Foetal hyperthyroidism is mediated by transplacental passage of thyroid stimulating antibodies (TSAbs) and affects mothers with autoimmune (AI) thyroid disease. We report a case of a 33-year-old woman with a history of AI hypothyroidism and raised TSI after 2 stillbirths with suspect foetal hyperthyroidism. At 20.5 gestational weeks (GW) of her third pregnancy, foetal tachycardia and goitre were detected. TSI levels were 30.9mUI/mL. Methimazole (MMI) was started and adjusted based on ultrasound signs (foetal heart rate and thyroid gland vascularisation). The neonate was born at 35GW and cord blood revealed decreased TSH and normal free T4. MMI was started in the neonate at 2 days of life due to the appearance of asymptomatic hyperthyroidism. This case illustrates a rare recurrence of foetal hyperthyroidism in a mother with AI hypothyroidism. Pregestational thyroidectomy, TSAbs determination, early ultrasound diagnosis and foetal therapy helped us to improve obstetric outcomes.
El hipertiroidismo fetal (HF) se produce por el paso transplacentario de anticuerpos estimulantes del tiroides (TSI). Presentamos el caso de una mujer de 33 años con hipotiroidismo autoinmune y TSI elevados, a quien se realizó una tiroidectomía después de 2 muertes fetales con sospecha de HF. A las 20,5 semanas de gestación, se detectó taquicardia y bocio fetal. Los niveles de TSI eran de 30,9mUI/ml. Se inició la administración de metimazol, ajustándose la dosis según los signos ecográficos (frecuencia cardiaca fetal y vascularización glandular). El neonato nació a las 35 semanas de gestación mediante cesárea. Inició tratamiento con metimazol a los 2 días de vida por hipertirodismo neonatal asintomático, hasta los 2 meses. Este caso ilustra una situación infrecuente de HF recurrente en una gestante con hipotiroidismo autoinmune. Los buenos resultados obstétricos pueden atribuirse a la tiroidectomía pregestacional, la determinación de TSI y al diagnóstico y tratamiento precoz del HF.
Foetal hyperthyroidism is a rare condition that affects foetuses of mothers with chronic autoimmune thyroid disease. It is mediated by the transplacental passage of maternal thyroid stimulating antibodies (TSAbs) that will consequently hyperactivate the foetal gland.1,2 These antibodies are commonly present in Graves’ disease (GD), but are rarely present in patients with chronic autoimmune thyroiditis.3,4
We report a case of recurrent foetal hyperthyroidism due to maternal transfer of TSAbs in a mother with autoimmune hypothyroidism and two previous stillbirths.
The patient was a 33-year-old Brazilian woman, with a personal history of chronic autoimmune hypothyroidism. Hypothyroidism was diagnosed at age 21 years and was treated with levothyroxine. She had no previous history of hyperthyroidism.
In her first pregnancy, 8 years ago, stillbirth occurred at 28 weeks of gestation (WG) of a foetus that previously presented tachycardia, pericardial effusion, hydrothorax and hepatosplenomegaly.5
In her second pregnancy, one year later, bilateral ventriculomegaly, mild tachycardia with cardiomegaly and tricuspid regurgitation were detected at 24 WG.5 In the following scans, hepatomegaly, hydrothorax and goitre were added. A cordocentesis was performed to study foetal thyroid function, infections, hemoglobinopathies, metabolopathies or foetal anaemia. Stillbirth occurred 2 days later. The foetal blood sample showed foetal hyperthyroidism (TSH <0.008μU/mL [0.55–4.78μU/mL], free T4 (FT4) 4.03ng/dL [0.80–1.76ng/dL]), while maternal TSAbs levels were elevated (422mUI/mL [0–1.75mUI/mL]).
After those events, she was submitted to total thyroidectomy in order to decrease the TSAbs levels and improve the obstetric results. The anatomopathological study showed chronic lymphocytic thyroiditis which supported Hashimoto's thyroiditis. After thyroidectomy, TSAbs decreased to 27–30mUI/mL, although they remained above the normal limit [0–1.75mUI/mL].
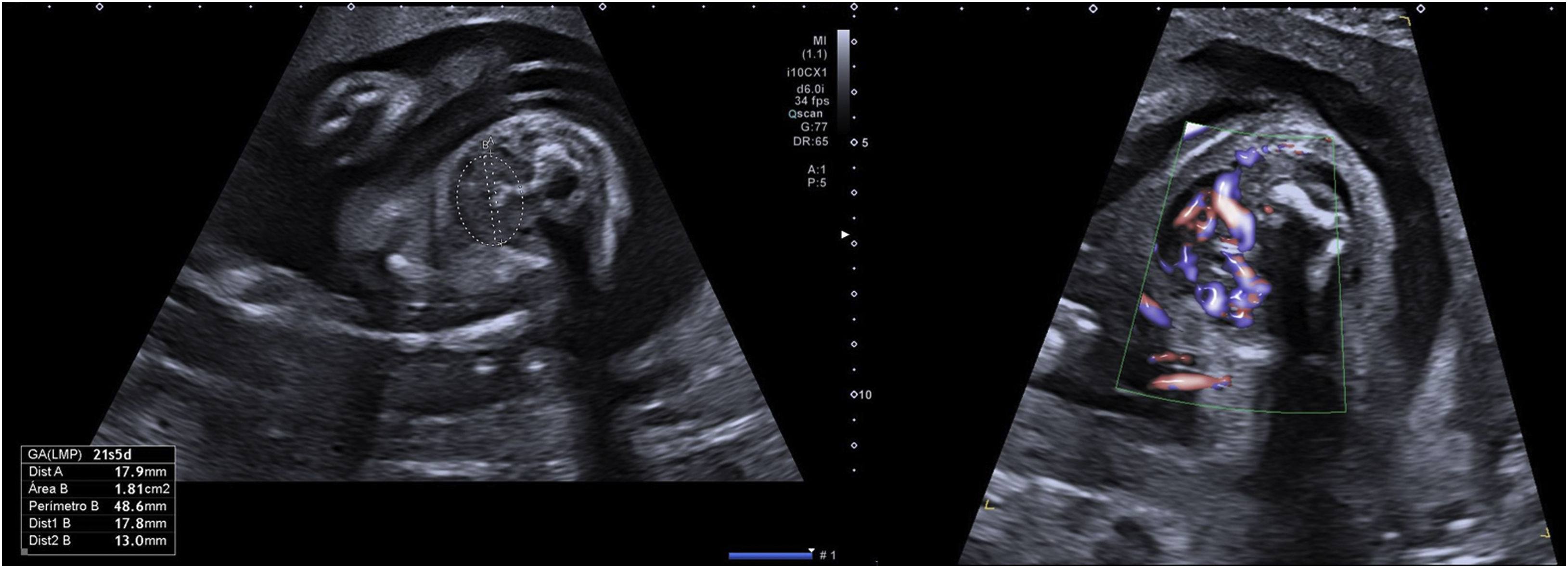
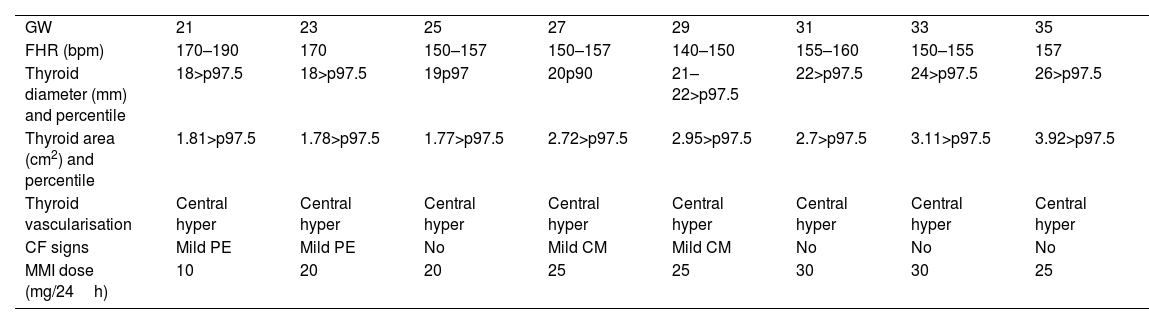
In the current gestation, she was referred at the beginning of pregnancy to our Maternal-Foetal Unit. She was euthyroid throughout the gestation. Hormone levels were TSH 2.29μU/mL and FT4 1.16ng/dL at the first trimester, TSH 3.45μU/mL and FT4 0.86ng/dL at the second trimester and TSH 2.59μU/mL and FT4 1.10ng/dL at the third trimester. She was initially supplemented with levothyroxine 150μg per day, which was increased up to 175μg per day at 26 WG, due to TSH >2.5μU/mL. Ultrasounds at 12, 16, and 17 WG were normal, but foetal goitre and tachycardia (170–180bpm) appeared at 20.5 WG. At 21.5 WG, tachycardia persisted and the thyroid gland diameter and area were above the 97th centile with central hypervascularisation (shown in Fig. 1).6 Mild pericardial effusion was also observed without other signs of cardiac dysfunction or foetal hydrops. Maternal TSAbs levels were 30.9mUI/mL [0–1.75mUI/mL]. At this point, as foetal hyperthyroidism was suspected, maternal treatment with methimazole (MMI) 10mg per day was started. Daily dosage was increased progressively until 25mg at 25 WG, due to the persistence of ultrasound findings. In the following weekly scans, foetal goitre presented stabilisation or slight increase in its diameter and area, with persistent central hypervascularisation. Improvement of the foetal heart rate (FHR) and pericardial effusion was also observed. Mild cardiomegaly was detected at 26 WG but disappeared at 31 WG. At 31 WG, the MMI dose was increased to 30mg per day due to a new mild increase of tachycardia, with subsequent improvement of FHR. Table 1 shows the evolution of the foetal thyroid diameter and area, FHR and maternal MMI dosage adjustment.
Evolution of foetal ultrasound findings and treatment adjustments.
| GW | 21 | 23 | 25 | 27 | 29 | 31 | 33 | 35 |
| FHR (bpm) | 170–190 | 170 | 150–157 | 150–157 | 140–150 | 155–160 | 150–155 | 157 |
| Thyroid diameter (mm) and percentile | 18>p97.5 | 18>p97.5 | 19p97 | 20p90 | 21–22>p97.5 | 22>p97.5 | 24>p97.5 | 26>p97.5 |
| Thyroid area (cm2) and percentile | 1.81>p97.5 | 1.78>p97.5 | 1.77>p97.5 | 2.72>p97.5 | 2.95>p97.5 | 2.7>p97.5 | 3.11>p97.5 | 3.92>p97.5 |
| Thyroid vascularisation | Central hyper | Central hyper | Central hyper | Central hyper | Central hyper | Central hyper | Central hyper | Central hyper |
| CF signs | Mild PE | Mild PE | No | Mild CM | Mild CM | No | No | No |
| MMI dose (mg/24h) | 10 | 20 | 20 | 25 | 25 | 30 | 30 | 25 |
CF: cardiac dysfunction; CM: cardiomegaly; FHR: foetal heart rate; GW: gestational week; MMI: methimazole; p: percentile; PE: pericardial effusion.
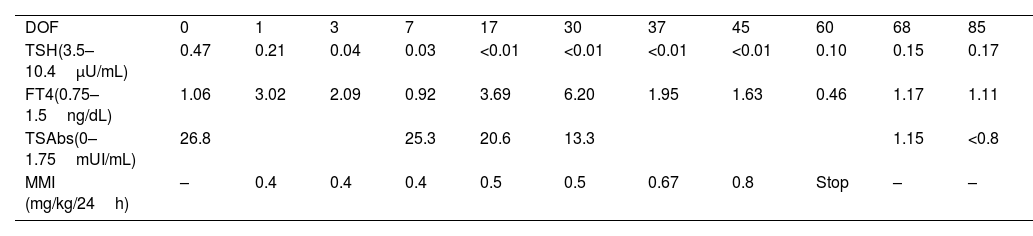
At 35 weeks and 2 days of gestation, the patient started labour and a caesarean section was performed due to front presentation and foetal distress. A newborn girl was delivered: weight 2.600g (>90th centile), length 48cm (90th centile) and cranial perimeter 32cm (50th centile). Apgar scores were 4–8–10 with a single umbilical pH of 7.08. She presented a normal physical examination. Cord blood levels of thyroid function revealed decreased TSH (0.47μU/mL [3.5–10.4μU/mL]) and normal FT4 (1.06ng/dL [0.75–1.5ng/dL]). After 2 days, TSH decreased and FT4 increased without clinical hyperthyroidism, and treatment with MMI was started at 0.5mg/kg daily. Thyroid ultrasound at 4 days of life showed an enlarged gland with hypervascularization. She was discharged at 4 days of life, following weekly outpatient controls. Serial blood tests were performed to adjust the treatment (shown in Table 2), mainly based on FT4 levels. MMI was stopped at the age of 2 months due to blood tests normalisation. At the age of 8 months, she presented normal growth and neurological development.
Evolution of newborn's hormones, antibodies and treatment dosage.
| DOF | 0 | 1 | 3 | 7 | 17 | 30 | 37 | 45 | 60 | 68 | 85 |
| TSH(3.5–10.4μU/mL) | 0.47 | 0.21 | 0.04 | 0.03 | <0.01 | <0.01 | <0.01 | <0.01 | 0.10 | 0.15 | 0.17 |
| FT4(0.75–1.5ng/dL) | 1.06 | 3.02 | 2.09 | 0.92 | 3.69 | 6.20 | 1.95 | 1.63 | 0.46 | 1.17 | 1.11 |
| TSAbs(0–1.75mUI/mL) | 26.8 | 25.3 | 20.6 | 13.3 | 1.15 | <0.8 | |||||
| MMI (mg/kg/24h) | – | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 | 0.67 | 0.8 | Stop | – | – |
DOF: days of life; MMI: methimazole.
Foetal hyperthyroidism is mediated by transplacental passage of maternal TSAbs, that can be found in pregnant women with chronic AI thyroid disease, especially GD,7 and overstimulate the foetal gland. Foetal goitre is an early sign of thyroid dysfunction. This condition can also produce foetal tachycardia (>160bpm), an increase in foetal movements, accelerated bone maturation, craniosynostosis, oligohydramnios, intrauterine growth restriction and signs of heart failure (cardiomegaly, tricuspid regurgitation, pericardial or pleural effusion).8 The goitre itself may worsen cardiac insufficiency due to oesophageal and tracheal compression, leading to polyhydramnios, hydrops, and even foetal death.
In our case, mild tachycardia and foetal goitre were the first signs of foetal hyperthyroidism, which were detected at the second-trimester scan. As foetal cardiac dysfunction signs, only mild pericardial effusion and mild cardiomegaly were temporarily observed during serial controls. Foetal growth was calculated every two weeks and, unexpectedly, the foetal centile was always above the 80th centile. The appearance of the distal ossification centre was not visible until 32 weeks, so we assumed that bone maturation was not accelerated.
In foetuses with goitre, the main clinical issue is determining foetal thyroid status to select the appropriate treatment. FHR, ultrasound vascularisation of the thyroid gland, bone maturation, and foetal movements have been described to differentiate between hypo or hyperthyroidism goitre.9,10 Regarding ultrasound colour Doppler, hypothyroidism is characterised by peripheral or absent vascularisation of the foetal gland, while hyperthyroidism is characterised by central hypervascularisation. The combination of maternal and foetal criteria usually makes it possible to distinguish between foetal hypothyroidism and hyperthyroidism.8 However, cordocentesis remains the gold standard to determine foetal thyroid status by measuring thyroid hormone levels directly in cord blood.11 It can be used for the diagnosis and for the adjustment of antithyroid treatment. It is only recommended when foetal thyroid status is unclear, due to associated foetal risks.12 In our case, we suspected foetal hyperthyroidism due to ultrasound findings (foetal tachycardia and foetal goitre with central hypervascularisation), the personal history of the mother and the high levels of TSAbs. For this reason, we decided not to perform a cordocentesis.
To our knowledge, only 3 cases have been previously reported of TSAbs-mediated foetal thyrotoxicosis in a mother with treated Hashimoto's thyroiditis and no history of hyperthyroidism.1,13,14 Chronic autoimmune thyroiditis (Hashimoto's disease) is one of the most common causes of hypothyroidism, with an estimated prevalence of 11%. It is characterised by a gradual thyroid failure due to lymphocytic infiltration and AI-mediated destruction of the thyroid gland.15 GD is another functional disorder of the thyroid gland which causes AI hyperthyroidism with the presence of TsAbs and may sometimes evolve into AI hypothyroidism. Only about 9% of Hashimoto's patients may also have TSAbs, as in our case, which usually are unable to stimulate maternal thyroid hormone synthesis because of thyroid cell destruction.16 However, foetal thyroid can be affected by TSAbs. Low levels of TSAbs are associated with a low risk of foetal hyperthyroidism, while elevated TSAbs levels (three times the upper limit of normal) are associated with a high risk of effects in the foetus. In our case, maternal TSAbs levels were around 30mUI/mL [0–1.75mUI/mL] throughout the pregnancy (26.8mUI/mL, 30.9mUI/mL and 31.6 in each trimester respectively). TSAbs plasma determination should be considered in pregnant women with autoimmune thyroid disease at the beginning of gestation and again at 20–24 WG,17 if they have a history of foetal death with unknown cause or signs of foetal hyperthyroidism.
Thyroidectomy has been described as an efficient measure to decrease the levels of TSAbs and to improve obstetric results.18 In our patient, the level of TSAbs in previous pregnancies was very high and it decreased to 26–29mUI/mL after the surgery.
Foetal ultrasound is recommended monthly, after 20 WG, in pregnant women who have positive TSAbs, to screen for goitre or other signs of foetal thyroid dysfunction, as foetal hyperthyroidism usually develops at or after this gestational age.6,19 In one case reported by Donnelly et al.,20 foetal hyperthyroidism was suspected early at 18 WG in a mother with severe GD after post-radioiodine ablation and thyroidectomy. In our case, ultrasound was normal at 17 WG but foetal goitre and tachycardia appeared at 20.5 weeks. Therefore, we considered beginning ultrasound follow-up at 18–19 GW.
The treatment of foetal hyperthyroidism consists of maternal administration of ATD drugs (propylthiouracil (PTU), carbimazole or MMI), which can effectively cross the placenta. Both of these have increased risk of malformations during organogenesis so they should be avoided in the first trimester of pregnancy, whenever possible.12 If treatment is required during the first trimester, PTU is preferred because the risk for severe birth defects is lower.21 In our case, treatment was started at 21 weeks and MMI was chosen because it was in the second trimester and to avoid the risk of liver failure in the mother secondary to PTU exposition. The recommended dose needed to treat foetal hyperthyroidism remains uncertain, but the general suggestion is to start with small doses: 10–20mg CM/MMI daily and 100–200mg PTU daily. These doses have not been associated with long-term adverse effects on intellectual development.22 The ATD dose should be periodically reviewed and adjusted by a multidisciplinary team depending on the foetal evolution (heart rate, thyroid Doppler vascularisation, foetal growth, …) and maternal criteria (TSAb titre).22 In our case, we started with a dose of 10mg daily, going up to 30mg at 31 GW, and our main tool to adjust the treatment was FHR.
In a previous case of TSAb-mediated thyrotoxicosis in a foetus with a mother with Hashimoto's thyroiditis, treatment adjustments were also based on foetal tachycardia.13 Although FHR normalised, a newborn was delivered at 36 WG, presenting clinical hyperthyroidism and respiratory distress. In contrast with our case, they used lower doses of MMI and stopped it at 35 WG. Moreover, we also used thyroid Doppler sign and the presence of signs of cardiac dysfunction as secondary tools to adjust the ATD dose. In the other two cases of foetal hyperthyroidism in mothers with autoimmune hypothyroidism with positive TSAbs, the diagnosis was postnatal, not using intrauterine treatment.1,14
Areas of uncertaintyOne of our concerns was exceeding the needs of the foetus and over-treating the hyperthyroidism, producing foetal hypothyroidism, which could affect neurological development.23 The literature reports that congenital hypothyroidism produced by ATD exposure is transient24 and reverts a couple of months after delivery. In our case, the fact that the mother was euthyroid throughout the gestation probably had a protective effect on foetal neurological development. On the other hand, it is also described that an undertreated GD or a maintained foetal hyperthyroidism may produce long-term neonatal central hypothyroidism. This is explained by decreased levels of TSH during the growth of the gland, preventing the normal development of the foetal hypothalamic–pituitary–thyroid axis.7 Matsumoto et al.2 and Donelly et al.20 described the appearance of central hypothyroidism in newborns of mothers with GD and previously treated foetal hyperthyroidism, during the first two months of life. In the case reported by Kiefer et al.13 in a mother with Hashimoto's thyroiditis, the newborn presented central hypothyroidism after the first days of life, which required levothyroxine replacement. In our case, the newborn showed no signs of central hypothyroidism at the age of 8 months, in contrast to these earlier cases.
Regarding neonatal treatment, MMI is preferred, at a dose of 0.5–1mg/kg daily, as was given to our patient. Sometimes, propranolol is also needed in order to improve the tachycardia, but it was not necessary in our case. As described in the literature, hormone levels were normalised and TSAbs were cleared at 2 months of life.
GuidelinesGuidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum were consulted.12 They strongly recommend monitoring the foetus of pregnant women with high levels of TSAbs (3 times the upper limit of normal for the assay) in the second half of pregnancy. If foetal hyperthyroidism is suspected in pregnant woman with hypothyroidism, a combination regimen of levothyroxine and an ATD is indicated. Cordocentesis is only recommended when the thyroid status of the foetus is unclear. Furthermore, we followed the review of Leger et al.,8 which helped us in the management of foetal and neonatal hyperthyroidism.
Conclusions and recommendationsThis case illustrates a rare recurrence of foetal hyperthyroidism in a mother with AI hypothyroidism and the use of medical foetal therapy to improve her obstetric results. The successful outcome of the current gestation can be explained by the diagnosis of the presence of TSAbs in the previous gestation,5 the pregestational thyroidectomy and the early diagnosis and treatment of foetal hyperthyroidism in this pregnancy. Our case indicates that TSAbs testing should be considered in pregnant women with any autoimmune thyroid disease and personal history of stillbirth. TSAbs plasma determination should also be considered in cases of unexplained foetal tachycardia.
We also suggest that ultrasound follow-up could begin at 18–19 GW, especially if the patient has very high TSAbs levels (exceeding two to three times the upper limit of the normal range). Furthermore, when foetal hyperthyroidism is suspected, antithyroid treatment can be started and it can be monitored by foetal ultrasound signs, such as FHR and thyroid gland vascularisation.
Ethical approvalWritten informed consent was obtained from the patient for publication of this case report and any accompanying images.
FundingThe authors received no financial support for the research, authorship, and/or publication of this article.
Authors’ contributionsThe first manuscript draft was written by LG-LF, redrafted by LG-LF, MCR and LAF, and reviewed by SPP, AMB, RCC, MZL and CLH. All authors have read and agreed to the published version of the manuscript.
Conflict of interestThe authors have no conflicts of interest to declare.