Few studies assessing the relationship between oxidative stress and glycemic variability in children with type 1 diabetes mellitus (T1DM) are available, and most of them reported no significant results.
ObjectiveTo assess the relationship between glucose control, glycemic variability, and oxidative stress as measured by urinary excretion of 8-iso-prostanglandin F2-alpha (8-iso-PGF2α) in children with T1DM.
Materials and methodsA cross-sectional study including 25 children with T1DM. Participants were evaluated during five days in two different situations: 1st phase during a summer camp, and 2nd phase in their everyday life at home. The following data were collected in each study phase:.
– Six capillary blood glucose measurements per day. Mean blood glucose (MBG) levels and glucose variability parameters, including standard deviation, coefficient of variation, and mean amplitude of glycemic excursions (MAGE), were calculated.
– Capillary HbA1c level.
– 24-h urine sample to measure 8-iso-PGF2α.
ResultsThere were no statistically significant differences in urinary 8-iso-PGF2α levels (142±37 vs. 172±61pg/mg creatinine) and glucose control and glycemic variability parameters between both phases. In the 2nd phase, statistically significant correlations were found between urinary 8-iso-PGF2α and HbA1c levels (r=0.53), MBG (r=0.72), standard deviation (r=0.49), and MAGE (r=0.42). No significant correlations between glucose control, glycemic variability and urinary 8-iso-PGF2α excretion were found in the 1st phase.
ConclusionsA significant correlation was found between glycemic variability and HbA1c level and urinary 8-iso-PGF2α excretion in a group of children with T1DM during their daily lives. Additional studies are needed to confirm this finding and to explore its long-term impact on health.
En niños con diabetes tipo 1 (DM1) hay pocos estudios que evalúen la relación entre estrés oxidativo y variabilidad glucémica, y la mayoría de ellos no encuentran resultados significativos.
ObjetivoEvaluar la relación entre control metabólico, variabilidad glucémica y estrés oxidativo medido por la excreción urinaria de 8-iso-prostaglandina F2 alfa (8-iso-PGF2α) en niños con DM1.
Material y métodoEstudio transversal que incluyó 25 niños con DM1. Los participantes fueron evaluados durante 5 días en 2 situaciones diferentes: 1.a fase durante un campamento de verano y 2.a fase durante su actividad habitual en domicilio. En cada fase se recogieron:
– Seis determinaciones de glucemia capilar diarias. Se calcularon glucemia media y parámetros de variabilidad glucémica: desviación estándar, coeficiente de variación y «mean amplitude of glycemic excursions» (MAGE).
– HbA1c capilar.
– Muestra de orina de 24h para la determinación de 8-iso-PGF2α.
ResultadosNo se encontraron diferencias estadísticamente significativas en excreción urinaria de 8-iso-PGF2α (142±37 vs. 172±61pg/mg creatinina) y parámetros de control y variabilidad glucémicos entre las fases. En la 2.a fase se observaron correlaciones estadísticamente significativas entre 8-iso-PGF2α urinario con HbA1c (r=0,53), glucemia media (r=0,72), desviación estándar (r=0,49) y MAGE (r=0,42). En la 1.a fase del estudio no se han detectado correlaciones significativas.
ConclusionesSe ha encontrado una correlación significativa entre parámetros de variabilidad glucémica y HbA1c con la excreción urinaria de 8-iso-PGF2α en un grupo de niños con DM1 evaluados durante su vida diaria. Son necesarios más estudios para confirmar estos resultados y evaluar el impacto a largo plazo sobre la salud.
There is evidence of an increase in mitochondrial free radical formation and a decrease of antioxidant capacity in subjects with diabetes, which lead to an oxidative damage of cellular components.1 This excess of oxidative stress causes endothelial dysfunction, which has been associated with the development and progression of diabetes related complications.2–4 Moreover, endothelial dysfunction has been reported to appear at early stages in subjects with type 1 diabetes mellitus (T1DM), even before the onset of structural and clinical signs of atherosclerosis and other macrovascular and microvascular complications.5–7
Isoprostanes are stable products from the free oxygen radical mediated oxidation of arachidonic acid and have been demonstrated to increase with oxidant injury; therefore they are considered to be good indicators of oxidative stress.8,9 Isoprostanes can be estimated from the measurement of 24-h urinary excretion of 8-iso-prostaglandin F2 alpha (8-iso-PGF2α).
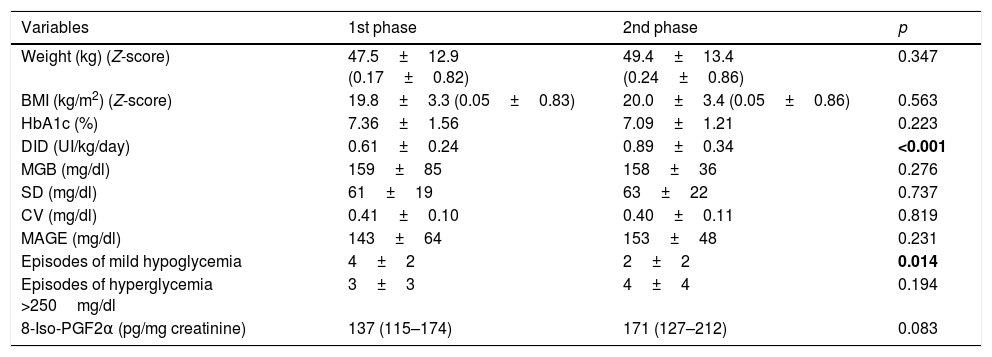
Monnier et al.10 reported that the urinary excretion rate of 8-iso-PGF2α was positively correlated with glycemic variability in 21 subjects with type 2 diabetes mellitus. However, there has been difficulty in confirming these findings in subjects with T1DM.11 In the pediatric population with T1DM, few studies aimed at evaluating the oxidative stress and its relationship with glycemic variability have been published, and most of them did not find positive results (Table 1).12–17 Specifically, in a previous study, our group analyzed the relationship between glycemic variability and oxidative stress in 14 children attending a summer camp, but we did not find any correlation.13
Studies aimed at evaluating glucose control, glycemic variability and oxidative stress measured by the urinary excretion of 8-iso-PGF2α in children with T1DM.
| Authors | Study design | nDM1 | 8-Iso-PGF2α in T1DM | ncontrol | 8-Iso-PGF2α in controls | 8-Iso-PGF2α T1DM vs. controls | Correlations 8-iso-PGF2α, HbA1c and GV |
|---|---|---|---|---|---|---|---|
| Gleisner et al., 200614 | Case–control | 13 | 1673±1706 | 16 | 741±551 | Not found | Not assessed |
| Schreiver et al., 201417 | Cross-sectional | 48 | MDI: 2870±180CSII: 2530±240 | – | – | – | Not found |
| Colomo et al., 201413 | Cross-sectional | 14 | 864 (723–1293) | – | – | – | Not found |
| Răchişan et al., 201416 | Case–control | 51 | 2091±3536 | 27 | 510±493 | Higher T1DM | Not found |
| Meng et al., 201515 | Case–control | 60 | Onset: 389±196Honeymoon: 301±93Long term: 673±180 | 20 | 130±103 | Higher T1DM | HbA1cMAGE |
| Altinik et al., 201612 | Case–control | 31 | 2809 (2161–3232) | 25 | 298 (199–345) | Higher T1DM | Not found |
In spite of previous controversial findings, we hypothesized that oxidative stress may have a relationship with metabolic control and glycemic variability in children with T1DM. Therefore, we designed a study in the pediatric population with T1DM, in which more participants were included and two different situations were evaluated: a first phase during a summer camp and a second phase in everyday life at home. The aim was to assess the relationship between glucose control, glycemic variability and oxidative stress measured by the urinary excretion of 8-iso-PGF2α in each scenario.
Material and methodsPatients and study designCross sectional study that included 25 children and adolescents with T1DM aged 8–15, followed up at the diabetes unit of the Hospital Materno-Infantil (Hospital Regional Universitario de Málaga) in Málaga (Spain). Participants were evaluated during five days in two different situations: the first phase in a summer camp and the second phase in everyday life at home.
The summer camp was organized by a local diabetes association (Asociación de diabéticos de Málaga-ADIMA) and followed the recommendations of the American Diabetes Association for the organization and management of camps for children with diabetes.18 It was held between the 7th and the 14th July 2013 in Coín (Málaga, Spain). The team comprised 10 physicians (two pediatric endocrinologists, two adult endocrinologists, two adult endocrinology fellows, two pediatric endocrinology fellows, two pediatric residents), one diabetes educator (nurse). The non-medical personnel included 10 free time monitors with diabetes, each monitor caring for five children to support glycemic control (performing checks and insulin administration) and five free time monitors without T1DM in charge of the leisure and sports activities.
The first phase of the study was performed in the last five days of the camp. The second evaluation was carried out in in December 2013.
Children and adolescents themselves gave their agreement orally to participate in the study and their parents provided written informed consent.
MeasurementsSociodemographic and clinical data, which included information about diabetes duration, diabetes treatment and the presence of microvascular complications, were collected. Diabetic retinopathy, nephropathy and neuropathy were defined as having retinal lesion detected by ophthalmoscopy, microalbuminuria requiring therapy with angiotensin converting enzyme inhibitors/angiotensin receptor blockers, and altered or diminished sensibility in hands and/or feet, respectively.
During each study phase, six daily blood glucose recordings (pre-meals and 2h post-meals) were obtained from all the participants using a One Touch Verio iQ® glucose meter (Lifescan). These capillary blood glucose values were used to calculate the mean blood glucose (MBG) and glycemic variability parameters: standard deviation (SD), coefficient of variation (CV) and mean amplitude of glycemic excursions (MAGE).
Daily insulin doses were collected. Episodes of documented hypoglycemia (blood glucose value <70mg/dl with or without symptoms of hypoglycemia), severe hypoglycemia (hypoglycemia with seizure or loss of consciousness),19 ketosis (ketonuria measured by urine test strips Ketostik® Bayer, in children on multiple daily injections [MDI] treatment, and ketonemia determined by MediSense Optium Xceed Meter® Abbott in children on insulin pump) and ketoacidosis occurring during the study were recorded.
Information about weight and height and HbA1c was obtained from medical records, always within two months of the camp date and of the daily life evaluation date. Body mass index (BMI) was calculated as weight (measured in kilograms) divided by the square of height (measured in meters). Z-score for weight and BMI was calculated using recently published Spanish growth Charts.20 HbA1c was measured in a capillary blood sample using a DCA 2000 Analyzer® (Bayer Corporation, Elkhart, IN, USA).
Oxidative stress was assessed by the measurement of 8-iso-PGF2α in a 24-h urine sample. All participants collected two 24-h urine samples, one the last day of the camp and the second on the 5th day of the daily life evaluation. Both urine samples were handled similarly: participants were asked to keep the urine sample at 4°C during the collecting process and to deliver it to the hospital within the first 24h after collection was finished. At the hospital, urine samples were stored at −80°C in the presence of 0.005% BHT because storage at −20°C was insufficient to prevent oxidative formation of 8-isoprostane. Urinary 8-iso-PGF2α levels were measured using an enzyme immunoassay (EIA) method (Cayman Chemical Company, Ann Arbor, MI, USA). The intra and inter-assay CV were 15.2 and 18.5 respectively. Urinary creatinine level was measured using an enzymatic spectrophotometric method based on alkaline picrate.
Statistical analysisContinuous variables are presented as mean and standard deviation or as median and interquartile range; categorical variable as percentages. Comparison of continuous variables between the two study phases was done with the non-parametric Wilcoxon test for paired data. Spearman correlation coefficient was employed in order to assess relationships between variables. Confidence levels of 95% were considered in two tail hypothesis tests.
ResultsParticipants were 14 boys (56%) and 11 girls (44%), mean age was 12±2 years and mean diabetes duration was 4±3 years. None of them had a previous diagnosis of diabetic retinopathy, nephropathy or neuropathy at inclusion. Only 4 children were on continuous subcutaneous insulin infusion (CSII) treatment, the rest of them were on multiple daily injections (MDI) with basal insulin (20 using insulin glargine and one using insulin detemir) and short acting insulin analogs (lispro or aspart). The diabetes related acute complications recorded were one episode of ketosis during camp, which was adequately treated. No episodes of severe hypoglycemia and no episodes of ketoacidosis occurred during the study.
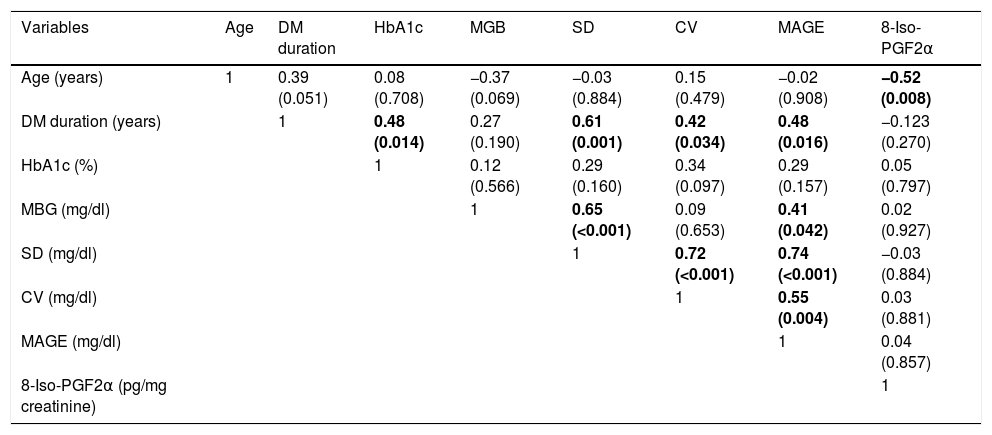
Table 2 shows the study variables during the first and second study phases. During the camp, daily insulin requirements were significantly lower and episodes of mild hypoglycemia were more frequent than in the daily life evaluation. The other study variables were not significantly different between both study phases including glycemic variability measures and urinary excretion of 8-iso-PGF2α.
Comparison of study variables between the first and second phases.
| Variables | 1st phase | 2nd phase | p |
|---|---|---|---|
| Weight (kg) (Z-score) | 47.5±12.9 (0.17±0.82) | 49.4±13.4 (0.24±0.86) | 0.347 |
| BMI (kg/m2) (Z-score) | 19.8±3.3 (0.05±0.83) | 20.0±3.4 (0.05±0.86) | 0.563 |
| HbA1c (%) | 7.36±1.56 | 7.09±1.21 | 0.223 |
| DID (UI/kg/day) | 0.61±0.24 | 0.89±0.34 | <0.001 |
| MGB (mg/dl) | 159±85 | 158±36 | 0.276 |
| SD (mg/dl) | 61±19 | 63±22 | 0.737 |
| CV (mg/dl) | 0.41±0.10 | 0.40±0.11 | 0.819 |
| MAGE (mg/dl) | 143±64 | 153±48 | 0.231 |
| Episodes of mild hypoglycemia | 4±2 | 2±2 | 0.014 |
| Episodes of hyperglycemia >250mg/dl | 3±3 | 4±4 | 0.194 |
| 8-Iso-PGF2α (pg/mg creatinine) | 137 (115–174) | 171 (127–212) | 0.083 |
Data are presented as mean±standard deviation or as median (interquartile range). Wilconxon test.
In bold, values below 0.05 (statistically significant).
BMI: body mass index; DID: daily insulin dose; MBG: mean blood glucose; SD: standard deviation; CV: coefficient of variation; MAGE: mean amplitude of glycemic excursions.
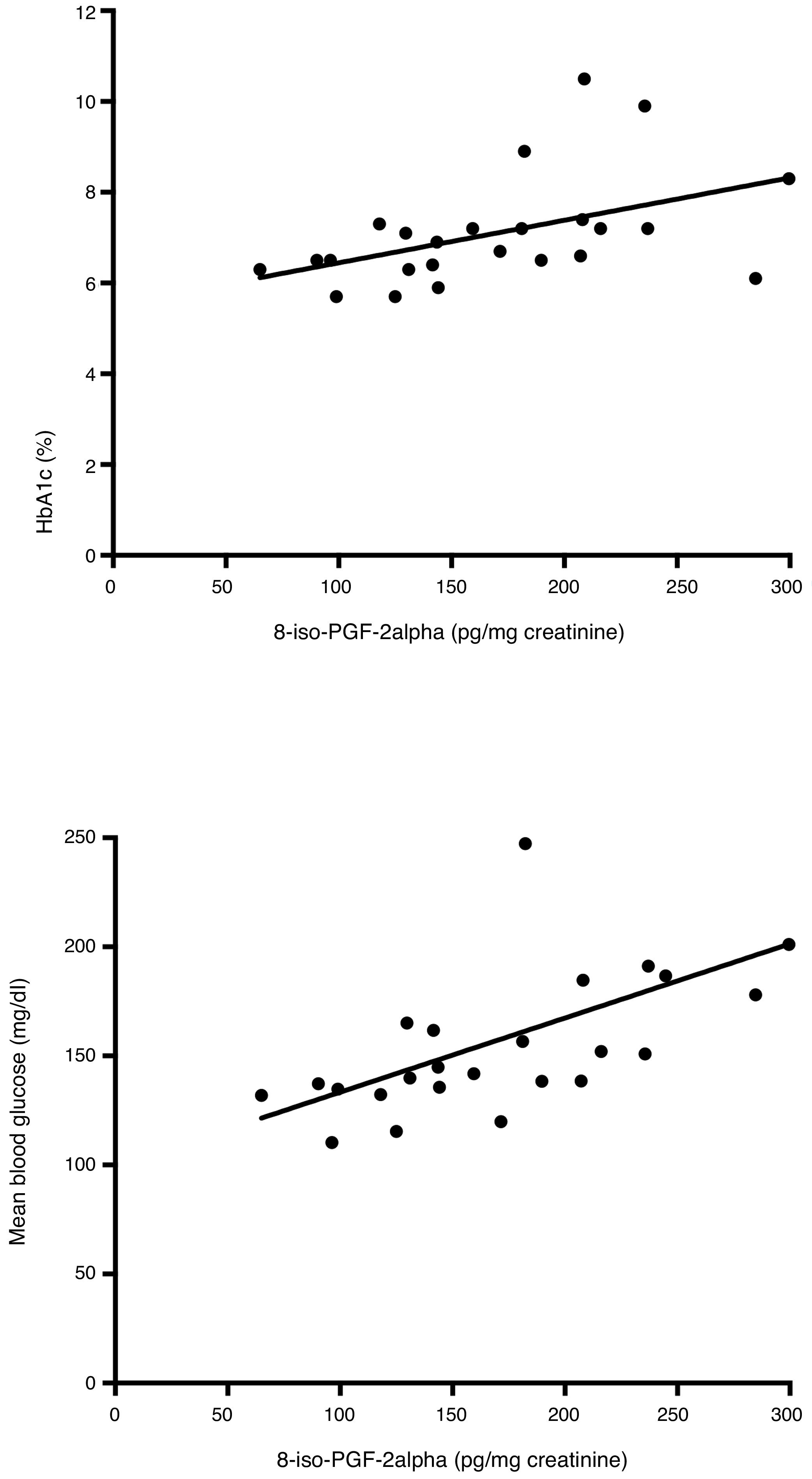
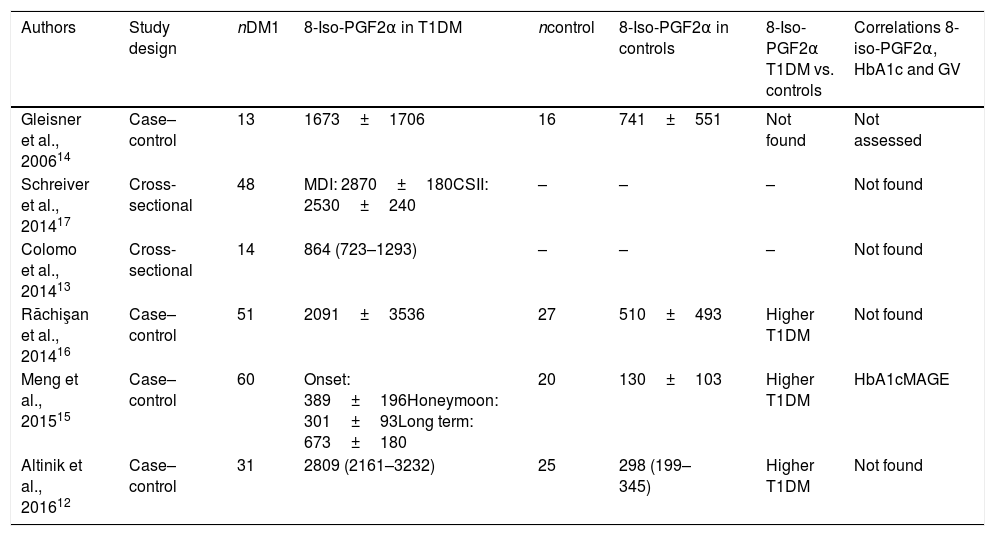
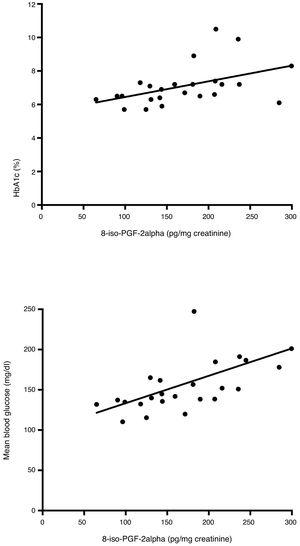
Correlations between glycemic control and glycemic variability variables and urinary excretion of 8-iso-PGF2α in each study phase were calculated. No significant correlations were found in the first study phase (Table 3). However, in the second phase, urinary 8-iso-PGF2α levels were significantly correlated with HbA1c (ρ=0.53), MGB (ρ=0.72), SD (ρ=0.49) and MAGE (ρ=0.42) (Table 4 and Fig. 1).
Correlations between glycemic control, glucose variability and urinary excretion of 8-iso-PGF2α in the first study phase.
| Variables | Age | DM duration | HbA1c | MGB | SD | CV | MAGE | 8-Iso-PGF2α |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 1 | 0.39 (0.051) | 0.08 (0.708) | −0.37 (0.069) | −0.03 (0.884) | 0.15 (0.479) | −0.02 (0.908) | −0.52 (0.008) |
| DM duration (years) | 1 | 0.48 (0.014) | 0.27 (0.190) | 0.61 (0.001) | 0.42 (0.034) | 0.48 (0.016) | −0.123 (0.270) | |
| HbA1c (%) | 1 | 0.12 (0.566) | 0.29 (0.160) | 0.34 (0.097) | 0.29 (0.157) | 0.05 (0.797) | ||
| MBG (mg/dl) | 1 | 0.65 (<0.001) | 0.09 (0.653) | 0.41 (0.042) | 0.02 (0.927) | |||
| SD (mg/dl) | 1 | 0.72 (<0.001) | 0.74 (<0.001) | −0.03 (0.884) | ||||
| CV (mg/dl) | 1 | 0.55 (0.004) | 0.03 (0.881) | |||||
| MAGE (mg/dl) | 1 | 0.04 (0.857) | ||||||
| 8-Iso-PGF2α (pg/mg creatinine) | 1 |
Data are presented as Spearman correlation coefficients (p value).
MBG: mean blood glucose; SD: standard deviation; CV: coefficient of variation.
Correlations between glycemic control, glucose variability and urinary excretion of 8-iso-PGF2α in the second study phase.
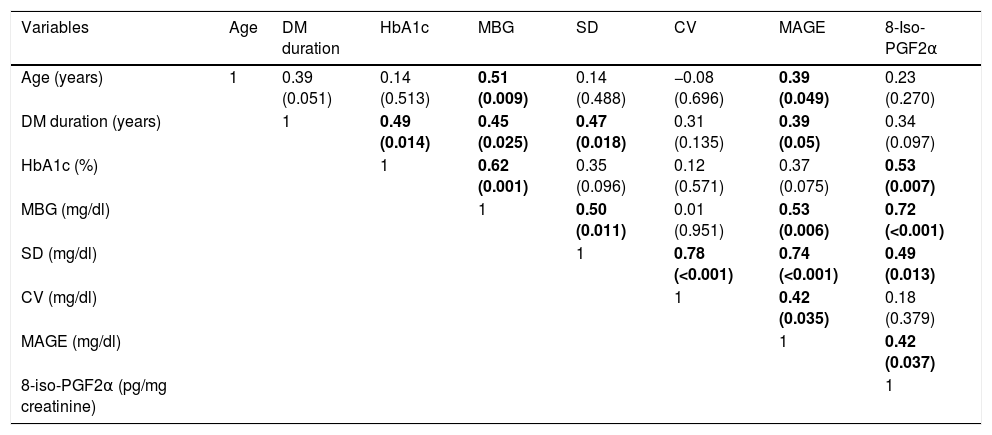
| Variables | Age | DM duration | HbA1c | MBG | SD | CV | MAGE | 8-Iso-PGF2α |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 1 | 0.39 (0.051) | 0.14 (0.513) | 0.51 (0.009) | 0.14 (0.488) | −0.08 (0.696) | 0.39 (0.049) | 0.23 (0.270) |
| DM duration (years) | 1 | 0.49 (0.014) | 0.45 (0.025) | 0.47 (0.018) | 0.31 (0.135) | 0.39 (0.05) | 0.34 (0.097) | |
| HbA1c (%) | 1 | 0.62 (0.001) | 0.35 (0.096) | 0.12 (0.571) | 0.37 (0.075) | 0.53 (0.007) | ||
| MBG (mg/dl) | 1 | 0.50 (0.011) | 0.01 (0.951) | 0.53 (0.006) | 0.72 (<0.001) | |||
| SD (mg/dl) | 1 | 0.78 (<0.001) | 0.74 (<0.001) | 0.49 (0.013) | ||||
| CV (mg/dl) | 1 | 0.42 (0.035) | 0.18 (0.379) | |||||
| MAGE (mg/dl) | 1 | 0.42 (0.037) | ||||||
| 8-iso-PGF2α (pg/mg creatinine) | 1 |
Data are presented as Spearman correlation coefficients (p value).
MBG: mean blood glucose; SD: standard deviation; CV: coefficient of variation.
Our results support the hypothesis that there is an association between glucose control, glucose variability and oxidative stress in children with T1DM. Furthermore, this is one of the first studies to show these associations in the pediatric population.
Meng et al.15 in 2015 found a significant correlation between urinary excretion of 8-iso-PGF2α and HbA1c levels and glucose fluctuations in children with T1DM. Additionally, they described a gradient in urinary 8-iso-PGF2α excretions in children with T1DM based on diabetes duration as follows: diabetes duration above 5 years (highest urinary 8-iso-PGF2α levels), diabetes onset and diabetes honeymoon (lowest urinary 8-iso-PGF2α levels). Our participants had a clinical and metabolic profile similar to the group of the long standing phase of the Meng study, but with lower HbA1c. However, the levels of urinary 8-iso-PGF2α that we described were lower than in this group and closer to the control group of that study. To our knowledge, the urinary 8-iso-PGF2α values that we report in the present study are the lowest published in children with T1DM (Table 1). We designed our study to collect two urine samples for each child in order to check internal validity in the measurement of urinary isoprostanos. As the values we obtained in both evaluations were similar, we could rule out methodological problems in the 8-iso-PGF2α determinations. It is noteworthy that the urinary 8-iso-PGF2α which was reported in children with T1DM had a wide range of values, although they all were measured by the same biochemistry assay (EIA method) (Table 1). Reference lab values should be proposed before the measurement of urinary 8-iso-PGF2α could be used to assess oxidative stress in daily clinical practice.
Meng et al.15 also observed increased urinary excretion of 8-iso-PGF2α in children with T1DM (independent of diabetes duration) when compared with age and sex matched control children. Previous studies comparing urinary excretion of 8-iso-PGF2α in children with and without T1DM consistently found higher levels in those with T1DM.12,16
We observed the relationship between MBG, HbA1c, glucose variability and oxidative stress in the daily life evaluation but not in the camp evaluation. Summer camps specialized for children with T1DM provide the opportunity to learn abilities for diabetes care and to acquire healthy life habits such as the Mediterranean diet and regular exercise.18,21 During the camp evaluation, habitual physical activity was increased, and therefore we observed that insulin requirements were reduced and more episodes of mild hypoglycemia were reported. Other authors and our group previously reported similar results in studies performed in children with T1DM attending summer camps.13,21 During camp, all study participants had the same physical activity and diet, but not at home. Diet and physical activity information during the study was not collected. It is known that acute exercise,22 insulin dose,23,24 events of hypoglycemia25 and diet might have an impact on oxidative stress. The homogenization of these factors during camp evaluation may be responsible of a lower variance in the urinary 8-iso-PGF2α values obtained in the camp compared with that obtained in the daily life evaluation (1343 vs. 3723, p=0.011, Levene test). The absence of correlation between glucose variability, glucose control and oxidative stress in the camp evaluation may be explained by a lower variance of the urinary 8-iso-PGF2α values during camp. It is noteworthy that the glucose variability during the camp was similar to the value measured during daily life which might be explained by the fact that the study was developed during the last days of the camp and because the insulin dose was always prescribed by a doctor.
Accordingly, we support the idea that not only long term glucose control measured by HbA1c but also glucose variability could affect oxidative stress. Cerriello and Ihnat pointed to evidence that glucose variability contributes to the accelerated formation of free radicals,26 and in this review27 the authors assessed the role of oxidative stress in the development of diabetes microvascular and cardiovascular complications. Monnier et al.28 suggested that glycemic variability might have more deleterious effects than sustained hyperglycemia in the development of diabetic complications as both upward (postprandial glucose increments) and downward (interprandial glucose decrements) changes activate oxidative stress. Studies with continuous glucose monitoring systems (CGMS) found a large range of glucose values in children with T1DM, even in those with excellent HbA1c values.29 They highlight the importance of glycemic variability evaluation in the clinical care of children with T1DM and the inclusion as a target of glycemic control.
Limitations of our study include:
- -
The absence of frequency and intensity of physical activity and diet records, factors that could impact on oxidative stress. Physical activity and diet were probably different between camp and daily life evaluation, and between the study subjects in the daily life evaluation. Therefore we could not rule out that differences in physical activity and diet between camp and daily life evaluations could be responsible of the absence of correlation between oxidative stress and glucose variability in the camp evaluation.
- -
The power to detect differences in urinary 8-iso-PGF2α values between camp and daily life evaluations is below 80%, therefore a type II error could not be rejected.
- -
An age and sex matched control group of children might be useful to compare the urinary excretion of 8-iso-PGF2α with the group of children with T1DM, and would help to propose a normal range of prostanoides urinary excretion in the future.
- -
Glucose variability was assessed using capillary blood glucose measurements instead of CGM, however, a study demonstrated that glycemic variability parameters calculated with four capillary blood glucose measurements in a group of children with T1DM had a good correlation with those estimated with data from CGM.30
In conclusion, we found a significant correlation between glucose control, glucose variability and urinary excretion of 8-iso-PGF2α in children with T1DM during daily life. Glucose variability along with HbA1c should be routinely assessed in the clinical care of children with T1DM. Further studies are needed to confirm these findings and to evaluate the long term effect on future health.
Abbreviations8-iso-PGF2 α, 8-iso-prostaglandin F2 alpha; BMI, body mass index; CGMS, continuous glucose monitoring system; CSII, continuous subcutaneous insulin infusion; CV, coefficient of variation; EIA, enzyme immunoassay; MAGE, mean amplitude of glycemic excursion; MBG, mean blood glucose; MDI, multiple daily injections; SD, standard deviation; T1DM, type 1 diabetes mellitus.
Contribution of authorsNC and JPLS contributed to the design of the study, acquisition, analysis and interpretation of data. IL and GOF contributed to the design of the study, analysis and interpretation of data. NF, ERB, AO, MG, MJT, BMT contributed to acquisition of data. MSRA contributed to interpretation of data. All authors revised the work and gave final approval of the version published.
FundingNone declared.
Conflict of interestThe authors declare no conflict of interests.
We wish to acknowledge the kind collaboration of the Asociación de diabéticos de Málaga (ADIMA), especially to Inmaculada Sánchez and José Sánchez.
LifeScan España (Madrid, España) kindly donated the glucometers and test strips for capillary glucose measurements.