Due to full automation, short result time, and high specificity and sensitivity, immunoassay platforms are currently the method of choice to measure thyroid function tests (TFTs) and parathormone (PTH) level. However, immunoassays are prone to different types of interferences that can result in conflicting interpretations. Recent immunization, transfusion, autoimmune disease, monoclonal therapy, or contact with pets are the main reasons predisposing towards this interference. In addition, currently, clinicians frequently encounter interventions associated with the use of biotin.1,2
Differences in the results obtained with the same assay and discrepancies associated with these results and imaging clinical findings are the main indicators to suspect interferences.3 After confirming the discordant results by repeating the analysis with the same method, a different assay method – precipitation with polyethylene glycol (PEG) – can be performed through serial dilution of the sample by adding commercially available blocking antibodies to rule out or identify the interfering agent.
Here we present four cases in which the proper diagnosis was achieved with minor interventions such as dilution of the sample and using a heterophile blocking tube. If the proper diagnosis was not achieved, we had the possibility of an intervention that could have led to macro problems.
TFTs and the PTH were measured with a sandwich electrochemiluminescence immunoassay method (ECLIA) by COBAS 8000 e801 (Roche diagnostics, Manheim, Germany).
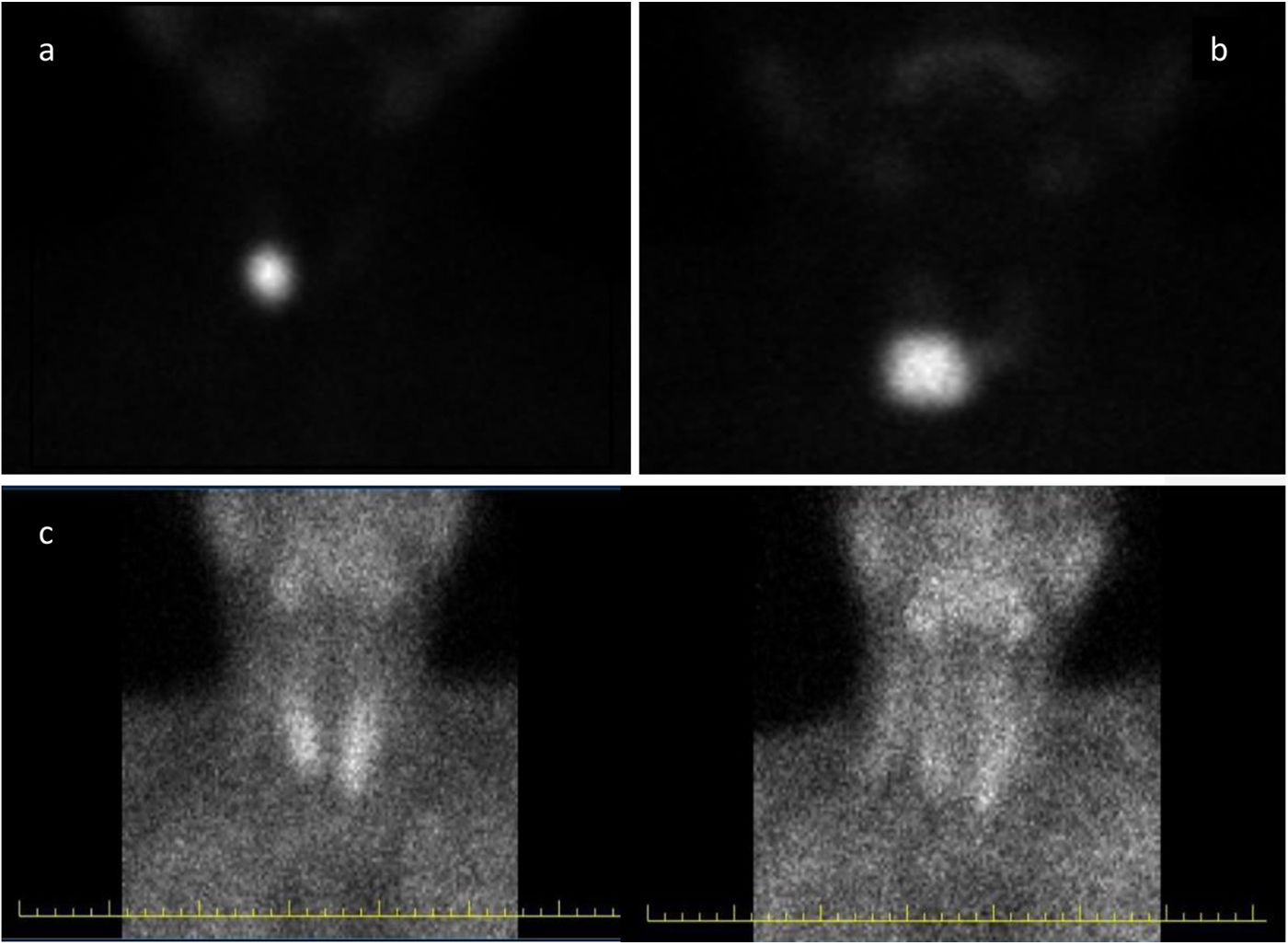
Case report #1: A 29-year-old woman presented with palpitations and fine tremor. At presentation, her thyroid stimulating hormone (TSH), free thyroxine (fT4), and free triiodothyronine (fT3) levels were 0.1μIU/mL (N: 0.51–4.3), 0.94ng/dL (N: 0.98–1.63), and 2.59pg/mL (N: 2.56–5.01), respectively, in two separate tests performed. She was not on biotin replacement therapy, and had no history of pet contact. A 3.0cm×2.0cm×1.5cm single, solid, isoechoic nodule with increased vascularity was found on the right lower lobe on sonography. Thyroid scintigraphy with technetium-99m (Tc-99m) was performed, and eventually turned out to be consistent with toxic adenoma (Fig. 1A). In this patient in whom clinical and imaging results were discordant with the lab test results, TFTs were reanalyzed with a 1:10–1:20 diluted sample in 0.9% NaCl, which revealed the presence of thyrotoxicosis with TSH levels of <0.05μIU/mL, fT4 levels of 4.6ng/dL, and fT3 levels of 15pg/mL. We decided to treat the patient based on her TSH level, and she was referred for radioactive iodine treatment. However, the patient declined radioiodine treatment, which eventually led to performing a right lobectomy. After surgery, the patient's TFTs normalized without the need for levothyroxine replacement.
Case report #2: A 43-year-old woman presented to the hospital with palpitations and tracheal compression symptoms. Upon admission, TFTs revealed the following values: TSH 0.02μIU/mL (N: 0.51–4.3), fT4 0.8ng/dL (N: 0.98–1.63), fT3 4.7pg/mL (N: 2.56–5.01) in two separate tests. She was not on biotin replacement therapy and had no history of pet contact. Sonography revealed the presence of a 3.5cm×2.4cm×0.5cm solid, isoechoic nodule with increased vascularity in the right lower lobe. Additionally, in the left lobe, multiple mixed nodules were identified, with the largest measuring 2.5cm×1.4cm×1.2cm. Afterwards, the thyroid scintigraphy with Tc-99m performed was consistent with a toxic adenoma (Fig. 1B). We reanalyzed the TFTs using a 1:10–1:20 diluted sample in 0.9% NaCl. These results revealed thyrotoxicosis with a TSH <0.05μIU/mL, fT4 4.1ng/dL, and fT3 16.7pg/mL. The patient was put on methimazole and referred for thyroidectomy. Postoperatively, levothyroxine was initiated, and 2 months later, TSH, fT4, and fT3 levels came back to normal.
Case report #3: A 65-year-old man presented with fatigue, and the lab test results were PTH 43pg/mL (N: 15–65); calcium, 13.6mg/dL (N: 8.5–10.5); phosphorus, 1.3mg/dL (N: 2.8–4.5), with a normal serum albumin level. Sonography revealed the presence of a 2.5cm×1.6cm×1.2cm diameter parathyroid adenoma located inferior to the left thyroid lobe, in the 99mTc-sestamibi (MIBI) scintigraphy (Fig. 1C). PTH levels were measured at 1:10 and 1:20 dilutions, revealing an increase up to 184pg/mL and 484pg/mL, respectively. After parathyroidectomy, the patient developed hungry bone syndrome and PTH level dropped down to 34pg/mL.
Case report #4: A 36-year-old woman presented to the endocrine outpatient clinic with persistently elevated PTH (>1000μg/L) in previous measurements. She remained asymptomatic and had normal calcium, phosphorus, eGFR and vitamin D levels. Three separate tests confirmed persistent PTH elevation (482, 884, and 373 pg/mL). Thyroid ultrasound revealed no lesion consistent with parathyroid adenoma. However, despite a high PTH level and the absence of clinical and radiologic findings, the presence of heterophile antibodies was suspected. To address this, her serum specimen was pretreated with a heterophilic blocking tube (Scantibodies Laboratory, Inc.). We tested two incubation periods: 1h (as recommended by the manufacturer) and 2h. After a 1-h incubation period (as recommended), the measured PTH level was 107μg/L. However, after a 2-h incubation period, the measured PTH level was 55μg/L, which fell within the reference range. The presence of a heterophile antibody was confirmed to be the cause of the elevated PTH level seen in our patient.
The discordant analytical levels with image and symptoms in cases with hyperthyroidism as seen in case report #4, and the discordance in adenoma size and PTH level case report #3 led us to consider interference.
Cobas c801 fT4 measurement consists of two consecutive incubation period. In incubation period #1, fT4 in the patient serum and T4-specific antibody labeled with a ruthenium complex form a complex. In incubation period #2, biotinylated T4 and streptavidin-coated microparticles are added into the reaction mixture. During this time, the still-free binding sites of the labeled antibody bind to the biotinylated T4 in the medium, and biotin combines with streptavidin and becomes immobilized. Unbound fractions are, then, washed away and chemiluminescent emission is measured. Therefore, in these two patients, it could be that interfering autoantibodies formed against fT4 prevented the ruthenium-labeled T4-specific antibodies from binding to fT4 in serum. We believe that the dilution performed may have reduced the concentration of these autoantibodies against fT4, thereby reducing their interfering properties. Another possible source of interference is anti-ruthenium antibodies. In a case report published by Favresse et al. on FT4 and FT3 measurement interference, anti-ruthenium antibodies caused low FT4 and FT3 results in the patient.4 The report noted that diluting the sample in ratios of 1:2, 1:4, and 1:8 resulted in significant increases in both FT4 (from <0.5pmol/L up to 8.8pmol/L) and FT3 (from <0.6pmol/L up to 2.3pmol/L) values. This finding is consistent with the general trend observed in the literature, where interfering antibodies tend to cause positive biases in FT4 and FT3 measurements. The rarity of reported negative interference further emphasizes the importance of considering this phenomenon in FT4 and FT3 analysis.
If parathyroid adenoma is not cystic, there is a significant correlation among PTH, calcium level, and parathyroid gland size.5 In case report #3, we checked PTH with dilution due to suspected hook effect and identified this interference by performing serial dilutions (1:10 and 1:20). Although the PTH kit insert states that dilution is unnecessary due to the wide measurement range, this statement seems to only apply to the range per se and not to potential interference issues. In case report #4, due to the nature of the antigen–antibody interaction, it was determined that a 2-h incubation period revealed the presence of heterophilic antibodies in the patient, while a 1-h incubation period did not. Therefore, in patients with suspected heterophilic antibody interference, evaluating the interference after a 2-h incubation period with heterophilic blocking tube will be more accurate.
In conclusion, due to the lack of a single definitive test for interference, accessible initial steps include precipitation with PEG and/or serial dilution of the sample. It is believed that dilution in free thyroxine measurements disrupts the equilibrium between free and bound forms, which is why it is not recommended in FT4 measurements. Antibodies formed against the components of the reagent, unlike interferences that lead to increased free T4 levels, can lead to decreased FT4 values. Serial dilution can provide a clue for the presence of this type of interference.