 years), female sex, and low body mass index (BMI).1,10,22,23 Little is known about the influence of race on the risk of statin-related myotoxicity, but in a large randomized trial with simvastatin 40mg/day, Chinese patients had a higher myotoxicity risk as compared to European patients (1.3 versus 0.4events/1000 patients per year).24 Simvastatin should therefore be used with caution in Chinese patients, especially when associated to niacin.10,25 Moreover, rosuvastatin bioavailability has been shown to be higher in Asian as compared to Caucasian patients.26
Systemic diseases and neuromuscular disorders may increase the risk of statin-related myotoxicity. The presence of the following systemic diseases, which can themselves cause myopathy, also increases the risk of statin-related myotoxicity: hypothyroidism, vitamin D deficiency, kidney failure (acute/chronic stages 3–5), impaired liver function, obstructive liver disease, acute infection, major surgery/trauma, organ transplant recipients, severe trauma, human immunodeficiency virus (HIV) infection, diabetes mellitus.1,10,23 Neuromuscular disorders appear to be related to statin myotoxicity in 3 possible ways. The first way would be triggering by statins of a new neuromuscular disorder: statin therapy has been associated to the occurrence of neuromuscular disorders, namely idiopathic inflammatory myopathies such as dermatomyositis, polymyositis, and inclusion body myositis, mononeuritis multiplex, malignant hyperthermia, and statin-related extraocular myopathy. A serious autoimmune disease potentially related to statin use is necrotizing myopathy, in which a macrophagocytic infiltrate destroys muscle fibers. Its exact pathogenesis is unknown, but autoantibodies against HMG CoA and MHC-1 upregulation without inflammation appear to be involved. Necrotizing myopathy responds to immune therapy; 1,27–29 The second proposed way would be detection triggered by statin treatment of a previously preclinical neuromuscular disease to which the patient is predisposed. In patients with a genetic muscle disease, statins may have greater toxicity and cause more frequent symptoms due to the patient's lower ability to overcome muscle toxicity of the drug.9,30–32 In these patients, muscle symptoms usually persist after statin withdrawal, and additional investigation with electromyography and muscle biopsy may be warranted;1,23 Finally, the third way would be increased statin toxicity and worsening of symptoms in patients with known neuromuscular disease. In patients with amyotrophic lateral sclerosis, statins were shown to be associated to symptom worsening. Although the exact mechanism is unknown, it seems that cholesterol lowering is harmful because cholesterol is required to respond to the hypermetabolic demand of amyotrophic lateral sclerosis. Dyslipidemia is associated to prolonged survival, and lipid-lowering drugs should be discontinued.33,34 Statins have also been related to potential worsening of symptoms in myasthenia gravis.23
In recent years, genetic factors have been increasingly thought to influence individual risk of statin-related myotoxicity.35 Genes related to statin metabolism and action have been studied, including those related to liver uptake, bile excretion, and liver metabolism of statins, as well as CK activity and hereditary metabolic myopathies.22 Although the mechanisms and genes involved are not extensively known, the following are illustrative examples: 1,22,36,37
- •
SLCO1B1 – this gene encodes the organic anion transporting polypeptide 1B1 (OATP1B1), which is responsible for liver uptake of most statins. Common variants of SLCO1B1 influence the risk of muscle toxicity in patients taking simvastatin.
- •
SLCO2B1 – this gene encodes a polypeptide called organic anion transporting polypeptide 1B1 (OATP2B1), which is found in the sarcolemma and participates in statin uptake into myocytes.
- •
MRP1 (also known as p-glycoprotein 1), MRP4, MRP5 – multidrug resistance-associated proteins found in skeletal muscle that act as statin efflux transporters.
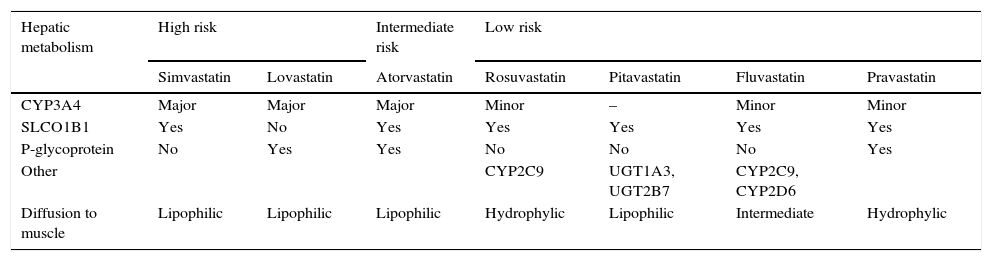
Not all statins have the same potential to cause myotoxicity, and this seems to be related to statin properties, including degree of lipophilicity and metabolism. Higher statin doses appear to be associated to a higher risk of muscle damage.1,10,23 As compared to hydrophilic statins (pravastatin, rosuvastatin), more lipophilic statins (simvastatin, atorvastatin, lovastatin, fluvastatin, pitavastatin) would diffuse more easily to striated muscle cells and could be more toxic to dividing myocytes, resulting in myositis (Table 1). However, this theory is not proven, and statin uptake by muscle cells seems to depend on other factors as well, namely individual patient characteristics.23 The hepatic cytochrome p450 (CYP450) enzyme system plays a key role in metabolism of most statins. Statins extensively metabolized by CYP3A4 (simvastatin, lovastatin and atorvastatin) have higher muscle toxicity. Rosuvastatin and pitavastatin, whose liver uptake is operated by transporters in the hepatocyte membrane, have fewer significant drug interactions and a lower risk of myotoxicity (Table 1).
Risk of myotoxicity with statins depending on hepatic metabolism.
| Hepatic metabolism | High risk | Intermediate risk | Low risk | ||||
|---|---|---|---|---|---|---|---|
| Simvastatin | Lovastatin | Atorvastatin | Rosuvastatin | Pitavastatin | Fluvastatin | Pravastatin | |
| CYP3A4 | Major | Major | Major | Minor | – | Minor | Minor |
| SLCO1B1 | Yes | No | Yes | Yes | Yes | Yes | Yes |
| P-glycoprotein | No | Yes | Yes | No | No | No | Yes |
| Other | CYP2C9 | UGT1A3, UGT2B7 | CYP2C9, CYP2D6 | ||||
| Diffusion to muscle | Lipophilic | Lipophilic | Lipophilic | Hydrophylic | Lipophilic | Intermediate | Hydrophylic |
CYP3A4: cytochrome P-450 CYP3A; SLCO1B1: organic anion transporter 1B1; CYP2C9: cytochrome P-450 CYP2C9; CYP2D6: cytochrome P-450 CYP2D6; UGT1A3: UDP-glucuronosyltransferase 1A3; UGT2B7: UDP-glucuronosyltransferase 2B7.
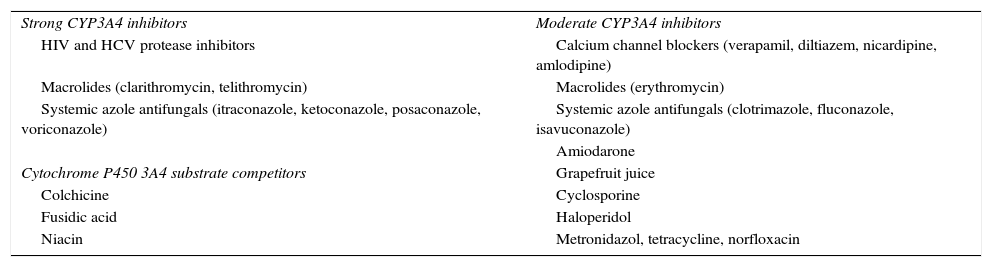
Drugs that interact in statin intestinal absorption, distribution, protein binding, metabolism, or excretion may lead to muscle toxicity.23 Concomitant treatments commonly prescribed with statins have pharmacokinetic profiles that suggest the interaction potential. The most common causes of drug–drug interaction increasing statin plasma concentrations are agents that inhibit statin metabolism by CPY450 or interfere with drug transporters such as OATP1B1 or P-glycoprotein, resulting in a higher risk of statin-associated adverse effects.38 Drugs that inhibit cytochrome P450 3A4 cause statin-related myotoxicity more frequently when patients take statins that are extensively metabolized by CYP450 3A4. Table 2 shows some of the most extensively used drugs that interact with CYP3A4.1,10,23 In patients in whom a CYP450 3A4 inhibitor cannot be discontinued, a statin with a low risk of muscle toxicity should be selected. Concurrent use of statins and fibrates is associated to an increased risk of myotoxicity and to a 10-fold greater risk of hospitalization for rhabdomyolysis as compared to statin monotherapy.9,23,39 Among fibrates, gemfibrozil inhibits glucuronidation and CYP2C8, thereby increasing serum statin levels by 1.9–5.7-fold, but fenofibrate does not interfere with this process.1,23,40,41 Approximately 40% of all statin-related cases of rhabdomyolysis have been reported to occur with statin plus fibrate combinations, but mainly when gemfibrozil is associated with statins.42 This combination should therefore be avoided, and fenofibrate is the drug of choice in patients who need combination therapy.1,23,40,41,43 Cyclosporine impairs statin metabolism through multiple inhibition mechanisms including CYP3A4, CYP2C9, and hepatocyte membrane efflux transporter OATP.23 Association with pitavastatin is formally contraindicated, and simvastatin and atorvastatin should be avoided.1 Other statins have dose limitation recommendations: fluvastatin 20–40mg/day, pravastatin ≤40mg/day, rosuvastatin ≤5mg/day, lovastatin 40–80mg/day. When statin use is needed, fluvastatin and pravastatin should be preferred.1,23
Most frequently used CYP3A4 inhibitors and substrate competitors.
| Strong CYP3A4 inhibitors | Moderate CYP3A4 inhibitors |
| HIV and HCV protease inhibitors | Calcium channel blockers (verapamil, diltiazem, nicardipine, amlodipine) |
| Macrolides (clarithromycin, telithromycin) | Macrolides (erythromycin) |
| Systemic azole antifungals (itraconazole, ketoconazole, posaconazole, voriconazole) | Systemic azole antifungals (clotrimazole, fluconazole, isavuconazole) |
| Amiodarone | |
| Cytochrome P450 3A4 substrate competitors | Grapefruit juice |
| Colchicine | Cyclosporine |
| Fusidic acid | Haloperidol |
| Niacin | Metronidazol, tetracycline, norfloxacin |
CYP3A4: cytochrome P-450 CYP3A; HIV: human immunodeficiency virus; HCV: hepatitis C virus.
In patients treated with drugs that may predispose to myopathy, risk is increased when statins are taken. Agents in this category include anti-inflammatory drugs (salicylates, nonsteroidal anti-inflammatory drugs, glucocorticoids), cyclosporine, azathioprine, daptomycin, antiviral drugs (zidovudine, ritonavir, didanosine), neuroleptic and antipsychotic agents (haloperidol, risperidone), anesthetics and neuromuscular blockers (ketamine, propofol, succinylcholine), alcohol and drug abuse (amphetamines, cocaine, opioids/heroin).9,10
ExerciseIn patients taking statins, the beginning of vigorous physical activity may lead to muscle toxicity, usually mild and even subclinical. Progressive exercise training may be beneficial, because it allows for muscle adaptation to exercise, with increased oxidative capacity and greater protection from the pro-oxidant effects of statins. However, even in patients who practice regular exercise, statins seem to have harmful effects on muscle when vigorous prolonged exercise is performed. Muscle damage is usually mild and statin discontinuation before exercise is not required, but it is not clear if statins should be discontinued before vigorous exercise, particularly when there are other potential causes of rhabdomyolysis.1,9,10,44
DiagnosisDiagnosis of statin-induced myotoxicity is based on medical history, clinical examination, and laboratory tests. Muscle biopsy may be helpful to diagnose an underlying disorder that predisposes the patient to statin-associated myotoxicity.
Clinical presentationStatin-related muscular disease may have several clinical presentations, but the most common is proximal and usually symmetric muscle pain/tenderness and/or weakness affecting major muscle groups such as the gluteal, calf, thigh (hip flexor, quadriceps and hamstring) and back muscles. Patients may also report stiffness and fatigue. Symptoms may be asymmetric and localized. Muscle symptoms may be associated to functional impairment of proximal muscles occurring as difficulty in raising arms, raising from a seated position, or climbing stairs.9,10,45 Tendon cramps and pain, decreased exercise tolerance (namely muscle strength, endurance and aerobic performance), prolonged recovery after exercise, and decreased fitness improvement with exercise are also possible symptoms of statin-related myotoxicity, but data available in this regard are conflicting, and additional studies are needed.13,46 Finally, not all patients with statin-related muscle disorders are symptomatic, and myotoxicity has been histologically documented in asymptomatic patients.47
Symptoms may occur at any time while taking statins, but usually appear in the first 4–6 weeks. Symptoms may also appear or worsen when statin dose is increased, when an interacting drug is introduced, or when a statin that has caused symptoms is restarted. In the latter situation, called “dechallenge and rechallenge”, taking patients off and on statins may help understand if the symptoms are caused by the drug. If they are, symptoms will recur in a majority of patients when the statin is restarted.9,10 In a study of 45 patients with statin-associated myotoxicity, symptoms appeared after a mean treatment time of 6.3 months (range, 0.25–48 months); two thirds of patients had symptoms after up to 6 months of treatment. In the same study, after statin discontinuation, symptoms resolved after a mean time of 2.3 months (range, 0.25–14 months); in more than half the patients (58%) symptoms disappeared in the first month, and almost all patients (93%) were free of symptoms within 6 months.48
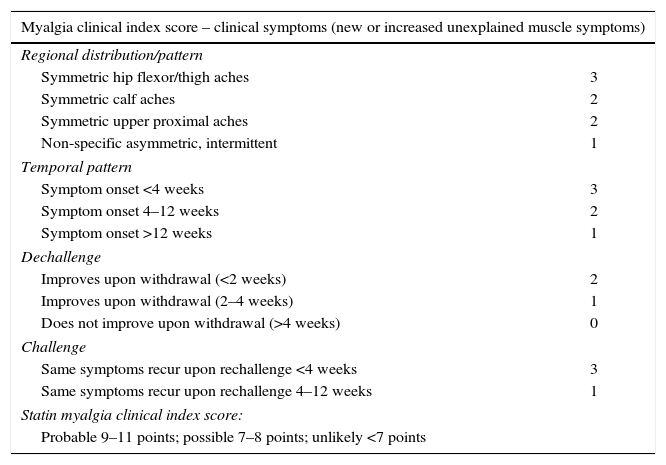
The European Atherosclerosis Society Consensus Panel proposed that the probability of statin-related myotoxicity should be based on the features of muscle symptoms, their relationship with statin start, statin discontinuation, and re-challenge.10 Diagnosis is more likely when symptoms markedly regress and elevated CK levels decrease in a few weeks after discontinuation of the statin or the interacting drug and/or reappear within a month of drug re-challenge. Differential diagnosis is however challenging for several reasons: symptoms are usually subjective; symptoms may have many possible causes; and proving causality based on the temporal relationship between statin start and discontinuation and symptom appearance and disappearance is usually difficult in the outpatient setting.9,10 Even so, diagnosis may not be clear, and a scoring system for statin-associated symptoms has been proposed (Table 3), but this has not been validated yet in clinical practice.9
Myalgia clinical index score.9
| Myalgia clinical index score – clinical symptoms (new or increased unexplained muscle symptoms) | |
|---|---|
| Regional distribution/pattern | |
| Symmetric hip flexor/thigh aches | 3 |
| Symmetric calf aches | 2 |
| Symmetric upper proximal aches | 2 |
| Non-specific asymmetric, intermittent | 1 |
| Temporal pattern | |
| Symptom onset <4 weeks | 3 |
| Symptom onset 4–12 weeks | 2 |
| Symptom onset >12 weeks | 1 |
| Dechallenge | |
| Improves upon withdrawal (<2 weeks) | 2 |
| Improves upon withdrawal (2–4 weeks) | 1 |
| Does not improve upon withdrawal (>4 weeks) | 0 |
| Challenge | |
| Same symptoms recur upon rechallenge <4 weeks | 3 |
| Same symptoms recur upon rechallenge 4–12 weeks | 1 |
| Statin myalgia clinical index score: | |
| Probable 9–11 points; possible 7–8 points; unlikely <7 points | |
A thorough examination assessing muscle performance may be used to diagnose myopathy, which is present when patients have proximal muscle weakness in lower and upper limbs ≤4 according to the Medical Research Council (MRC) score, a scoring system for muscle strength.9,49 For comparison, in patients with prior muscle symptoms, it would be beneficial to perform a muscle strength examination before statin treatment is started.9
In asymptomatic patients taking statins, no routine muscle strength assessment is advised. However, patients with mild symptoms and CK levels up to four times the upper limit of normal and who continue on statin therapy should be assessed annually. In patients with more severe symptoms and/or more increased CK levels, regular muscle strength assessment should be considered and a dechallenge/rechallenge approach may clarify if the symptoms are due to statins.9
Creatine kinase levelsCK is a muscle enzyme involved in cell energy storage and transfer which is released when cell damage occurs.9,10 It is the most commonly used biochemical marker of muscle disease because of its high sensitivity and high serum levels in the presence of muscular damage. It is also a good marker to monitor the course of muscle disease.50 It should be noted, however, that individual CK levels are affected by many factors such as age, gender, ethnicity, and physical activity (type, intensity, duration, and regular versus intermittent practice).9 It has been suggested that CK levels should be interpreted based on baseline values of the patient or on normative upper limit of normal adjusted for age, race, and gender. According to this, a >4-fold increase in baseline CK level would be rated as “mild myonecrosis”, a ≥10-fold increase would be “moderate myonecrosis”, and a ≥50-fold increase would be considered “severe myonecrosis”.9 Rhabdomyolysis, which is the most severe presentation of myonecrosis, is characterized by severe muscle pain and weakness associated with very high CK levels, myoglobinemia, myoglobinuria, and acute renal failure due to myoglobin precipitation in renal tubules.9,10
Most symptomatic patients with statin-associated muscle disease have normal or mildly to moderately elevated CK levels (<4x upper limit of normal – ULN), and CK levels >10x ULN are considered substantially elevated and related to the presence of myopathy/myositis.10 Muscle symptoms are more likely to be caused by statin when elevated CK levels decrease after statin discontinuation, and the clinical significance of an asymptomatic increase in CK levels in patients taking statins has not been elucidated yet.10 On the other hand, muscle enzymes may be elevated in many neuromuscular disorders or in situations not related to muscular disease such as iatrogenic muscle damage, exercise, kidney disease, and seizures.9,51,52
Since CK levels are rarely increased during statin therapy, typical symptoms occur in the absence of increased CK levels, and levels of the enzyme do not necessarily indicative statin-induced muscle damage, routine measurement of CK is not indicated in patients on statin therapy because it is not cost-effective and to avoid unnecessary statin discontinuation.10,53,54 However, as normal CK reference ranges vary depending on patient age, race and gender, measurement of baseline CK levels would be convenient when a new statin is started as a reference for comparison if patients develop symptoms, especially in individuals considered to be at an increased risk of adverse muscle events.55 CK levels should be evaluated in patients with muscle symptoms or generalized fatigue, and should be measured again after statin discontinuation/dose reduction and rechallenge.
Muscle biopsyMuscle biopsy has no value for diagnosis of statin-associated myotoxicity because it is invasive and its sensitivity and specificity are unknown. Muscular biopsy findings vary depending on disease stage, but histological criteria to classify the severity of statin-associated myotoxicity have not been defined. A muscle biopsy is recommended in patients with persistent muscle symptoms after statin discontinuation, especially if muscular tenderness, weakness, or persistently elevated CK levels exist. It should be noted, however, that muscle symptoms may persist for longer than one year after statin discontinuation, and CK levels usually normalize afterwards. It cannot be predicted which patients will have a longer recovery.9
ManagementManagement of statin-related myotoxicity may be challenging and depends on the specific signs of the condition. In the absence of symptoms, incidental CK elevation may be related to statin therapy, but its clinical significance is unclear. Statin therapy may therefore be continued, if indicated, and CK measurement should be repeated in appropriate conditions. If there are muscle complains, initial management should include assessment of potential interactions with concomitant drugs and risk factors that may predispose to statin-related myotoxicity; exclusion of other causes of muscle symptoms, including hypothyroidism, vitamin D deficiency, and other common causes of myopathy; and review of the medical indication for statin use. The European Atherosclerosis Society Consensus Panel recently suggested a specific approach for patients with muscle symptoms based on CK levels and cardiovascular risk (CVR)10:
- •
In patients with marginal elevations of CK levels (<4x ULN), if CVR is low, need for this medication should be reevaluated and a healthy lifestyle should be promoted; on the other hand, if CVR is high, it should be assessed whether treatment benefits outweigh its side effects and the most suitable alternative should be selected (see below).
- •
If CK levels are moderately elevated (between 4x and 10x ULN) and the patient has a low CVR, statin therapy should be stopped and its need should be reassessed. If CVR is high, treatment may be continued and CK levels should be closely monitored.
- •
If CK levels are higher than 10x ULN, statin therapy should be discontinued due to risk of rhabdomyolysis, and the same treatment regimen should not be restarted. After CK levels return to normal, an alternative approach with/without statin may be considered. Due to the high risk of recurrence, patients with previous statin-induced rhabdomyolysis should not be treated with another statin unless an acute reversible cause is identified for that episode of rhabdomyolysis.
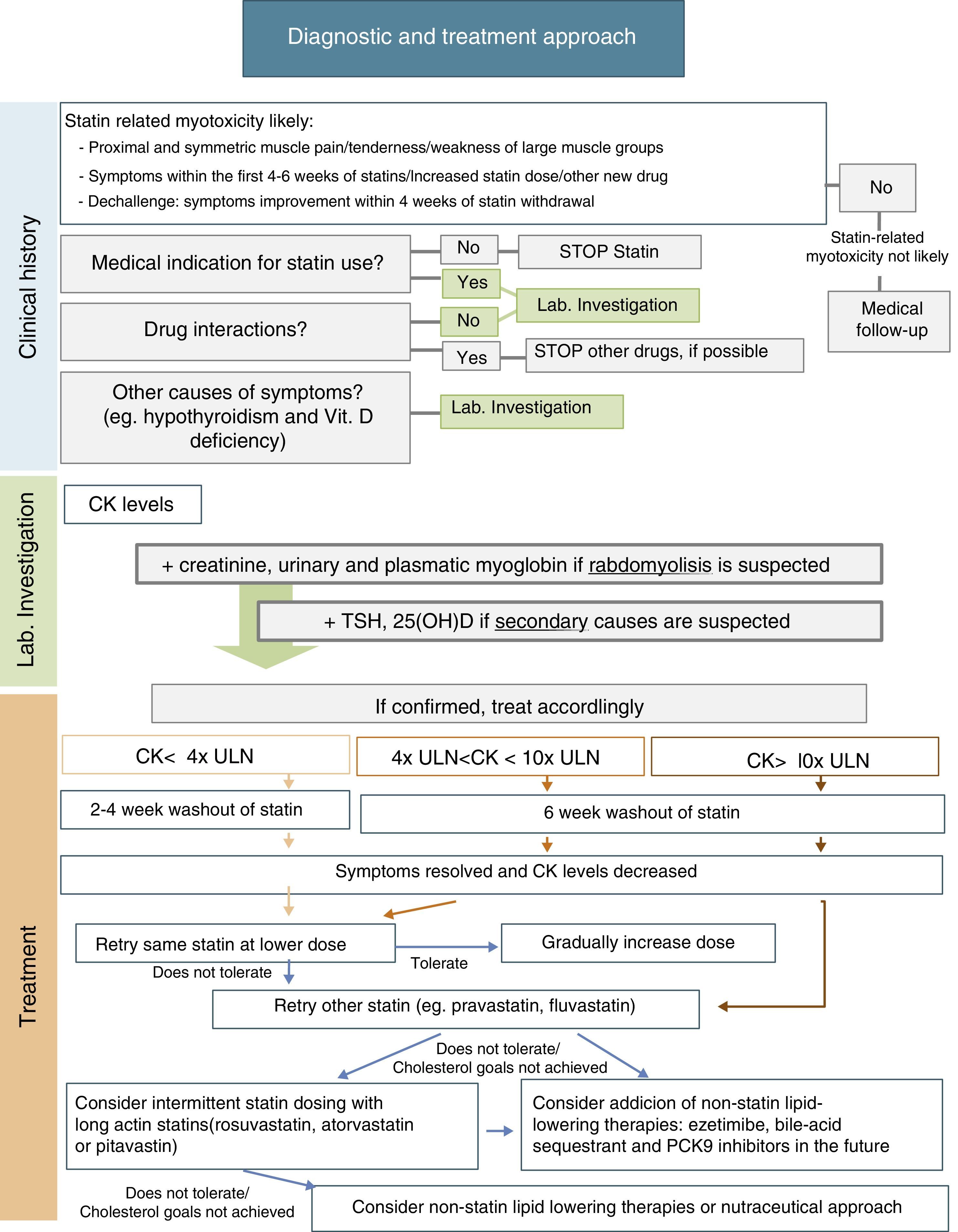
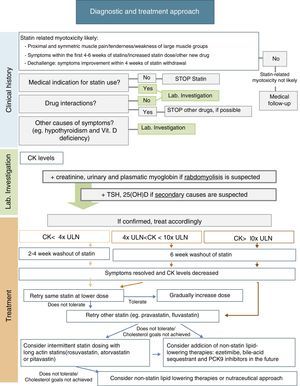
After statin discontinuation and resolution of symptoms and CK elevation, there are several options for specific drug management of these patients: maintaining the same statin at a reduced daily dosage; maintaining the same statin, given every-other day at the same or a lower dose; decreasing dosing frequency even more (e.g. once weekly); changing to a different statin, alone or in combination with non-statin lipid-lowering agents; and using a nutraceutical approach.9Fig. 1 includes a scheme for stepwise selection of different therapeutic options. Because of the associated cardiovascular benefit of statins, the first pharmacological approach is maintenance of statin treatment, considering use of the same statin at a lower dose or switching to another statin (preferably pravastatin, fluvastatin, or pitavastatin) and, if the patient does not tolerate these strategies, alternate day or less frequent dosing should be considered, preferably using a high intensity and long-acting statin such as atorvastatin, rosuvastatin, or pitavastatin. However, to guarantee a satisfactory LDL cholesterol (LDL-C) goal, any of these therapeutic approaches may also be used in combination with non-statin lipid-lowering therapy such as ezetimibe or bile acid sequestrants, and the new lipid-lowering drug proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies. Nutraceuticals and supplemental therapies, either alone or with drug therapy, may finally be considered. The evidence available for the different strategies is summarized below.
It has been suggested that the first therapeutic option is rechallenge with the same statin therapy at lower doses. Dose may be uptitrated based on patient tolerance. Using this strategy, 70–80% of patients can tolerate treatment.9
It is also reasonable to switch to another statin, especially if the patient does not tolerate the same statin at a lower dose. In this case, it is important to select a statin with an acceptable tolerance. Statins of the lower risk group (independent of cytochrome P450 3A4 metabolism), including pravastatin, fluvastatin, rosuvastatin, or pitavastatin, should be selected. Pravastatin and fluvastatin appear to have less intrinsic toxicity. A randomized clinical trial including 199 patients with history of statin-related myotoxicity analyzed prevalence of recurrent muscle symptoms when therapy was changed to extended-release fluvastatin monotherapy (80mg/day), ezetimibe (10mg/day) or a combination of fluvastatin plus ezetimibe. The proportions of patients who developed muscle symptoms in the fluvastatin, ezetimibe and combined therapy groups were 17%, 24%, and 14% respectively.56 It has been reported that 92% of patients tolerate a second statin and 72.5% tolerate even a third statin after statin discontinuation for side effects.9,14
Another option is alternate day or less frequent dosing (e.g. once or twice weekly). A potent long-acting statin such as atorvastatin, rosuvastatin, or pitavastatin should be chosen. This approach may be especially useful in patients fearing side effects. Some studies have reported tolerance in 70–75% of formerly intolerant patients and a LDL-C decrease up to 40–45%.9,58,59 A randomized clinical trial of 50 patients given a mean dose of rosuvastatin 10mg once weekly showed a high tolerance rate (74%) and a non-negligible LDL decrease (23%) during a mean follow-up of 4 months.60 Although alternate day dosing has not been shown to reduce cardiovascular events, it may be a feasible option. These patients should be carefully monitored, and statin dose titration should be attempted as tolerated.
Non-statin lipid-lowering therapyCombination therapy may be an option to achieve the LDL-C goal with lower statin doses. Ezetimibe is considered the drug of first choice for combination with statins. In the Stein el al. trial, ezetimibe lowered LDL-C levels by 15.6%, fluvastatin by 32.8%, and fluvastatin plus ezetimibe by 46.1%.56 Alternatively, bile acid sequestrants (e.g. colesevelam) can lower LDL-C by 15–19%. Fenofibrate is another safe option for combination therapy with statins. In a large randomized clinical trial of treatment with fenofibrate in 9795 patients, incidence of muscle side effects was less than 1%, similar to the incidence found in those taking placebo or also a statin.57 In statin-intolerant patients, bile acid sequestrants and fenofibrate may be associated with ezetimibe, with additional LDL-C reduction.9,10
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are a promising lipid-lowering therapy. PCSK9 is a serine protease that leads to degradation of hepatocyte LDL-C receptors, increasing LDL-C levels. Monoclonal antibodies that inhibit PCSK9 may markedly decrease LDL-C levels. The GAUSS-2 study, a randomized, placebo-controlled, phase 3 clinical trial, showed that evolocumab effectively decreased LDL-C levels in patients who cannot tolerate effective doses of at least 2 statins. LDL-C reduction was 53–56% in the evolocumab group, as compared to 37–39% in the ezetimibe group, and incidence of myalgia was lower in the PCSK9 inhibitor group (7–9%, as compared to 18% in the ezetimibe group).61 The ODYSSEY ALTERNATIVE, an ongoing phase 3, multicenter, randomized, double-blind trial, is testing the efficacy of alirocumab in patients intolerant to 2 different statins.62 Thus, this is a very hopeful therapy for statin-intolerant patients.
Nutraceuticals and supplemental therapiesNutraceuticals represent an essential approach in all patients with dyslipidemia, whether treated or not with lipid-lowering drugs. All these patients must follow a low saturated fat diet with avoidance of trans fats.10 Red yeast rice has been shown to decrease LDL-C by 20%-30% because it contains monacolin K, a product similar to lovastatin.63 However, pharmacokinetic interactions and safety of red yeast rice use are unknown, and it has not yet been proved to be superior to statins for lowering recurrence of statin-related muscle side effects.63 CoQ10 and vitamin D have been suggested as potential approaches to improve statin tolerability. CoQ10 depletion may play a role in the pathogenesis of statin-related myotoxicity. However, a meta-analysis of 6 randomized clinical trials including 302 patients did not support use of CoQ10 supplementation for statin-related muscle side effects (CK activity and muscle pain).64 Based on the role of vitamin D in muscle cell function, it has been postulated that vitamin D deficiency and statins, additively or synergistically, may cause muscle complaints. Several authors have shown an association between low vitamin D levels and statin-related myotoxicity.65–67 A recent prospective study of 146 patients with low vitamin D levels and intolerance to at least 2 statins found that 88%-95% of patients tolerated statins with no muscle complaints after supplementation and repletion of vitamin D serum levels.68 However, other reports do not support this association.69 A placebo-controlled, double-blind study is needed to clarify this association. Until then, vitamin D supplementation may be tried in patients with low vitamin D levels and statin-related muscle side effects.55,70
ConclusionStatin-related myotoxicity is a common, probably underdiagnosed problem in clinical practice. Its diagnosis is essentially clinical, and temporal relation between statin use and symptom occurrence may be useful. An effort should be made to identify individual factors predisposing to this condition. Management should be individualized based on severity of myotoxicity and cardiovascular risk of the patient. Treatment should combine a maximally tolerated or even non-daily statin dose with non-statin-based lipid-lowering therapies in order to achieve the LDL-C goal. Preclinical and clinical studies to understand the potential pathophysiological link between statins and muscle symptoms, and long-term, rigorously designed randomized clinical trials comparing different treatment options are a priority to further develop therapies to prevent statin-related myotoxicity and to provide optimal drug treatment for patients with statin-related muscle side effects.
Conflicts of interestThe authors state that they have no conflicts of interest regarding the contents of the manuscript.