At present, the majority of patients with type 1 diabetes mellitus do not achieve the recommended glycemic control goals to reduce the risk of acute and chronic complications. Hybrid closed-loop systems or automated insulin infusion systems emerged as an opportunity to improve metabolic control, quality of life and reduce the psychosocial impact of type 1 diabetes. This article analyzes the evidence regarding their effectiveness and safety, the challenges they pose and best practices to optimize results when implemented in clinical practice.
En la actualidad, la mayoría de los pacientes con diabetes mellitus tipo 1 no logra alcanzar los objetivos de control glucémico recomendados para reducir el riesgo de complicaciones agudas y crónicas. Los sistemas híbridos de asa cerrada o sistemas de infusión automatizada de insulina emergen como una oportunidad para mejorar el control metabólico, la calidad de vida y reducir el impacto psicosocial de la diabetes tipo 1. En este artículo se analiza la evidencia en relación con su eficacia y seguridad, los retos que plantean y las mejores prácticas para optimizar los resultados en su implementación en la práctica clínica.
Type 1 diabetes mellitus (T1DM) is a chronic and demanding condition that poses a constant challenge for children and adolescents with diabetes and their families, representing a significant burden for health care systems. The goal of treatment in pediatric age is to achieve and maintain glycemic management within recommended targets to reduce the risk of acute and chronic complications, as well as the potential harmful effects of hypoglycemia and hyperglycemia on cognitive development and brain structure, while also improving the quality of life for children and adolescents with T1DM.1
However, even today, despite technological advances and the development of new insulin analog formulations, most patients fail to achieve the glycemic targets advocated by international societies. Indeed, only 21% maintain levels of glycated hemoglobin (HbA1c) < 7.0%, according to recent data from over 25,600 children included in the SWEET initiative registry (Better control in Pediatric and Adolescent diabeteS: Working to crEate CEnTers of Reference).2 The mean HbA1c levels among patients aged 1 to 15 years is 8.6%, according to the T1D Exchange registry report for the period 2021–2022, and among those under 6 years, 8.0%.3,4 Additionally, it is estimated that 1.4 million children and youth under 20 years of age are diagnosed with T1DM worldwide, and the global number of people living with T1DM is projected to go from 66% up to 116% from 2020 through 2040.5
All of this highlights the need, which is still not fully met, for more effective methods to achieve glycemic control targets. In the past five years, significant advances have been made in automated insulin delivery (AID) systems. Thus, hybrid closed-loop systems or AID systems are gradually transforming the clinical management of T1DM, opening a window of opportunity to improve the health and quality of life of people with diabetes and reduce the psychosocial impact of the disease.
The objective of this review is to analyze the current evidence regarding their safety and efficacy profile, the challenges they present, and best practices to optimize the results obtained in clinical care.
Automated insulin delivery systems: functioning, components, and clinical resultsAID systems consist of a continuous glucose monitoring (CGM) device connected to an algorithm that responds in real-time to variations in glucose levels by adjusting the doses of insulin delivered by an insulin pump. Current AID systems are considered hybrid systems because they require user input regarding meals and exercise.6 The control algorithm may be housed in the insulin pump or a mobile application, and fundamentally, there are three types: Proportional-Integrative-Derivative (PID), Model Predictive Control (MPC), or fuzzy logic.
- 1
PID algorithms adjust insulin delivery based on glucose monitoring data, relying on three elements: the difference between measured and target glucose levels (the proportional component), the area under the curve between measured and target glucose (the integral component), and the rate of change in measured glucose levels over time (the derivative component).
- 2
MPC algorithms use a predictive mathematical model that determines glucose excursions and adjusts insulin delivery to reach the target taking into account estimated insulin sensitivity.
- 3
Fuzzy logic-based algorithms modulate insulin delivery according to a set of rules designed to mimic the knowledge and reasoning of diabetes treatment experts.
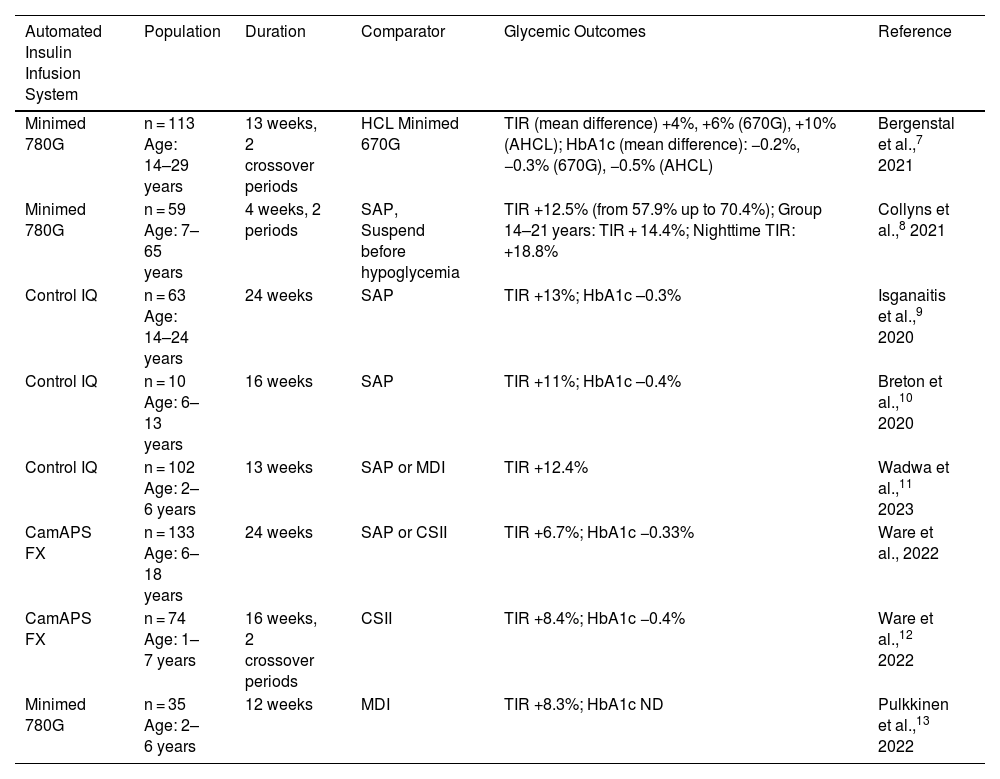
AID systems have shown a clinically relevant and homogeneous increase in time in range (TIR, 70–180 mg/dL) between 9% and 14% vs standard therapy in randomized controlled clinical trials in children and adolescents with diabetes,7–14 even in children under six years of age (Table 1). The observed improvement in TIR is accompanied by a reduction in mean glucose and HbA1c between 0.3% and 0.5%, being greater during the nighttime and overall in trials in which the study group started with a previously higher HbA1c. Regarding time in hypoglycemia, some studies show no differences, while others indicate a significant reduction, even vs systems integrated with automatic suspension in prediction of low glucose.8,10
Randomized Clinical Trials with Automated Insulin Infusion Systems (AID Systems).
| Automated Insulin Infusion System | Population | Duration | Comparator | Glycemic Outcomes | Reference |
|---|---|---|---|---|---|
| Minimed 780G | n = 113 Age: 14–29 years | 13 weeks, 2 crossover periods | HCL Minimed 670G | TIR (mean difference) +4%, +6% (670G), +10% (AHCL); HbA1c (mean difference): −0.2%, −0.3% (670G), −0.5% (AHCL) | Bergenstal et al.,7 2021 |
| Minimed 780G | n = 59 Age: 7–65 years | 4 weeks, 2 periods | SAP, Suspend before hypoglycemia | TIR +12.5% (from 57.9% up to 70.4%); Group 14–21 years: TIR + 14.4%; Nighttime TIR: +18.8% | Collyns et al.,8 2021 |
| Control IQ | n = 63 Age: 14–24 years | 24 weeks | SAP | TIR +13%; HbA1c –0.3% | Isganaitis et al.,9 2020 |
| Control IQ | n = 10 Age: 6–13 years | 16 weeks | SAP | TIR +11%; HbA1c –0.4% | Breton et al.,10 2020 |
| Control IQ | n = 102 Age: 2–6 years | 13 weeks | SAP or MDI | TIR +12.4% | Wadwa et al.,11 2023 |
| CamAPS FX | n = 133 Age: 6–18 years | 24 weeks | SAP or CSII | TIR +6.7%; HbA1c −0.33% | Ware et al., 2022 |
| CamAPS FX | n = 74 Age: 1–7 years | 16 weeks, 2 crossover periods | CSII | TIR +8.4%; HbA1c −0.4% | Ware et al.,12 2022 |
| Minimed 780G | n = 35 Age: 2–6 years | 12 weeks | MDI | TIR +8.3%; HbA1c ND | Pulkkinen et al.,13 2022 |
AHCL: advanced hybrid closed-loop; HbA1c: glycated hemoglobin; HCL: hybrid closed-loop; CSII: continuous subcutaneous insulin infusion; MDI: multiple daily insulin doses; SAP: sensor augmented pump; TIR: time in range (70–180 mg/dL).
In patients previously treated with multiple daily insulin injections (MDI) using a structured 10-day initiation protocol, Petrovski et al. demonstrated in a 12-week prospective study involving children and adolescents aged 7 to 17 years an improvement in TIR of +36.7% (from 42.1% ± 18.7% at baseline up to 78.8% ± 6.1% at the end of the study) with a decrease in HbA1c from 8.6% ± 1.7% down to 6.5% ± 0.7%.15 In newly diagnosed T1DM patients, AID systems show an improvement in TIR at both 12 and 24 months vs standard treatment in a randomized controlled trial; however, this does not translate into improved preservation of pancreatic reserve.16,17
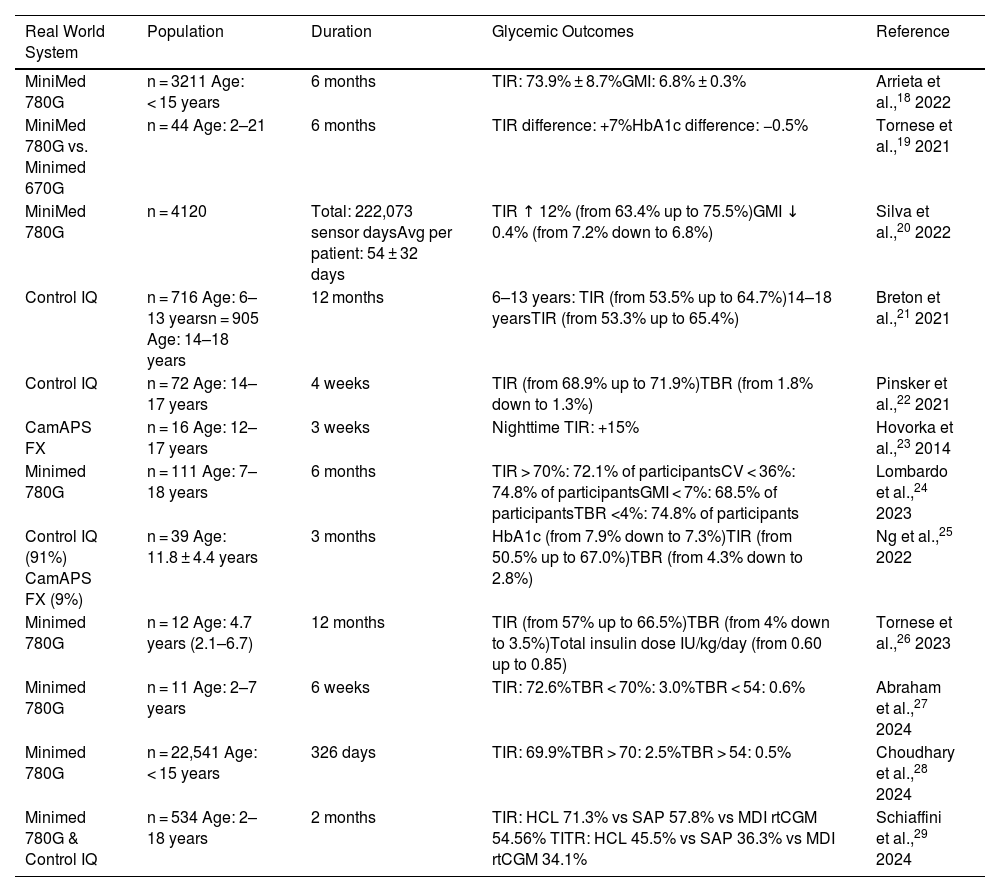
Real-life study data also demonstrate the superiority of AID systems for improving TIR, Time in Tight Range (TITR, 70–140 mg/dL), HbA1c, mean glucose, and reducing time in hypoglycemia and hyperglycemia in the mid-term, across large population groups, even in children under six years of age18–29 (Table 2).
Real-World Studies with Automated Insulin Infusion Systems (AID Systems).
| Real World System | Population | Duration | Glycemic Outcomes | Reference |
|---|---|---|---|---|
| MiniMed 780G | n = 3211 Age: < 15 years | 6 months | TIR: 73.9% ± 8.7%GMI: 6.8% ± 0.3% | Arrieta et al.,18 2022 |
| MiniMed 780G vs. Minimed 670G | n = 44 Age: 2–21 | 6 months | TIR difference: +7%HbA1c difference: −0.5% | Tornese et al.,19 2021 |
| MiniMed 780G | n = 4120 | Total: 222,073 sensor daysAvg per patient: 54 ± 32 days | TIR ↑ 12% (from 63.4% up to 75.5%)GMI ↓ 0.4% (from 7.2% down to 6.8%) | Silva et al.,20 2022 |
| Control IQ | n = 716 Age: 6–13 yearsn = 905 Age: 14–18 years | 12 months | 6–13 years: TIR (from 53.5% up to 64.7%)14–18 yearsTIR (from 53.3% up to 65.4%) | Breton et al.,21 2021 |
| Control IQ | n = 72 Age: 14–17 years | 4 weeks | TIR (from 68.9% up to 71.9%)TBR (from 1.8% down to 1.3%) | Pinsker et al.,22 2021 |
| CamAPS FX | n = 16 Age: 12–17 years | 3 weeks | Nighttime TIR: +15% | Hovorka et al.,23 2014 |
| Minimed 780G | n = 111 Age: 7–18 years | 6 months | TIR > 70%: 72.1% of participantsCV < 36%: 74.8% of participantsGMI < 7%: 68.5% of participantsTBR <4%: 74.8% of participants | Lombardo et al.,24 2023 |
| Control IQ (91%) CamAPS FX (9%) | n = 39 Age: 11.8 ± 4.4 years | 3 months | HbA1c (from 7.9% down to 7.3%)TIR (from 50.5% up to 67.0%)TBR (from 4.3% down to 2.8%) | Ng et al.,25 2022 |
| Minimed 780G | n = 12 Age: 4.7 years (2.1–6.7) | 12 months | TIR (from 57% up to 66.5%)TBR (from 4% down to 3.5%)Total insulin dose IU/kg/day (from 0.60 up to 0.85) | Tornese et al.,26 2023 |
| Minimed 780G | n = 11 Age: 2–7 years | 6 weeks | TIR: 72.6%TBR < 70%: 3.0%TBR < 54: 0.6% | Abraham et al.,27 2024 |
| Minimed 780G | n = 22,541 Age: < 15 years | 326 days | TIR: 69.9%TBR > 70: 2.5%TBR > 54: 0.5% | Choudhary et al.,28 2024 |
| Minimed 780G & Control IQ | n = 534 Age: 2–18 years | 2 months | TIR: HCL 71.3% vs SAP 57.8% vs MDI rtCGM 54.56% TITR: HCL 45.5% vs SAP 36.3% vs MDI rtCGM 34.1% | Schiaffini et al.,29 2024 |
CV: coefficient of variation; GMI: Glucose Management Indicator; HbA1c: glycated hemoglobin; HCL: hybrid closed-loop; MDI: multiple daily injections of insulin; rtCGM: Real Time Continuous Glucose Monitoring; SAP: sensor augmented pump; TBR: time below range (< 70 mg/dL); TIR: time in range (70–180 mg/dL); TITR: Time In Tight Range (70–140 mg/dL).
Finally, there are few studies comparing outcomes between the different current AID systems. Bassi et al., in a retrospective study, describe a slightly greater increase in TIR in the group of patients treated with the Minimed™ 780G system (Medtronic, United States) vs the group treated with the Control IQ™ system (Tandem Diabetes Care, United States) 1 year after staring using the system.30 Meanwhile, Schiaffini et al. found no differences in TIR between the two systems in a study involving 31 children and adolescents with a mean age of 13.05 ± 2.4 years after four weeks of treatment.31 Beato-Víbora et al., in a multicenter retrospective study including 150 patients aged 16 to 72 years comparing the same systems, also found no significant differences in TIR or HbA1c.32 Santova et al. compared results from a total of 512 children and adolescents under 19 years of age treated with the Medtronic 780G systems, Tandem Control IQ, and the Do It Yourself Android APS system, and found a lower HbA1c in the Android APS group, followed by the Control IQ group, although they reported significant differences between the three groups concerning diabetes duration, age, insulin dose, body mass index, and number of patients in each treatment group.33
Impact on quality of lifeRegarding quality of life, although results are not as homogeneous as those described concerning metabolic control, AID systems have demonstrated, in randomized controlled trials, improvements in technology satisfaction, sleep quality, and diabetes-related quality of life vs treatment with multiple doses or systems integrated with suspension in prediction of hypoglycemia.34,35 Compared to first-generation AID systems, the level of satisfaction with technology is higher with advanced systems and increases with greater use of the automatic mode.36
Parents of children with T1DM experience improvements in sleep quality, as well as a decrease in fear of hypoglycemia and diabetes-related stress.37
Technical characteristics of automated insulin delivery systemsMinimed 780G (Medtronic)This system utilizes an interoperable PID and fuzzy logic algorithm, the Minimed 780G™ insulin pump, and the Guardian 4™ sensor, and soon the Simplera™ sensor (Medtronic, United States). Upon system initiation, it requires, at least, 48 hours of use in manual mode. In automatic mode, the algorithm automatically calculates the basal dose every 5 minutes based on sensor glucose, trend, target, and insulin history. It allows programming of glucose targets at 100, 110, or 120 mg/dL. It delivers automatic correction boluses up to every five minutes, targeting 120 mg/dL if the maximum basal has been reached. The duration of active insulin (DIA), programmable from two to eight hours, also influences the insulin dose calculations by the algorithm.
The calculation of preprandial boluses is based on the carbohydrate-to-insulin ratio. The dose is adjusted based on the glucose detected by the sensor at the time of bolus administration, using a correction factor calculated by the system. This system includes a function called Safe Meal Bolus, whereby the system can reduce the corresponding insulin dose for meals in case of predicting hypoglycemia. In manual mode, it has an automatic insulin suspension function in anticipation of hypoglycemia.
Tandem control-IQThis system combines the Control IQ algorithm, an MPC type, the Tandem tX2™ pump (Tandem Diabetes Care, United States), and the Dexcom G6/G7™ sensor (Dexcom, United States) or the FreeStyle Libre™ (Abbott Diabetes Care, United Kingdom). This system administers user-programmed basal doses with the capability to automatically increase them if glucose prediction within the next 30 minutes is outside the target glucose range (112.5–160 mg/dL). The system can deliver an automatic correction bolus (60% of the calculated bolus based on the correction factor) if glucose prediction exceeds 180 mg/dL within the next 30 minutes. In case of predicting glucose levels < 80 mg/dL, the system suspends insulin infusion. In automatic mode, the glucose target for correction dose calculations is 110 mg/dL, and the DIA is five hours, without the option to modify these values.
The system allows for extended bolus administration with a maximum duration of two hours and use of temporary basal measurements.
CamAPS FXThis is currently the only AID system approved for children from 1 year of age. It uses the CamAPS FX™ algorithm (CamDiab Ltd., United Kingdom), the Ypsopump™ insulin pump (Ypsomed, Switzerland), and the Dexcom G6™ sensor (Dexcom, United States) or the FreeStyle Libre 3™ (Abbott Diabetes Care, United Kingdom). It utilizes an MPC-type algorithm. It automatically administers basal insulin in the form of extended boluses every 8 to 12 minutes based on sensor data. The algorithm is not housed in the pump but in a mobile application. The glucose target can be customized in hourly segments between 80 and 198 mg/dL. It does not deliver automatic correction boluses. Boluses for meals or corrections are administered from the application, and over-correction is permitted without exiting automatic mode. It has pre-loaded insulin cartridges.
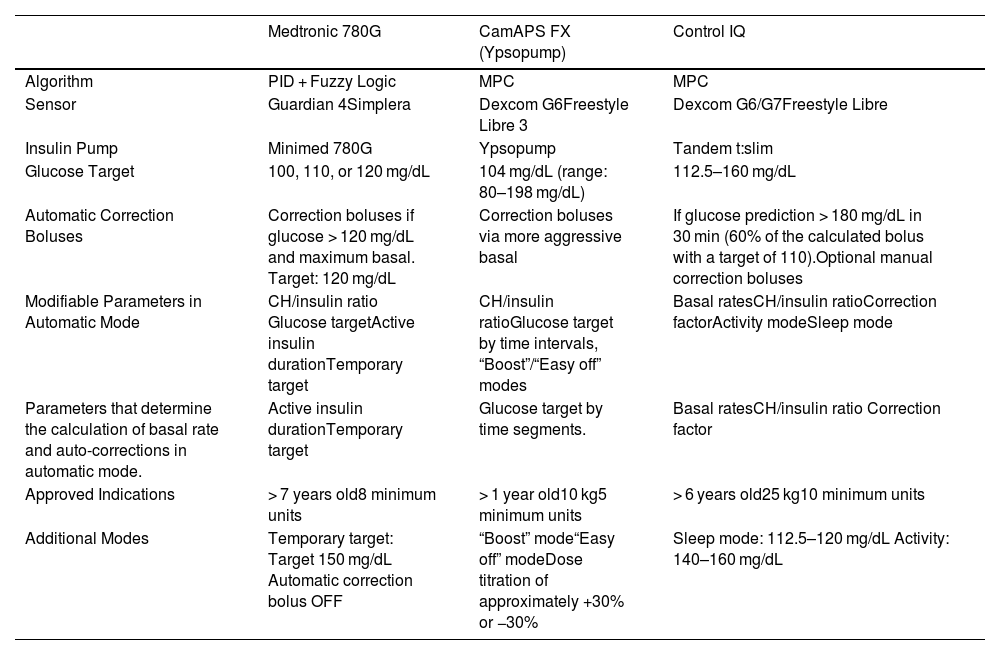
Table 3 describes the technical characteristics of the AID systems currently available in Spain for use in pediatric age.
Hybrid Closed-Loop/Automated Insulin Infusion Systems Available in Spain.
| Medtronic 780G | CamAPS FX (Ypsopump) | Control IQ | |
|---|---|---|---|
| Algorithm | PID + Fuzzy Logic | MPC | MPC |
| Sensor | Guardian 4Simplera | Dexcom G6Freestyle Libre 3 | Dexcom G6/G7Freestyle Libre |
| Insulin Pump | Minimed 780G | Ypsopump | Tandem t:slim |
| Glucose Target | 100, 110, or 120 mg/dL | 104 mg/dL (range: 80–198 mg/dL) | 112.5–160 mg/dL |
| Automatic Correction Boluses | Correction boluses if glucose > 120 mg/dL and maximum basal. Target: 120 mg/dL | Correction boluses via more aggressive basal | If glucose prediction > 180 mg/dL in 30 min (60% of the calculated bolus with a target of 110).Optional manual correction boluses |
| Modifiable Parameters in Automatic Mode | CH/insulin ratio Glucose targetActive insulin durationTemporary target | CH/insulin ratioGlucose target by time intervals, “Boost”/“Easy off” modes | Basal ratesCH/insulin ratioCorrection factorActivity modeSleep mode |
| Parameters that determine the calculation of basal rate and auto-corrections in automatic mode. | Active insulin durationTemporary target | Glucose target by time segments. | Basal ratesCH/insulin ratio Correction factor |
| Approved Indications | > 7 years old8 minimum units | > 1 year old10 kg5 minimum units | > 6 years old25 kg10 minimum units |
| Additional Modes | Temporary target: Target 150 mg/dL Automatic correction bolus OFF | “Boost” mode“Easy off” modeDose titration of approximately +30% or −30% | Sleep mode: 112.5–120 mg/dL Activity: 140–160 mg/dL |
PID: PID algorithm (Proportional, Integral, and Derivative); MPC: Model Predictive Control algorithm; CH/insulin ratio: carbohydrate/insulin ratio.
Currently, the use of AID systems is recommended for all children and adolescents with T1DM who can safely use the device (either independently or with the assistance of caregivers) (Grade A recommendation from the American Diabetes Association [ADA]).38 This should especially be considered for those with glycemic control outside target levels, problematic hypoglycemia, or elevated glycemic variability, basing the treatment choice on the circumstances, desires, and needs of the individual and their family.6,38,39
The decision to initiate the system should be agreed with the patient and their family based on comprehensive information regarding expectations related to outcomes and patient requirements. Both the patient and family must commit to completing the training program, following safety recommendations for preventing ketoacidosis, and making the necessary efforts to adequately meet insulin needs for meals.39
Training and initiation of the automated insulin delivery system from multiple daily insulin injectionsPatients starting to use an AID system, along with their families and/or caregivers, should receive specific training in a structured program that addresses the aspects detailed in Table 4 in a gradual and systematic manner, specifically recommending using the system manually before activating its automatic mode, even in systems that do not necessarily require this prior step.39
Essential Content in the Training Program for Initiating Hybrid Closed-Loop Systems.
| Before Starting the System in Manual Mode | Before Starting the System in Automatic Mode |
|---|---|
| System Components: Introduction to system components, including the insulin pump, continuous glucose monitor (CGM), and relevant applications for caregivers. | System Components: Same as in manual mode, ensuring understanding of all technical components and caregiver applications. |
| Infusion Set Change: Instructions on changing the infusion set, including insulin reservoir replacement every 2–3 days. Emphasis on rotating sites and ensuring proper tubing fill based on length and cannula fill. | Infusion Set Change: Same procedure as in manual mode. Rotating sites, proper tubing fill, and cannula fill. |
| Operation in Manual Mode: - Explanation of basal and bolus insulin concepts. - Use of temporary basal rates. - Bolus calculator settings: carbohydrate ratio (grams/unit), correction factor, target (single value or range), definition of active insulin, and its implications on bolus calculation. | Operation in Automatic Mode: - Understanding the algorithm for automatic mode: how automatic basal rates and boluses function. - Parameters influencing the algorithm. - Concept of user-initiated boluses and insulin doses administered by the system algorithm. - Situations requiring deactivation of automatic mode. - When and how to override system bolus recommendations. |
| Hypoglycemia Management: - Typically, the carbohydrate doses required are lower than with multiple daily injections (MDI), and slow-absorbing carbs are often unnecessary. - Use of suspend before hypoglycemia feature: criteria for suspension and reactivation. - Avoid overreacting to automatic suspensions; avoid duplicating actions. - In case of severe hypoglycemia, administer glucagon and disconnect the pump until blood glucose > 70 mg/dL. | Hypoglycemia Management: - Similar to manual mode; lower carbohydrate doses typically needed. Slow-absorbing carbs are usually unnecessary. - In case of severe hypoglycemia, administer glucagon and disconnect the pump until blood glucose > 70 mg/dL. |
| Hyperglycemia Treatment: - For blood glucose > 250 mg/dL lasting over 2 hours with negative ketones: change infusion set, including insulin reservoir, and administer a correction bolus. - For blood glucose > 250 mg/dL lasting over 2 hours with positive ketones: administer correction boluses via pen (not pump). Change the infusion set, including insulin reservoir. Follow ketosis protocol. - In cases of diabetic ketoacidosis (DKA), remove the system and manage with IV fluids and insulin infusion. | Hyperglycemia Treatment: - For blood glucose > 250 mg/dL lasting over 2 hours with negative ketones: change the infusion set and insulin reservoir; consider administering a correction bolus. - For blood glucose > 250 mg/dL lasting over 2 hours with positive ketones: administer correction boluses via pen, not the pump. Change the infusion set and insulin reservoir. Keep the system in manual mode while administering pen boluses. Follow the ketosis protocol. - In cases of DKA, remove the system and manage with IV fluids and insulin infusion. |
| Safety Strategies for DKA Prevention: - Always suspect a blockage in case of unexplained hyperglycemia or glucose > 250 mg/dL for 2 hours. - Sick days: adjust the system’s settings to account for increased insulin needs (+10–50%). Monitor blood glucose and blood ketones frequently. | Safety Strategies for DKA Prevention: - Similar to manual mode; always suspect a blockage with unexplained hyperglycemia or glucose > 250 mg/dL for 2 hours. - Sick days: adjust the system settings to account for increased insulin needs (+10–50%). Monitor blood glucose and blood ketones frequently. Consider deactivating automatic mode if optimal results are not achieved. |
| Exercise Adjustments: - Use lower temporary basal rates starting one hour before aerobic exercise. - Recommendations to prevent hyperglycemia related to anaerobic exercise. - Action plan for disconnection during exercise. - Carbohydrate intake for prolonged exercise. | Exercise Adjustments: - Use activity mode or temporary target: when to set and deactivate it to avoid hypo- or hyperglycemia. - Recommendations to prevent hyperglycemia related to anaerobic exercise. - Action plan for disconnection during exercise (suspending insulin infusion). - Carbohydrate intake for prolonged exercise. |
| Alternative Protocol with MDI in Case of System Failure: Comprehensive plan on reverting to MDI if the system fails. | Alternative Protocol with MDI in Case of System Failure: Same as in manual mode. |
DKA: diabetic ketoacidosis; HC: carbohydrates; MDI: multiple daily injections.
This table outlines the essential training components for users starting hybrid closed-loop systems, distinguishing between manual and automatic modes to ensure optimal understanding and system management.
This approach allows the patient to learn to use the system in manual mode, which may be essential in specific situations, such as the absence of sensor data or the need to apply maximum or minimum doses for an extended period of time during which the system cannot operate automatically. Additionally, it allows for early adjustments in basal doses and parameters for calculating boluses, contributing to a faster adaptation of the algorithm later on.
Overall, the transition from manual to automatic mode can be carried out over a period of two to seven days if manual mode is chosen first.39 In the Medtronic 780G system, a period of, at least, 48 hours in manual mode from the 24 hours of the starting day is required by the system as a prerequisite for activating automatic mode. In the CamAPS-Ypsopump and Tandem Control IQ systems, automatic mode can be activated from the start. However, it is crucial to note that this process should be individualized, while considering the preparation of both the patient and their family or caregivers. In cases where the system is started manually, evaluating the insulin doses administered in the days prior to the transition to automatic mode is especially relevant. In situations where the total daily dose in the previous days does not reflect the patient’s usual needs, the system algorithm will require a longer period to adequately adapt to the patient’s needs, which will subsequently impact glycemic control outcomes within the early weeks.
Start of the automatic mode and optimization of glycemic controlThe programmable parameters that determine the operation of automatic mode differ among various systems and are detailed in Table 3.
In all cases, it is essential that patients receive specific training on how the algorithm works and variations in recommendations for preventing episodes of hypoglycemia and hyperglycemia before activating the automatic mode.
The concepts of “patient-administered insulin” and “algorithm-administered insulin” are more relevant in the context of automatic mode than the concepts of “basal insulin” and “bolus insulin”, as they facilitate the understanding of the impact of the patient’s actions on the system response and the importance of not overreacting in the case of hypo or hyperglycemia.6
Patient’s goals must remain aligned with the programmed glucose target in automatic mode. This is essential because if the patient acts to achieve higher glucose levels, he/she will cause the system to administer insulin to reach the target, resulting in increased glycemic variability and risk of hypoglycemia. Similarly, implementing strategies to prevent postprandial hyperglycemia, such as optimizing the timing between insulin administration and meal onset or adjusting the carbohydrate-to-insulin ratio, will prevent the system from over-administering insulin in response to postprandial glucose peaks and contribute to better glycemic outcomes.
The percentage of insulin administered by the system in the form of automatic basal doses and/or automatic correction boluses can serve as an indicator for evaluating the need to adjust the automatic mode parameters or implement strategies aimed at reducing blood glucose during the postprandial period. Overall, and empirically, for the Medtronic 780G system, a self-correction percentage > 20% indicates that it is necessary to reduce the target or DIA or that meal-related insulin needs are not being adequately covered. In this latter case, it is necessary to evaluate optimizing the timing between bolus administration and meal onset, and the need to reduce the carbohydrate-to-insulin ratios. With the CamAPS-YPsopump system, as a reflection of the need for optimization of preprandial boluses, we might see a high percentage of basal doses vs the total daily dose for the patient’s needs. Finally, with the Tandem Control IQ system, in this situation, we might observe both an increase in self-correction and basal dose.
It is also important to periodically update parameters that do not impact the algorithm, such as the basal pattern in the case of the Medtronic 780G system or the CamAPS FX system, as well as all parameters of the bolus calculator. In the event of automatic mode interruption, the system will revert to these parameters to operate in manual mode.
Start of the automatic mode in the Medtronic 780G systemFor most patients starting automatic mode with the Medtronic 780G system, the best results are obtained by starting the system with a target of 100 mg/dL and a DIA of 2 hours.18,40 In patients with prior glycemic control outside target levels, starting with a higher target and progressively adjusting it may be considered.
In patients with higher insulin sensitivity, especially younger children, it is recommended to initially consider a longer DIA to avoid the tendency toward hypoglycemia related to the dawn phenomenon. Subsequently, it is recommended to program the lowest tolerated target.
In this system, meal boluses administered by the patient are determined by the programmed carbohydrate-to-insulin ratio and the adjustment made by the algorithm. Occasionally, the algorithm may apply a reduction to the bolus for a meal, resulting in a smaller amount than what would correspond to the programmed ratio (Safe Meal Bolus).36 The Safe Meal Bolus function considers the total daily dose, the programmed ratio, the amount of total carbohydrates, the active insulin, and the estimated risk of hypoglycemia within the following four hours to decide on the reduction of the calculated bolus. The system bolus suggestion cannot be overcorrected. If, at any time, the system recommends boluses lower than those corresponding to the programmed ratio and lower than those necessary for the patient, instead of entering false carbohydrate amounts, it is preferable to exit automatic mode, administer the bolus using the bolus wizard, and then reactivate the automatic mode once the bolus has been administered. Under these circumstances, entering false carbohydrates will generally be ineffective for increasing the suggested bolus. Additionally, the daily carbohydrate amount is a parameter considered by the algorithm to adapt its response, so this practice may negatively influence the outcome later on. Reducing the ratio will also be ineffective if the adjustment is due to the Safe Meal Bolus function.
Other features of the Medtronic 780G system algorithm that are useful to understand the system’s responses include:
Safe Correction Bolus: the system reduces each automatically calculated correction bolus considering the risk of low glucose within the following two hours.
Meal Detection Module: when the system detects a missed or underestimated meal (for which it uses a weighted average of the glucose variation rate), the algorithm lowers the threshold for predicting low glucose to allow for a smaller reduction of automatic correction boluses.41 In other words, it modifies the effect of the Safe Correction Bolus function.
Self-Correction Limit: a total of 8% of the total daily insulin dose as automatic correction within a 45-minute period. Once this limit has been reached, when the patient enters a certain blood glucose level, self-corrections will be administered again if the criteria are met.42
Start of the automatic mode in the Tandem-Control IQ systemWith the Tandem-Control IQ system, the best results are obtained when the basal pattern, carbohydrate-insulin ratios, and correction factors are correctly adjusted prior to initiating automatic mode. To avoid hypoglycemia due to insulin accumulation in the case of boluses administered with intervals shorter than the DIA, it is essential for the patient to understand the concept of active insulin and how the bolus calculator uses it. With this system, the calculator will only subtract the active insulin from previous (manual or automatic) boluses from the bolus corresponding to the meal if glucose level is underneath the target. If glucose level is above the target, it will subtract active insulin only from the part of the bolus corresponding to correction. Adjusting the correction factor is considered the most important determinant in relation to the increasing TIR and reducing glycemic variability.43
Start of the automatic mode in the CamAPS-Ypsopump systemThe CamAPS system allows for the programming of an individualized glucose target by time segments. The algorithm is capable of learning from the results (an initial learning period of several weeks is a common thing). The algorithm progressively adapts its response by simultaneously considering: the mean total insulin dose, the insulin need for each hour of the day, and postprandial glucose. In the latter case, if the patient consistently overestimates or underestimates carbohydrates, the system will start adjusting the administration of insulin after meals.44 In young children, who are highly sensitive to insulin and have very low dosing requirements, it is preferable to start with higher targets and progressively reduce them. Initially, while the system is learning, which can go on for 2 to 3 weeks, it is recommended to avoid overreacting to both low and high glucose levels, abstaining from using the “boost mode” and administering additional manual correction boluses, as these practices often contribute to increased glycemic variability in the pediatric population.
Main challenges of current automated insulin delivery systemsCurrent AID systems still face various challenges, including the high frequency of skin-related issues arising from the use of sensor adhesives and infusion systems,45,31 as well as the absence of algorithms that satisfactorily automate insulin infusion concerning meals and adapt automatically to unannounced physical activity. Regarding meals, although the best results with current AID systems are achieved by optimizing carbohydrate counting, programmed ratios, and bolus timing, studies have shown that it is possible to reach TIRs > 70% with simplified meal announcements46 and the system could manage small unannounced snacks of up to 20 g of carbohydrates without glucose increases > 50 mg/dL.47 On the other hand, while significant advances have been made, such as the definition of specific categories for interoperable systems by the U.S. Food and Drug Administration (FDA), the current possibilities on the interoperability of system components remain very limited. Finally, it is important to emphasize that ensuring equitable access for all patients with T1DM remains perhaps the most relevant challenge concerning these systems and, in general, regarding the treatment of T1DM.
FundingNone declared.