Although sodium-glucose cotransporter-2 inhibitors (SGLT2i) were shown to lower hyperuricemic events in patients with type 2 diabetes mellitus (T2DM), the extent of this effect in the general population is yet to be elucidated. We performed an updated systematic review and meta-analysis on a large sample of patients with and without T2DM to evaluate the influence of SGLT2i therapy on clinically relevant hyperuricemic events, defined as the composite of acute gout flare episodes, acute anti-gout management or urate-lowering therapy initiation. Furthermore, we conducted a multivariate meta-regression to assess the relationship between different covariates and the pooled effect size.
Materials and methodsWe systematically searched all reported outcomes of interest in patients on SGLT2i (PROSPERO: CRD42023442077) across PubMed, Scopus and Cochrane databases looking for randomized controlled trials, observational studies and post-hoc analyses since inception until August 2023.
ResultsData from seven randomized controlled trials and seven observational studies were included for a total of 464,009 patients, 13,370 of whom did not have T2DM. A total of 50% of the patients included were on SGLT2i. The pooled analysis demonstrated that SGLT2i reduce clinically relevant hyperuricemic events by 33% (HR, 0.67; 95% CI, 0.59–0.77; I2=83%) regardless of the concomitant diagnosis of T2DM. The multivariate meta-regression on chronic kidney disease (CKD) showed a positive correlation on the pooled effect size.
ConclusionsSGLT2i reduce the risk of developing hyperuricemic events regardless of the concomitant diagnosis of T2DM. The multivariate meta-regression on CKD showed a significant impact on the main outcome. Further studies are essential to investigate more conclusively the extent of these beneficial effects.
Los inhibidores del cotransportador de sodio-glucosa tipo 2 (iSGLT2) disminuyen los eventos hiperuricémicos en los pacientes con diabetes mellitus tipo 2 (DMT2). Sin embargo, el grado de este efecto en la población general aún debe ser investigado. Presentamos un metaanálisis actualizado en una gran población con y sin DMT2 que evalúa los efectos de los iSGLT2 sobre los eventos hiperuricémicos clínicamente relevantes, definidos como el compuesto de episodios de gota aguda, comienzo del manejo de la crisis de gota o del tratamiento hipouricemiante, con una metarregresión multivariante dirigida a evaluar la relación entre diferentes covariables y el resultado.
Materiales y métodosRealizamos la búsqueda sistemática (PubMed, Scopus y Cochrane) para estudios aleatorizados, observacionales y análisis post hoc, desde su concepción hasta agosto de 2023, que comunican los resultados de interés en pacientes que reciben iSGLT2 (PROSPERO: CRD42023442077).
ResultadosSe recogieron datos de 7 estudios aleatorizados y 7 estudios observacionales en 464.009 pacientes, de los cuales 13.370 eran pacientes sin diagnóstico de DMT2. El 50% de los pacientes incluidos recibieron iSGLT2. El resultado principal muestra que los iSGLT2 reducen los eventos hiperuricémicos clínicamente relevantes en un 33% (HR: 0,67; IC 95%: 0,59-0,77; I2=83%) independientemente del diagnóstico concomitante de DMT2. La metarregresión multivariante sobre la enfermedad renal crónica (ERC) reveló una correlación positiva en el tamaño del efecto.
ConclusionesLos iSGLT2 reducen el riesgo de desarrollar eventos hiperuricémicos independientemente del diagnóstico concomitante de DMT2. La metarregresión multivariante sobre la ERC mostró un impacto significativo en el resultado principal. Se requieren estudios adicionales para obtener conclusiones más detalladas respecto a la magnitud de estos efectos beneficiosos.
Gout is the most common inflammatory arthritis, affecting 2% up to 4% of adults from high-income countries,1 with an incidence rate that has doubled in the past 30 years.2 The clinical impact of gout is underscored by its strong associations with hypertension, obesity, diabetes, and cardiovascular disease, in addition to accelerated mortality.3–7 Population data suggests that gout is significantly undertreated, presumably because of the paucity of existing data related to the safety and efficacy profile of the classic urate-lowering therapy (ULT).8–10
The relationship between type 2 diabetes mellitus (T2DM) and gout is explained by the fact that most urate is reabsorbed by the urate transporter 1 (URAT1) protein and ATP-binding cassette subfamily G member 2 (ABCG2), both of which are upregulated during hyperinsulinemia.11,12 Also, the urinary excretion rate of uric acid is strongly correlated with glucosuria via the GLUT9 of the renal tubules, demonstrating the correlation between serum uric acid (SUA) and glycosuric effects of sodium-glucose cotransporter-2 inhibitors (SGLT2i).13
Heart failure (HF) is another important comorbidity associated with elevated levels of SUA and gout. The increased oxidative stress and inflammatory conditions that are so typical of HF pathophysiology are somehow correlated with the activity of xanthine oxidase, which also participates in uric acid generation.14,15 Importantly, SGLT2i can lead to the downregulation of xanthine oxidase via enhancement of the SIRT-1 signalling pathway, hence decreasing SUA levels.16
SGLT2i are currently the standard intervention to stop the progression of diabetic kidney disease and cardiovascular disease in patients with T2DM, representing the foundational therapy to prevent cardiovascular death or hospital admissions in patients with HF.17–21 SGLT2i have also been found to have beneficial effects on renal function, which promotes their use against chronic kidney disease (CKD).22
Former studies have demonstrated that SGLT2i can lower SUA levels23,24 and also decrease the incidence of gout in patients with T2DM.25,26 A recently published meta-analysis that included a relatively small population without T2DM also showed similar results.27
Our aim is to perform an updated meta-analysis using all the data of interest available and include a larger population with important cardiovascular comorbidities, since new studies have been conducted on patients with and without T2DM. It will also allow us to measure the impact of different clinical conditions, such as diuretic use, obesity and CKD on the obtained pooled analysis results.
Materials and methodsThis systematic review and meta-analysis were conducted and reported in full compliance with the Cochrane Collaboration Handbook for Systematic Review of Interventions28 and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement guidelines.29 The prospective meta-analysis protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database under protocol no. CRD42023442077.30 Statement of human and animal rights was deemed unnecessary.
Search strategyThree independent authors conducted a systematic literature search across the PubMed, Scopus and Cochrane databases looking for studies published in English and other languages from inception until August 30, 2023, including, among others, the following terms: “sodium-glucose cotransporter 2 inhibitors”, “sglt2 inhibitors”, “Empagliflozin”, “Dapagliflozin”, “Canagliflozin”, “Ertugliflozin”, “Gout”, “Uric Acid”, “Uricemia”. The complete electronic search strategy is described in the supplementary file. The references of both the studies included, and the systematic reviews were evaluated for additional studies that report the outcomes of interest. Furthermore, a thorough review was conducted to find the data of interest through a manual search in case of being reported as a secondary or tertiary outcome. Disagreements were resolved by consensus among the three authors.
Selection criteriaInclusion in this meta-analysis was limited to studies that met the following eligibility criteria: (1) randomized controlled trials (RCTs), post-hoc analysis of RCTs, observational studies or non-randomized cohorts and abstracts; (2) trials comparing treatment with SGLT2i vs non-SGLT2i regimens; (3) all patients on SGLT2i; (4) trials that reported the outcome of interest in hazard ratios.
We excluded (1) studies without control groups; (2) overlapping patient populations; (3) case reports or case series; (4) review articles, comment articles, editorials, and letters to the editor.
Main endpoint variablesThe primary endpoint was clinically relevant hyperuricemic events defined as the composite of acute gout flare episodes, acute anti-gout management or ULT initiation. The acute anti-gout management mainly consisted of the administration of colchicine, intra-articular or oral corticosteroids, or nonsteroidal anti-inflammatory drugs (NSAIDs) dispensed within 1–2 weeks after gout diagnosis.
Prespecified subgroup analyses included data limited to (1) studies that reported gout flares only, (2) RCTs with the outcomes that included episodes of acute gout flares, (3) studies with a specific SGLT2i used in the intervention group, (4) studies that were not conducted exclusively on patients with T2DM and (5) studies conducted on patients with and without prior ULT regimen.
Risk of bias assessmentWe evaluated the risk of bias of the RCTs using version 2 of the Cochrane Risk of Bias assessment tool.31 Non-randomized clinical trials were assessed with the Risk of Bias in Non-randomized Studies of interventions tool (ROBINS-I).32 Two authors independently completed quality assessments while disagreements were resolved through consensus after discussing the reasons for discrepancy. Publication bias was investigated using the Funnel-plot analysis and Egger's regression test.
Statistical analysisWe analyzed data using hazard ratios (HR) with their corresponding 95% confidence intervals to compare treatment effects for the primary endpoints and subgroup analyses. We assessed heterogeneity using the I2 statistics and Cochran Q test; p-values <0.10 and I2>25% were considered significant. Meta-regression tests and subgroup analyses were performed to explore possible causes of heterogeneity among the study results. We used DerSimonian and Laird random-effects models for all outcomes. We also performed GOSH analysis and “leave-one-out” sensitivity analyses by removing each individual study from the outcome assessment. Review Manager 5.4 (Cochrane Center, The Cochrane Collaboration, Denmark), Comprehensive Meta-Analysis version 3 and R version 4.3 were used for statistical analysis.
ResultsStudy selection and baseline characteristicsAs illustrated in the PRISMA flow diagram (Fig. 1) our initial search yielded a total of 1267 results. After removing duplicate records and ineligible studies based on their titles and abstracts, a total of 37 results remained and were fully reviewed based on the inclusion criteria. One additional study was identified through a manual search, reporting data of interest as a tertiary endpoint.33 One cohort study was excluded because of the alternative study design approach.34 Three out of the five meta-analyses identified had the outcome of interest, two of which were performed with studies conducted exclusively on patients with T2DM25,26 and one meta-analysis with post-hoc trail-level data.27
A total of 13 studies were included in our meta-analysis of 464,009 patients with a mean follow-up from 9 months to 5.6 years. Although five studies included represented post-hoc analysis of RCTs that compared the SGLT2i effects with placebo,35–39 one of them was conducted using data obtained from two different RCTs.38 Two out of the seven retrospective electronic health record-linkage cohort studies compared SGLT2i with a glucagon-like peptide-1 receptor agonists (GLP-1 RA) regimen in the control group with subsequent subgroup analyses using dipeptidyl peptidase 4 inhibitors (DPP4i) as the control.40,41 Four cohort studies included DPP4i in the control group.42–45 One cohort study included both GLP-1 RA and DPP4i in the control group.46 Finally, 1 study included was the recently published large double-blinded RCT comparing empagliflozin with placebo.33
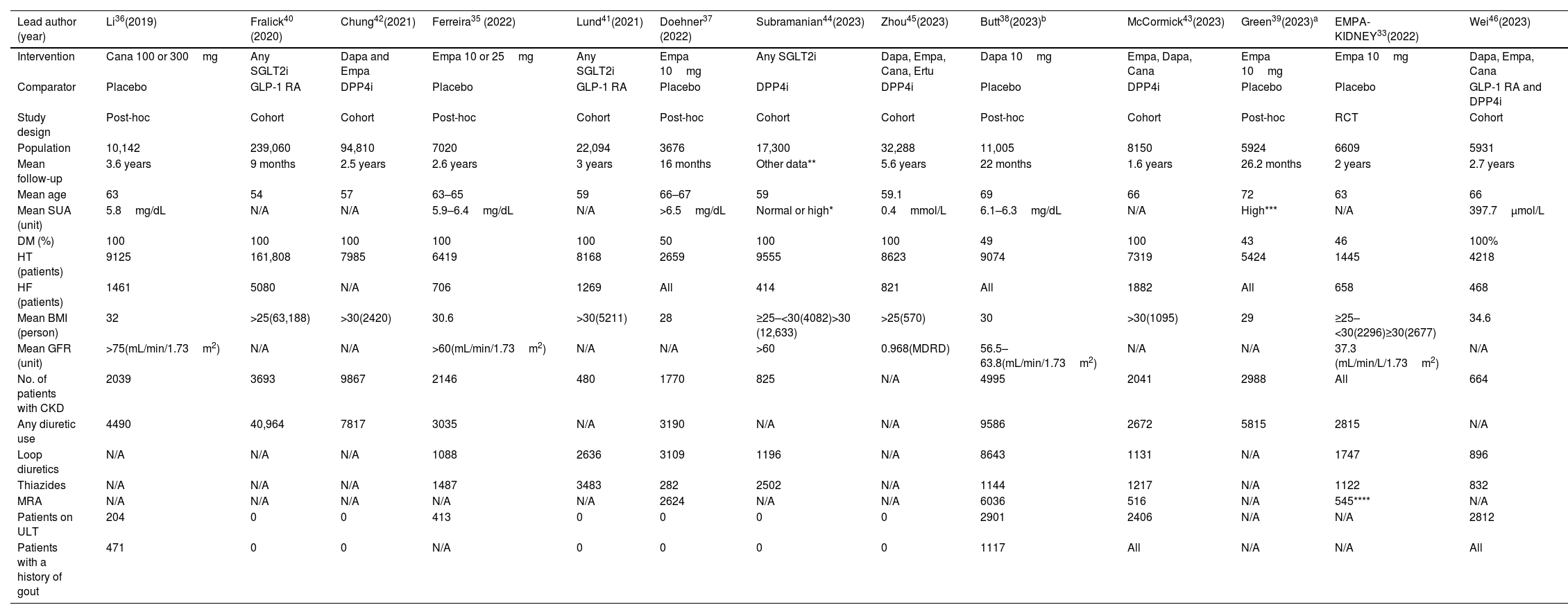
Full study characteristics are shown in Table 1. The details of the definitions of the outcomes in each study included with the specific methods used for gout diagnosis are shown in the supplementary file. To avoid confounding effects from baseline comorbidities and drugs used from the observational studies we included data from the 1:1 propensity score-matched analysis. A total of 50% of the population remained on SGLT2i which constituted the intervention group while the remaining patients from the control group were on regimens without SGLT2i. The population without T2DM consisted of approximately 13,370 patients. The mean age of the patients included ranged from 54 up to 72 years old, 137,870 patients (30%) had overweight or obesity, 33,364 patients (7.19%) a diagnosis of HF and approximately 38,117 (8.21%) had CKD. Notably, at least, 80,384 patients (about 17%) were on diuretics (loop, thiazide, mineralocorticoid receptor antagonists, potassium sparing and other), which are associated with a higher risk of hyperuricemia and gout flares. There was significant variability between the duration of follow-up, mean SUA levels, body mass index (BMI), the number of patients with HF, the number of patients with diuretic treatment, and the number of patients without T2DM.
Individual study characteristics.
| Lead author (year) | Li36(2019) | Fralick40 (2020) | Chung42(2021) | Ferreira35 (2022) | Lund41(2021) | Doehner37 (2022) | Subramanian44(2023) | Zhou45(2023) | Butt38(2023)b | McCormick43(2023) | Green39(2023)a | EMPA-KIDNEY33(2022) | Wei46(2023) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Cana 100 or 300mg | Any SGLT2i | Dapa and Empa | Empa 10 or 25mg | Any SGLT2i | Empa 10mg | Any SGLT2i | Dapa, Empa, Cana, Ertu | Dapa 10mg | Empa, Dapa, Cana | Empa 10mg | Empa 10mg | Dapa, Empa, Cana |
| Comparator | Placebo | GLP-1 RA | DPP4i | Placebo | GLP-1 RA | Placebo | DPP4i | DPP4i | Placebo | DPP4i | Placebo | Placebo | GLP-1 RA and DPP4i |
| Study design | Post-hoc | Cohort | Cohort | Post-hoc | Cohort | Post-hoc | Cohort | Cohort | Post-hoc | Cohort | Post-hoc | RCT | Cohort |
| Population | 10,142 | 239,060 | 94,810 | 7020 | 22,094 | 3676 | 17,300 | 32,288 | 11,005 | 8150 | 5924 | 6609 | 5931 |
| Mean follow-up | 3.6 years | 9 months | 2.5 years | 2.6 years | 3 years | 16 months | Other data** | 5.6 years | 22 months | 1.6 years | 26.2 months | 2 years | 2.7 years |
| Mean age | 63 | 54 | 57 | 63–65 | 59 | 66–67 | 59 | 59.1 | 69 | 66 | 72 | 63 | 66 |
| Mean SUA (unit) | 5.8mg/dL | N/A | N/A | 5.9–6.4mg/dL | N/A | >6.5mg/dL | Normal or high* | 0.4mmol/L | 6.1–6.3mg/dL | N/A | High*** | N/A | 397.7μmol/L |
| DM (%) | 100 | 100 | 100 | 100 | 100 | 50 | 100 | 100 | 49 | 100 | 43 | 46 | 100% |
| HT (patients) | 9125 | 161,808 | 7985 | 6419 | 8168 | 2659 | 9555 | 8623 | 9074 | 7319 | 5424 | 1445 | 4218 |
| HF (patients) | 1461 | 5080 | N/A | 706 | 1269 | All | 414 | 821 | All | 1882 | All | 658 | 468 |
| Mean BMI (person) | 32 | >25(63,188) | >30(2420) | 30.6 | >30(5211) | 28 | ≥25–<30(4082)>30 (12,633) | >25(570) | 30 | >30(1095) | 29 | ≥25–<30(2296)≥30(2677) | 34.6 |
| Mean GFR (unit) | >75(mL/min/1.73m2) | N/A | N/A | >60(mL/min/1.73m2) | N/A | N/A | >60 | 0.968(MDRD) | 56.5–63.8(mL/min/1.73m2) | N/A | N/A | 37.3 (mL/min/L/1.73m2) | N/A |
| No. of patients with CKD | 2039 | 3693 | 9867 | 2146 | 480 | 1770 | 825 | N/A | 4995 | 2041 | 2988 | All | 664 |
| Any diuretic use | 4490 | 40,964 | 7817 | 3035 | N/A | 3190 | N/A | N/A | 9586 | 2672 | 5815 | 2815 | N/A |
| Loop diuretics | N/A | N/A | N/A | 1088 | 2636 | 3109 | 1196 | N/A | 8643 | 1131 | N/A | 1747 | 896 |
| Thiazides | N/A | N/A | N/A | 1487 | 3483 | 282 | 2502 | N/A | 1144 | 1217 | N/A | 1122 | 832 |
| MRA | N/A | N/A | N/A | N/A | N/A | 2624 | N/A | N/A | 6036 | 516 | N/A | 545**** | N/A |
| Patients on ULT | 204 | 0 | 0 | 413 | 0 | 0 | 0 | 0 | 2901 | 2406 | N/A | N/A | 2812 |
| Patients with a history of gout | 471 | 0 | 0 | N/A | 0 | 0 | 0 | 0 | 1117 | All | N/A | N/A | All |
Dapa: dapagliflozin; Empa: empagliflozin; Cana: canagliflozin; Ertu: ertugliflozin; SGLT2i: sodium-glucose cotransporter-2 inhibitors; DPP4i: dipeptidyl peptidase 4 inhibitors; GLP-1 AR: glucagon-like peptide-1 receptor agonists; DM: diabetes mellitus; HT: hypertension; HF: heart failure; BMI: body mass index; SUA: serum uric acid; ULT: urate-lowering therapy; MRA: mineralocorticoid receptor antagonist; N/A: not available.
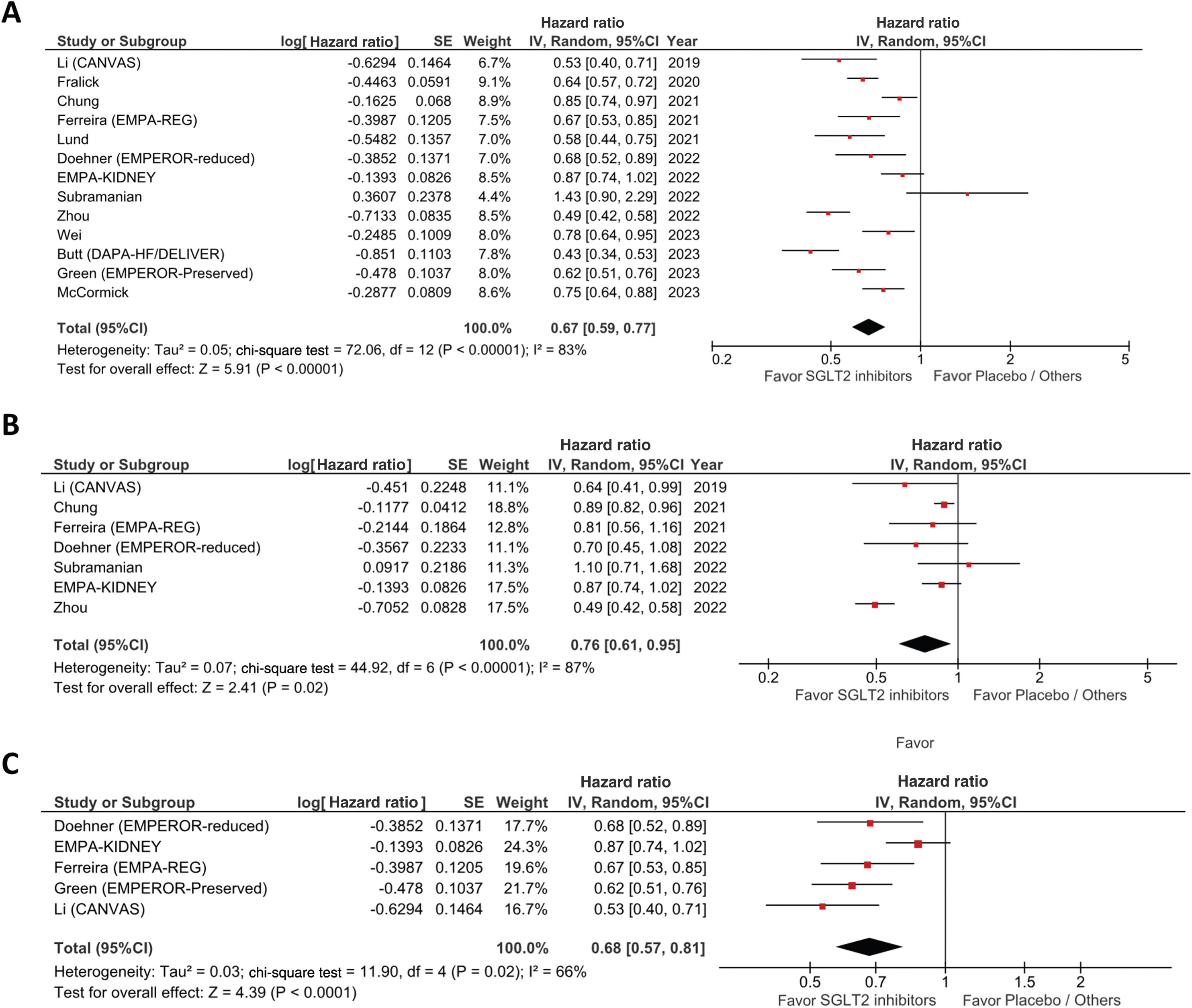
As described in the forest plot of Fig. 2A, the pooled analyses of all studies showed a decrease of clinically relevant hyperuricemic events in patients on SGLT2i vs the non-SGLT2i treatment group (HR, 0.67; 95% CI, 0.59–0.77; p<0.00001) with a significant heterogeneity across the studies included (I2=83% and p<0.00001).
In the subgroup analysis of the episodes of gout flares only, a lower risk of developing gout with SGLT2i use was reported vs no SGLT2i therapy (HR, 0.76; 95% CI, 0.61–0.95, I2=87%, p<0.00001, Fig. 2B).
To explain the high heterogeneity reported, we performed a subgroup analysis with RCTs that reported outcomes including documented episodes of acute gout flares (Fig. 2C). The results showed a decreased HR, 0.68; 95% CI, 0.57–0.81 with lower heterogeneity (I2=66%, p=0.02). Moreover, we conducted a subgroup analysis on the RCTs that provided similar primary composite outcomes of gout events and the beginning of anti-gout treatment that yielded nearly identical results to the primary endpoint (HR, 0.63; 95% CI, 0.56–0.71; I2=0%, p=0.60). In addition, separate subgroup analyses were performed with RCTs that provide outcomes of only the risk of developing gout flares (HR, 0.82; 95% CI, 0.72–0.94; I2=0%, p=0.52) and only anti-gout management or ULT initiation (HR, 0.55; 95% CI, 0.44–0.70; I2=67%, p=0.03) presented in Fig. 3 of the supplementary file.
Furthermore, we conducted a subgroup analysis of three different patient groups on three different SGLT2i from each group (empagliflozin, dapagliflozin and canagliflozin). In these subgroup analyses – as shown in Fig. 4 of the supplementary file – a lower risk of developing clinically relevant hyperuricemic events in each group of 20%, 43% and 39% respectively was reported vs no SGLT2i therapy (HR, 0.80; 95% CI, 0.69–0.94; I2=69%, p=0.02 for empagliflozin, HR, 0.57; 95% CI, 0.35–0.92; I2=96%, p<0.00001 for dapagliflozin and HR, 0.61; 95% CI, 0.47–0.80; I2=44%, p=0.18 for canagliflozin).
To enhance the robustness of results, we performed two additional subgroup analyses: one with the studies conducted exclusively among the T2DM population and subsequently excluding them and another subgroup analysis including studies with patients on a prior ULT regimen comparing them to studies without ULT use. As shown in Fig. 5 of the supplementary file, the results remain statistically significant regardless of the baseline existence of T2DM (HR, 0.69; 95% CI, 0.59–0.80; I2=82%, p<0.00001 for studies exclusively with T2DM patients and HR, 0.63; 95% CI, 0.46–0.86; I2=89%, p<0.00001 for studies without T2DM exclusivity), and a prior ULT regimen (HR, 0.62; 95% CI, 0.50–0.78; I2=82%, p<0.0001 for patients with prior ULT and HR, 0.64; 95% CI, 0.54–0.78; I2=82%, p<0.0001 for patients without prior ULT regimen).
A forest plot with detailed information, including results of the subgroup analysis conducted on each study, and subgroup analyses of the characteristics of the studies included are shown in the Fig. 6 of the supplementary file.
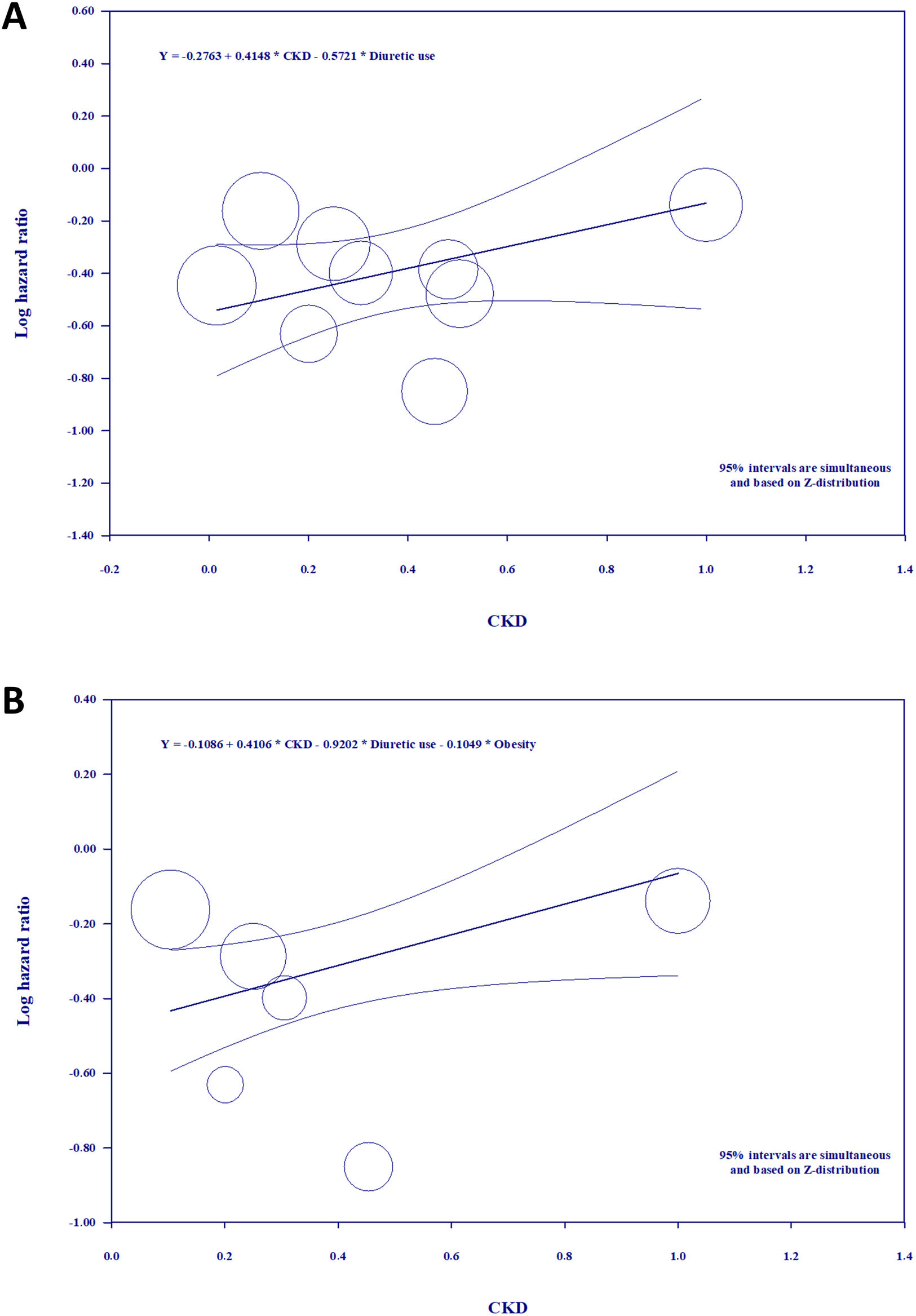
Meta-regressionWe performed random-effects model univariate and multivariate meta-regression tests to explain the high heterogeneity and some variation in the results of each study. For that matter, we looked for a relationship between the pooled analyses effect and certain covariates that were available for most studies included, which would also presumably prompt important changes on primary endpoints, such as BMI, HF, CKD and use of diuretics. To perform the meta-regression analyses we calculated the ratios of the respective covariate data for each included study. Although the results of the univariate meta-regression did not show any statistically significant moderator effects for these confounders, we should still mention the details of meta-regression on the use of diuretics because of the apparently negative correlation (Fig. 7 of the supplementary file). The multivariate meta-regression of CKD with the “use of diuretics” constant (Fig. 3A) kept a positive correlation with a significant p-value of 0.0133 and a Z-distribution of 1.98 (Fig. 8 of the supplementary file). In this model, the CKD covariable accounted for the 0.0199 units of variance, which amounts to 55% of the between-study variance (supplementary file). A different multivariate meta-regression of CKD controlling for the use of diuretics and obesity (Fig. 3B) showed a statistically significant impact in the form of positive correlation, explaining 100% of the total variance in true effect sizes of the studies included in the meta-regression (Fig. 9 of the supplementary file).
Quality assessmentVersion 2 of the Cochrane Risk of Bias assessment tool31 was used to check both the quality and risk of bias of the RCTs included, which were all rated as having low risk of bias (Fig. 1 of the supplementary file).
The observational studies included were assessed using the Risk of Bias in Non-randomized Studies of interventions tool (ROBINS-I).32 Five observational studies were categorized as having serious risk of bias and two as having a moderate risk of bias according to the above-mentioned tool (Fig. 2 of the supplementary file).
Publication bias of the primary endpoint was investigated using the Funnel-plot analysis showing a symmetric distribution of studies with similar weights. Furthermore, the point estimates converged toward the pooled treatment effect as weight increased. We also conducted Egger's regression test showing that the p-value was not statistically significant and therefore, there was no evidence of publication bias. Results are shown in the supplementary file.
DiscussionIn this systematic review and meta-analysis of RCTs and retrospective cohort studies, we compared the risk of developing clinically relevant hyperuricemic events defined as the composite of acute gout flare episodes, acute anti-gout management or ULT initiation between patients on SGLT2i and patients who were not on this group of drugs. The main findings were (1) the risk of developing clinically relevant hyperuricemic events (33% lower among patients on SGLT2i); (2) the risk of developing gout flares alone was also significantly lower in patients on SGLT2i; (3) subgroup analyses of RCTs with outcomes that included documented acute gout flare episodes showed a lower risk of 32% with SGLT2i; (4) the risk reduction effects of each of the three different types of SGLT2i separately, namely empagliflozin, dapagliflozin and canagliflozin showed a significant difference towards reducing the clinically relevant hyperuricemic events; and the statistical significance of results remained constant regardless of concomitant T2DM (4) and prior ULT (5).
There was evidence of high heterogeneity in the pooled estimates (I2=83%) due to the diversity of the population involved in the studies included regarding the variability in comorbidity status, treatment regimens or history of previous gout flare episodes. To explain it, different subgroup analyses were performed with the RCTs only with heterogeneity rates from 66% down to 0%. The strength of this study was increased by the large population involved that made it possible to perform multivariate meta-regressions. The results obtained on CKD showed a significant impact of this covariate. Although the univariate meta-regression on the use of diuretics did not show a statistically significant moderator effect it would suggest a direction for additional research.
Gout and hyperuricemia are suggested to be associated with higher risks of hypertension, obesity, and T2DM.47,48 Gout also proved to be associated with an approximately 30% higher rate of cardiovascular disease and all-cause mortality vs patients without a history of gout.7,49 Furthermore, hyperuricemia was shown to be a common comorbidity in patients with HF, while elevated SUA levels turned out to be an independent predictor of advanced disease and poor prognosis.7,50
Given the lifestyle and dietary changes of the 21th century, the prevalence of gout continues to increase.1,50 Although gout may be prevented through diet and lifestyle changes, many patients with gout require long-term pharmacologic therapy, which in turn can lead to serious cardiovascular and renal adverse effects.3 This may explain the fact that over the past two decades, the use of ULT has not increased51 and that adherence to ULT is the lowest among treatments for seven common chronic medical conditions.52 Furthermore, former studies have shown an increase in the rate of flares shortly after the introduction of ULT; also that flares can persist for years after the SUA target has been achieved.35 This is not known to happen with the use of SGLT2i, hence the importance of further evaluating the potential anti-gout effects of these drugs, considering the high morbidity and mortality rate of patients who are usually affected.
Gout is typically diagnosed using criteria from the American College of Rheumatology and the European Alliance of Associations for Rheumatology,53 however, some of the studies included established the gout outcomes using diagnostic codes from the ICD-9-CM and ICD-10-CM. The diagnostic codes for gout are commonly used in observational studies, and prior validation studies demonstrated the accuracy and completeness of these codes in identifying patients with gout.54,55 The combination of gout visit and flare drug dispensing/procedure was also found to accurately ascertain gout flares in former studies.56 Some retrospective cohort studies included44,46 used The Read classification system to code specific diagnoses while drugs were coded using a dictionary based on the Multilex classification system.
The retrospective open cohort study of Subramanian44 lacked the values of HbA1c, the eGFR, and uric acid which were imputed using multiple imputation by chained equations that could lead to information bias or confounding. Also, the possibility of information bias resulting from incorrect documentation of outcomes or covariates cannot be ruled out in this study. These limitations could explain the findings reported in the study of Subramanian,44 even though these results did not achieve statistical significance.
As far as we know, the current meta-analysis includes the largest sample of the overall population at high risk of developing gout. In a former meta-analysis conducted on T2DM populations26 there was a 34% lower risk of developing gout. On top of being consistent with the outcomes of the former study, our meta-analysis demonstrates that these beneficial effects remain constant regardless of the coexistence of T2DM and other important comorbidities. Moreover, it benefited from the most recent observational studies and rigorously conducted RCTs33,39,43,46 which increase the robustness of results.
The importance of the coexisting benefit of SGLT2i in reducing clinically relevant hyperuricemic events is also emphasized in polymedicated elderly patients, an important limitation for adding new drugs with prolonged treatment regimens, particularly ULT. Although, the reduction of SUA with SGLT2i seen in former studies was substantially less than the one seen with classic ULT, the reduction of clinically relevant hyperuricemic events seen with SGLT2i in our meta-analysis was almost similar to the results obtained with the traditional agents of appropriate gout management such as allopurinol or febuxostat.57 Such ambiguity in the reduction in SUA and gout is seen, for example, with canakinumab58 and can be explained by the anti-inflammatory action of SGLTi, characterized by a lower oxidative stress16 and the inhibition of interleukin-1β (IL-1β),59 considering that acute gout flares activate the NLRP3 inflammasome and promote interleukin-1β.60
Finally, a significant number of these patients were also on diuretics and our findings suggest that the effect of SGLT2i on preventing clinically relevant hyperuricemic events remained unaffected by this covariate and was consistent across several subgroup analyses.
LimitationsOur study has some limitations. First, data provided by the RCTs were obtained from the corresponding post-hoc analyses to the point that only 1 of them was available as an abstract since the full study had not been published when this meta-analysis was conducted. None of the RCTs included had the gout flare events as pre-specified primary endpoint therefore, some event misclassification may have occurred. It is possible that some gout flare events or concomitant prescriptions of ULT have gone unreported, even though a significant association of a lower risk of gout with SGLT2i was still observed.
ConclusionsOur findings indicate that SGLT2i lowers the risk of developing clinically relevant hyperuricemic events by 32% up to 37% in patients with and without T2DM. In addition, there was a significantly lower risk of developing gout flares. The multivariate meta-regression suggested a significant impact of CKD on the pooled estimate. The consistency of the findings across several subgroups increases the robustness of results and highlights the practical utility of SGLT2i in the treatment of clinically relevant hyperuricemic events, demonstrating that the anti-gout effect is a class effect of the SGLT2i. Further RCTs are still needed to investigate the efficacy of these drugs in gout treatment vs the traditional ULT regimens.
Ethical considerations and consent to participateNot applicable.
FundingNone declared.
Authors’ contributionsHamlet Ghukasyan contributed to the study conception and design. Material preparation, data mining and analysis were performed by Hamlet Ghukasyan, Denilsa Dinis Pedro Navalha, Ignacio Pérez Romero, Maria Vitória Prato Wolwacz, and Artur Ghahramanyan. The first manuscript was drafted by Hamlet Ghukasyan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflicts of interestNone declared.