To evaluate the long-term efficacy of percutaneous ethanol injection treatment (PEIT) as an alternative to thyroid surgery in symptomatic thyroid cysts.
Patients and methods100 subjects (48±12 years; 58% women) with symptomatic thyroid cysts relapsing after drainage were prospectively included. PEIT was conducted using an established procedure, and the initial cyst volume, symptoms and pain perceived by the patient were assessed. The volume of instilled alcohol was ≤2ml without re-extraction in all cases. Patients were followed-up for more than 3 years and final cyst volume and symptom improvement were assessed.
ResultsMean maximum cyst diameter before drainage was 3.1±1.2cm. In 71% of patients ≤2 PEIT sessions were required. Median maximum cyst volume was 12.7 (5.4–21.7)ml before the first drainage and median total volume extracted from the cysts was 13.0 (6.2–37.0)ml. After a mean follow-up period of 52±10 months, 98% of patients reported a complete absence of symptoms. The final median volume for the whole group was 0.8 (0.1–2.0)ml with a median volume reduction of 94 (81–99)%. A final volume reduction greater than 65% was observed in 90% of cases. Reported pain during the procedure was absent or mild in 76.4% of cases.
ConclusionsPEIT is a safe and well-tolerated first-line treatment for symptomatic thyroid cysts with long-term effectiveness.
Evaluar la eficacia a largo plazo del tratamiento de inyección percutánea de etanol (IPE) como alternativa a la cirugía tiroidea en quistes tiroideos sintomáticos.
Pacientes y métodosSe incluyeron 100 sujetos (48-12 años; 58% mujeres) con quistes tiroideos sintomáticos recidivados. Se realizó una IPE utilizando un procedimiento establecido, evaluando los síntomas, el volumen inicial del quiste y el dolor percibido por el paciente. El volumen de alcohol administrado fue ≤2ml sin reextracción en todos los casos. Se realizó un seguimiento superior a los tres años y se evaluó el volumen final del quiste y la mejoría sintomática.
ResultadosEl diámetro máximo medio de los quistes previo al drenaje fue de 3,1±1,2cm. En el 71% de los pacientes se requirieron ≤2 sesiones de IPE. La mediana del volumen máximo de los quistes previo al drenaje fue de 12,7 (5,4-21,7) ml y la del volumen total extraído de 13,0 (6,2-37,0)ml. Después de un período medio de seguimiento de 52±10 meses en el 98% de los pacientes se consiguió la desaparición completa de los síntomas. El volumen final de todo el grupo fue de 0,8 (0,1-2,0)ml con una reducción del volumen del 94 (81-99)%. En el 90% de los casos se observó una reducción media del volumen final superior al 65%. El dolor notificado periprocedimiento fue nulo o leve en el 76,4% de los casos.
ConclusionesLa IPE es un tratamiento seguro y bien tolerado y por tanto de primera línea en el tratamiento de quistes tiroideos sintomáticos con eficacia demostrada a largo plazo.
Thyroid cysts are mostly benign lesions that can produce compressive or esthetic symptoms depending on size and location and are characterized by their liquid content. After percutaneous drainage, the majority refill and symptoms reappear.1 In the last two decades, a new minimally invasive sonography-guided approach known as percutaneous ethanol injection treatment (PEIT) has been proposed as a safe and effective conservative alternative to open surgical excision2–5 with significant improvement of symptoms and health-related quality of life.8,9 Although PEIT is currently considered a first-line treatment for symptomatic thyroid cysts, this therapeutic is not always included in clinical guidelines10,12 and publications on its long-term results are scarce.13,14 Additionally, a standardized procedure has not been established and there are significant differences among authors regarding important issues such as optimal needle diameter, the volume of alcohol instilled, the re-aspiration or retention of alcohol, the number and interval of PEIT treatment sessions, and the length of follow-up.15,16 Here we report on the long-term effectiveness of our own PEIT protocol in a group of patients with symptomatic recurrent thyroid cysts.
Material and methodsA series of consecutive patients referred for evaluation of a cervical nodule or nodular goiter with cystic component and treated at the outpatient clinic of Endocrinology and Nutrition Department of Germans Trias i Pujol University Hospital in Badalona, Spain, were prospectively included in the study. A case was considered for PEIT if it met all the following criteria: (1) age >18 years; (2) normal thyroid function tests; (3) no major comorbidities; (4) no history of neck irradiation; (5) cystic or predominantly cystic (>80% cystic component) thyroid nodule relapsed after the first simple drainage; (6) a benign cytopathological study and (7) compressive symptoms or esthetic complaints. All patients were followed-up for more than 3 years. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local Human Research Ethics Committee of the Hospital Germans Trias i Pujol. All participants gave their written informed consent.
In all cases, the patient's medical history was reviewed, a physical examination was performed and blood samples were obtained for hormone measurement (serum-free thyroxine, fT4 and TSH), and thyroid anti-peroxidase and anti-thyroglobulin antibodies.
Ultrasound examinationAn echographic examination was performed on each patient by an experienced operator (JLR) using a 12–15MHz linear transducer device (General Electric Logiq E9®). Morphological evaluation included description of the thyroid echostructure and measurement of the diameters and ultrasound characteristics of each detected nodule. Volumetric assessment of the nodules was based on the use of an ellipsoid model.17 With this rotating ellipsoid model, the height, width and depth of each nodule were measured and used to calculate the nodule's volume, with the obtained result then multiplied by the mathematical correction factor 0.524.10 Fine-needle aspiration (FNA) was performed in all cases to obtain cytologic samples and rule out malignancy in the capsule or solid portion of the cyst10 and also to drain the liquid content, and cytological analysis was performed. Patients were offered PEIT if (1) the cyst presented significant regrowth as indicated by the reappearance of compressive symptoms or esthetic complaints after the first drainage, (2) the cytological analysis of the first aspiration had been benign and (3) the patient preferred not to undergo thyroid surgery after extensive explanation of the procedure.
Percutaneous ethanol injection treatment procedureAll PEIT procedures were performed by the same operator (JLR) with 10 years of experience in ultrasound-guided techniques. The positions of patient and operator were similar to those adopted during the FNA procedure, with the patient lying supine with their neck in hyperextension. After skin sterilization, and under ultrasound guidance, a 0.5–0.8mm needle mounted on a connector with a line extension and a 20ml syringe were used to empty the contents of the cyst. Subsequently, 99% ethanol was injected in successive 0.2ml amounts, with a slow movement of the needle to reach the major part of the inner face of the cyst capsule. The patient was instructed to signal any feeling of pain. The amount of ethanol injected was approximately 30% of the volume of liquid extracted, with a maximum of 2ml. The needle tip was constantly monitored during the procedure in order to ensure that it remained within the cyst. In no case were more than 2ml injected. The ethanol was not re-extracted and patients were discharged after a 20–30-min observation period.
Follow-upPatients were followed up biweekly for one month, monthly for three months and every three months thereafter. Ultrasound examination was performed at each appointment to evaluate the characteristics and volume of the residual lesion. TSH and thyroid auto-antibodies were performed annually.
In cases of symptomatic cyst regrowth confirmed by echography, a new PEIT was performed with the same protocol followed by weekly appointments This procedure was repeated until a permanent and significant reduction of the cyst was achieved, defined as a complete resorption or a reduction of at least 50% compared to the initial value with a significant reduction in symptoms. No pre-established limits were set on the number of PEIT procedures.
Response parametersIn each patient, the percentage of volume reduction was calculated by the formula [(V1−V2)/V1]×100 in which V1 is the initial cyst volume and V2 the final cyst volume.
Patients were asked to rate the degree of pain they had felt during with the alcohol injection immediately after the procedure, using a 10-cm visual analog scale (VAS) with one end indicating ‘no pain’ (0) and the other ‘excruciating pain’ (10) and each third of the scale corresponding, from lowest to highest, to mild pain, moderate pain and severe pain.
In order to assess the specific symptoms of neck cysts, patients were administered a 10-item questionnaire related to the symptoms of goiter most frequently described in the literature, namely visible enlargement of the neck (cosmetic complaint), a feeling of pressure in the neck, throat pressure, sore throat, pain in the throat radiating to the ears, difficulty in swallowing, throat clearing often, shortness of breath and hoarseness. Patients had to rate each item for frequency (‘1 none of the time, 2 very occasionally, 3 some of the time, 4 most of the time and 5 all of the time’), and the final score was the sum of the ten items. These scores were then classified as ‘absence of symptoms’ (<10), ‘mild symptoms’ (11–20), ‘moderate symptoms’ (21–30), ‘intense symptoms’ (31–40) and ‘worst possible symptoms’ (40–50). Compressive symptoms involving the trachea, if present, were evaluated by chest X-ray.
Statistical analysisAll continuous normally distributed values were given as mean±SD and all other values were given as median (interquartile range) and categorical variables as percentages. Student's t-test was used for comparisons between continuous variables. For comparison of categorical variables, the chi-square test or Fisher's exact test was used where appropriate. For the correlation studies it was required that Pearson's coefficient be greater than r≥.25. A p value <.05 was considered statistically significant. Data analyses were carried out using the Statistical Package for Social Sciences, Version 15.0 (SPSS©, Chicago, IL, USA).
ResultsOf the initial cohort of 102 patients, two cases (1.9%) presented no recurrence of thyroid cyst after the first FNA drainage and sample collection for cytological evaluation. Therefore, a total of 100 subjects with symptomatic thyroid cysts (48±12 years; 58% women) were included in the protocol. Mean duration of symptoms was 8 (1–12) months and in all cases. Mean TSH was within the normal range (2.0±1.4mU/L) and positive anti-thyroid peroxidase and/or anti-thyroglobulin antibodies were detected in 4.5% of patients. The most frequent symptoms reported by patients were compressive (91%) and esthetic (10%) symptoms, and throat pain (8%). The reported intensity of symptoms was ‘mild’ in 7% of patients, ‘moderate’ in 40% and ‘intense’ in 53%. With respect to type of cyst, 58% were pure liquid lesions with a capsule, 16% were septate cysts and 26% were mixed but predominantly cystic nodules. The cytological studies of samples obtained by FNA from the cystic nodules were benign (hemorrhagic-cystic lesion, colloid cyst or non-malignant lesion) in all cases. One case required liquefaction of the thick colloid that could be subsequently drained without difficulty with the 21G needle.
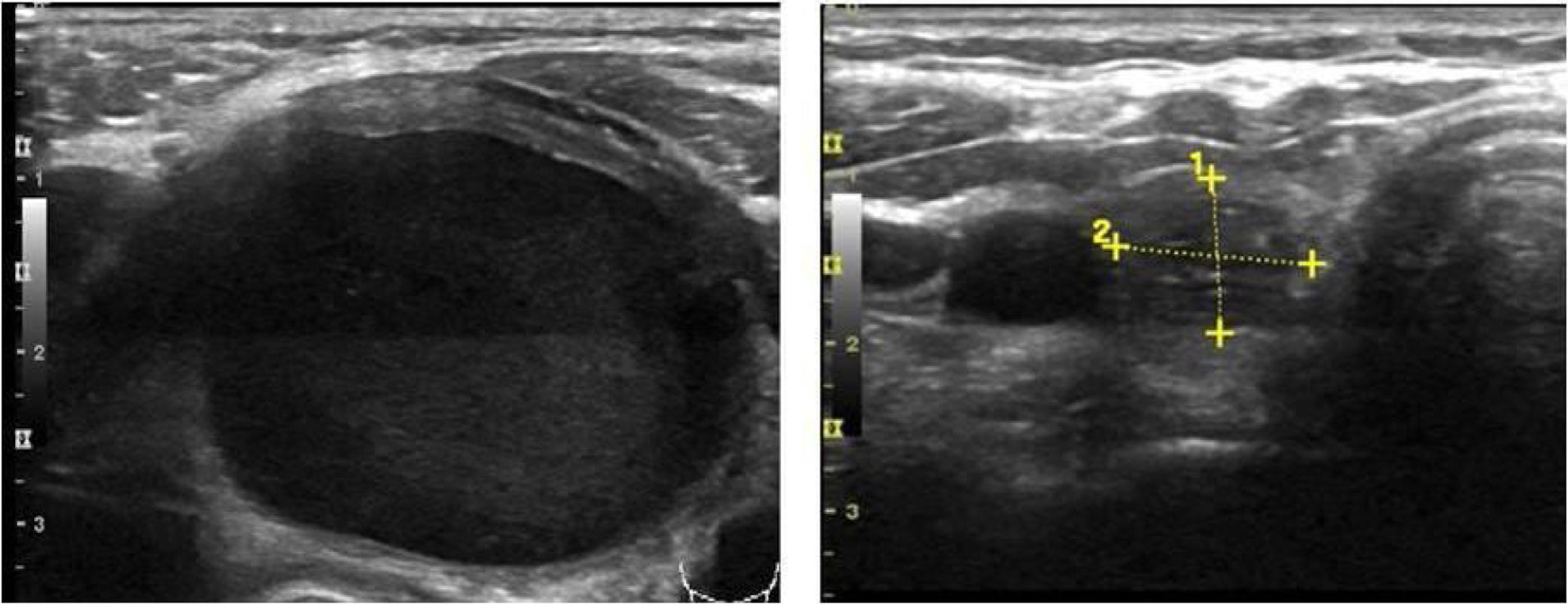
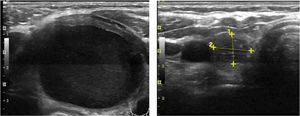
Mean maximum cyst diameter before drainage was 3.1±1.2cm with a calculated median initial volume of 12.7 (5.4–21.7)ml. Median total volume extracted from the cysts in all procedures performed in one patient was 13.0 (6.2–37.0)ml. Panels A and B in Fig. 1 show respectively the initial and final ultrasound appearance of a treated thyroid cyst.
After a follow-up period of 52±10 months, all patients reported significant symptom improvement. After treatment, 98% of patients reported a score corresponding to ‘absence of symptoms’, the remaining 2% perceiving ‘mild symptoms’. No cases of hypo or hyperthyroidism or de novo appearance of anti-thyroid autoantibodies were observed.
The number of PEITs required for a permanently symptom-free situation was one in 38% of cases, two in 33%, three in 15%, and more than three in 14%. Patients requiring more than one PEIT presented significant greater initial cyst volume compared to those who required only one procedure [14.0 (8.5–33.9)ml vs. 7.6 (2.6–18.0)ml, respectively; p<.01]. The mean final volume for the whole group was 0.8 (0.1–2.0)ml with a mean volume percentage reduction of 94 (81–99)%. A final volume reduction greater than 65% was observed in 90% of the patients and a reduction greater than 85% in 70% of all cases. In 4 patients, follow-up ultrasound examination showed total disappearance of the lesion.
With regard to nodule characteristics, cystic nodules were significantly greater than mixed nodules in baseline volume [14.2 (8.1–24.7) vs. 8.5 (3.9–14.7)ml, respectively; p=.01]. The number of PEIT sessions required were similar [2.4 vs. 2.1, p=.5; respectively]. At the end of the follow-up, the final volume were similar [0.8 (0.1–1.5) vs. 1.1 (0.1–2.1)ml, respectively; p=.02] but pure cysts showed greater volume reduction with respect to mixed nodules [96 (86–99) vs. 87.6 (68–97)%, respectively; p=.007].
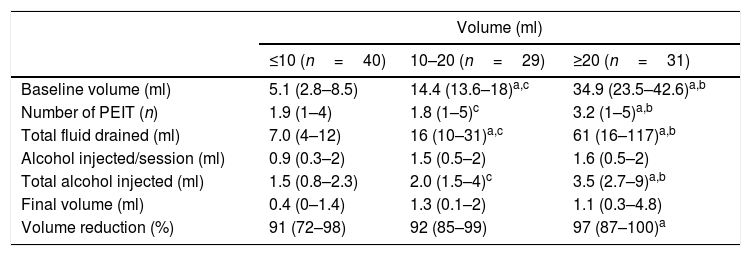
Table 1 summarizes the final volume observed and reduction relative to the initial volume of the cysts. No significant differences were observed between cysts of ≤10ml, 10–20ml and ≥20ml regarding the final volume achieved. As expected, volume reduction and the number of PEITs required was significantly greater in nodules ≥20ml with respect to those ≤10 (p <.01), but similar compared to cysts with initial volume ranging from 10 to 20ml (p=.2). Larger nodules required a significantly greater number of PEITs with respect to smaller ones (p<.01) but not with respect to intermediate nodules (p=.6).
Results obtained in patients treated with percutaneous ethanol injection treatment (PEIT) according to the initial cyst volume. Values are presented as median (interquartile range).
| Volume (ml) | |||
|---|---|---|---|
| ≤10 (n=40) | 10–20 (n=29) | ≥20 (n=31) | |
| Baseline volume (ml) | 5.1 (2.8–8.5) | 14.4 (13.6–18)a,c | 34.9 (23.5–42.6)a,b |
| Number of PEIT (n) | 1.9 (1–4) | 1.8 (1–5)c | 3.2 (1–5)a,b |
| Total fluid drained (ml) | 7.0 (4–12) | 16 (10–31)a,c | 61 (16–117)a,b |
| Alcohol injected/session (ml) | 0.9 (0.3–2) | 1.5 (0.5–2) | 1.6 (0.5–2) |
| Total alcohol injected (ml) | 1.5 (0.8–2.3) | 2.0 (1.5–4)c | 3.5 (2.7–9)a,b |
| Final volume (ml) | 0.4 (0–1.4) | 1.3 (0.1–2) | 1.1 (0.3–4.8) |
| Volume reduction (%) | 91 (72–98) | 92 (85–99) | 97 (87–100)a |
In correlation studies, final volume was significantly related to initial volume (r=.3), volume reduction (r=−0.5), and instilled ethanol (r=.5) (p<.05 in all the cases).
The two cases in which the cyst did not recur after the first simple evacuation remained asymptomatic and ultrasound examinations showed the resolution of the liquid part with a follow-up similar to that of the treated patients.
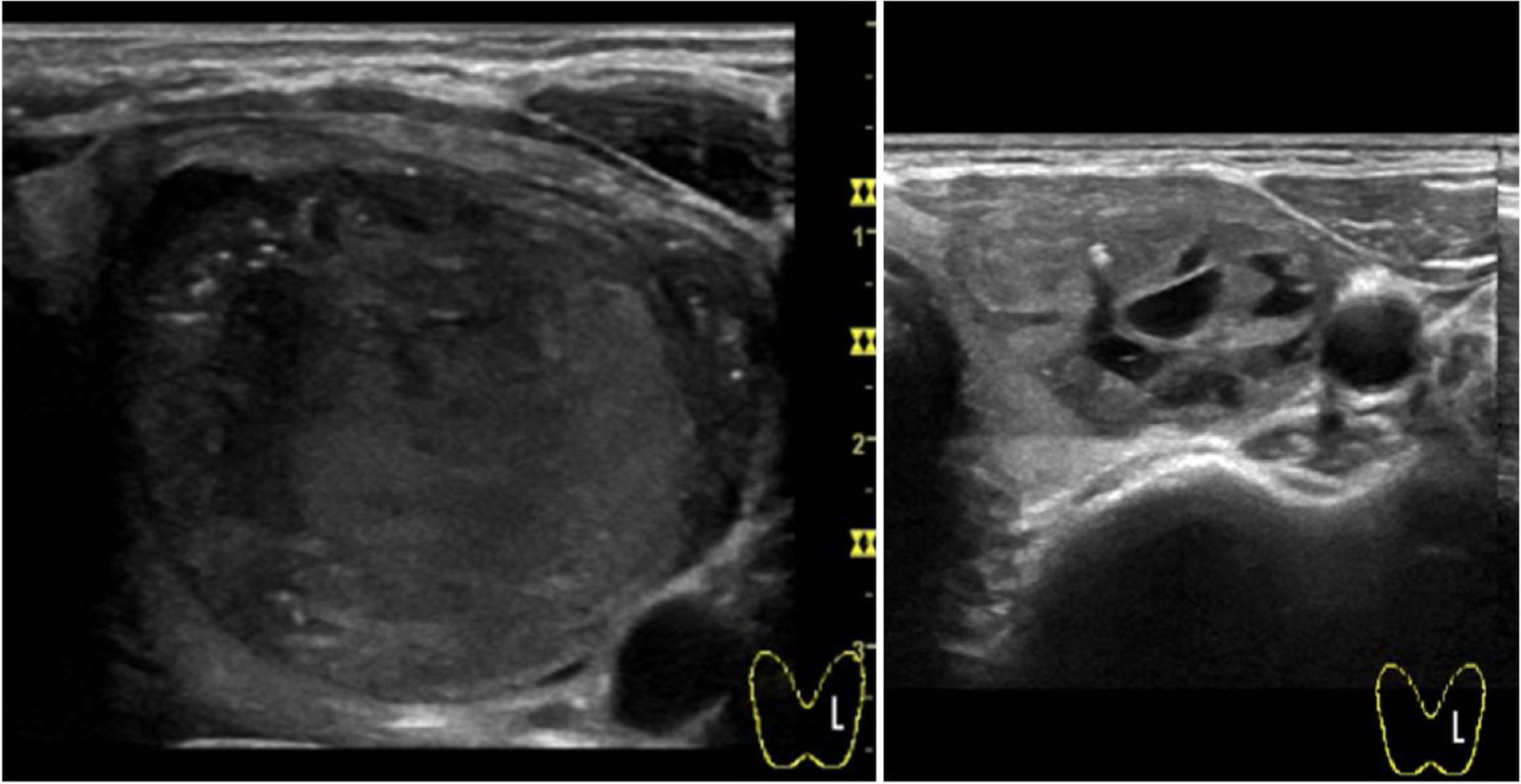
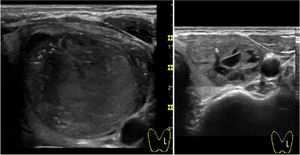
With regard to complications, reported pain during the procedure was mild in 55.2% and moderate in 23.6% of cases. Of note, virtually absent pain perception was observed in 21.2%. In all cases, this pain sensation was transient with relief within minutes. Two patients presented intranodular bleeding in one PEIT, which was self-limited with external compression and local cold application with spontaneous partial reabsorption and totally with a subsequent PEI performed two weeks after (Fig. 2). Cyst recurrence in cases treated more than once occurred from hours to a few days after each PEIT procedure (Fig. 3).
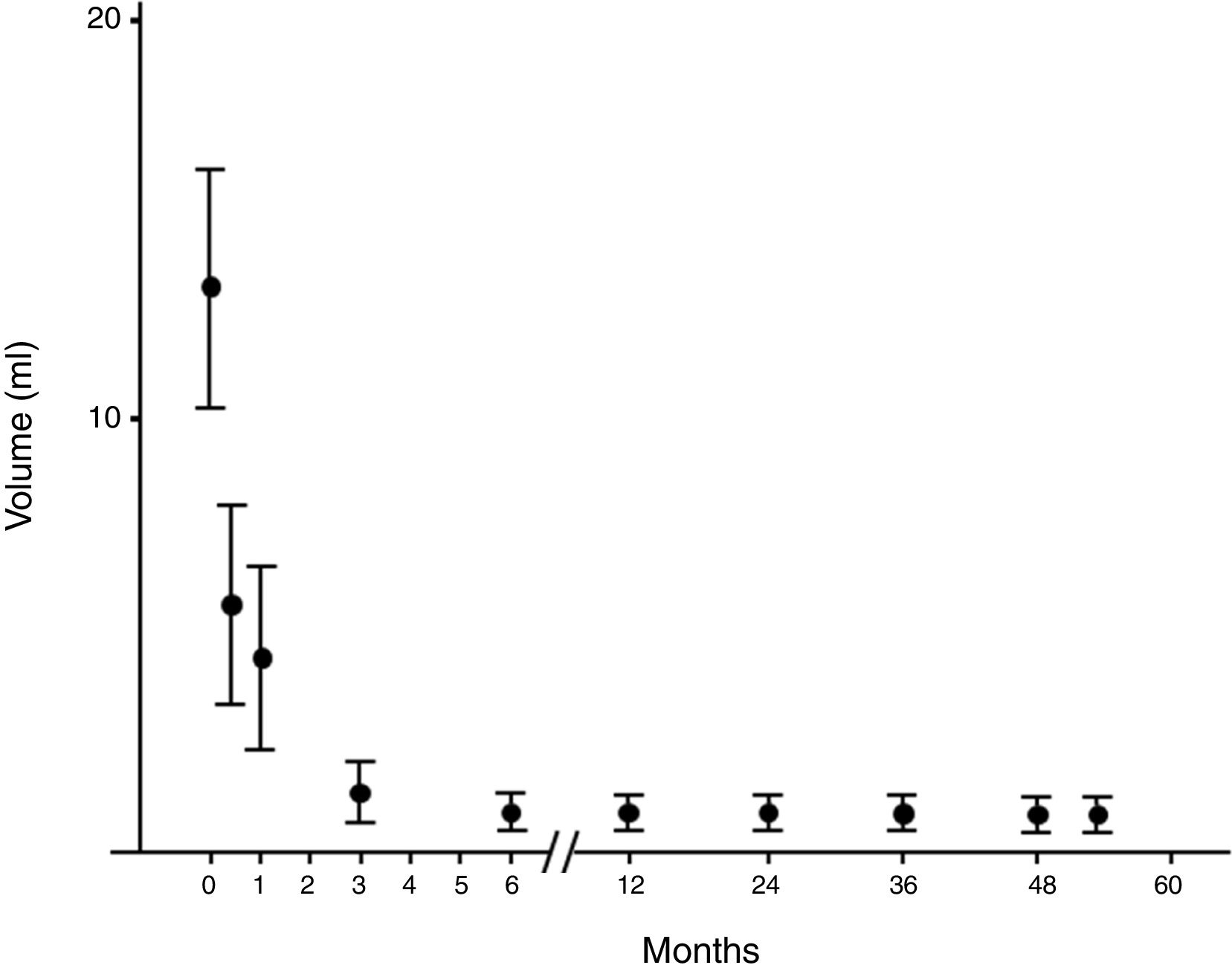
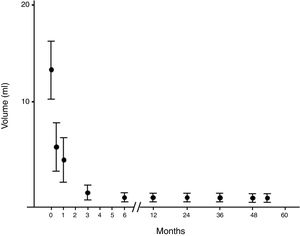
Evolution of the volume of cysts treated by percutaneous percutaneous ethanol injection. Evolution of the volume expressed as median (interquartile range) of the cysts treated by percutaneous alcoholization during the follow-up period. It is observed that once a significant reduction has been obtained, it persists over time.
The results presented in this report clearly demonstrate that percutaneous ethanol treatment is a safe procedure with long-term effectiveness in cases of symptomatic benign thyroid cysts which achieves significant volume reduction of cysts and a total disappearance of symptoms in practically all patients without significant complications. With respect to patient comfort, with our protocol pain was reported to be virtually absent or only mild in most patients and in no case was reported to be greater than moderate. This well-tolerated discomfort is similar to what we8 and other groups have previously reported16,2 and confirm that PEIT is safe and comfortable and no local anesthesia is necessary. The following characteristics of our protocol deserve to be highlighted and in our experience they have proven to be safe and effective
Needle diameterWe used a needle diameter not greater than 0.8mm, which was good enough to remove the cyst contents even when it was dense colloid. Liquefaction through the introduction of alcohol allowed in one of our cases the extraction of the content, and it was not necessary to consider thicker needles. The pain caused by such a needle gauge tends to be limited. Furthermore, in cases in which the first drainage extracted a clear liquid, a fine 0.5mm needle diameter was used with efficacy. This technique is similar to that described in some published protocols18 while others report using larger diameter needles, with greater risk of pain and complications.16,19 In light of our results, we do not advise the use of needles over 0.8mm in diameter.
Needle connectorIn our protocol, the needle was connected to a line extension. The flexibility of this set-up allows the needle to be mobilized without difficulty and without causing injuries while also allowing it to be collapsed to change extraction syringes and for the instillation of alcohol. To the best of our knowledge, there is no description of this technique elsewhere in the literature.
Alcohol retentionIn our experience, retention (as opposed to re-aspiration) of the ethanol inside the cyst cavity is safe and effective and reduces PEIT time. Some authors have reported similar results comparing ethanol retention and aspiration but have found less reported pain with the aspiration technique.16 The 2–5min with the needle inserted in place, increases the duration of the procedure and probably produces discomfort in the patient. The retention technique reduces the number of injections and minimizes the risk of needle protrusion from the cyst cavity due to longer procedure. The consistent results obtained here in volume reduction and the considerable number of patients who expressed having suffered no pain at all or only mild pain are clear arguments in favor of using alcohol retention as a technique.
Initial simple aspiration and cytologyWe offered PEIT after confirming the benignity of the lesion by fluid aspiration and cytological examination of the solid parts of the cyst, and the refilling of cysts that occurred in all cases except two. Previous studies have not described this aspect clearly.4,6,7,9,13 Although we confirm a high recurrence rate (98%), it is reasonable to perform a first simple drainage and wait for cytological results and eventual refill, which tends to be the case, or stable cyst collapse.
Number of PEITIn our protocol, though the number of PEIT sessions required ranged from one to eight, it should be noted that 71% of patients showed disappearance of symptoms and significant cyst reduction after just one or two procedures. This result is similar to previous reports20,21 and, depending on the initial cyst volume, patients can be advised in advance about the number of procedures that may prove necessary.
Volume of ethanol injectedThere is a great diversity of opinion in the literature regarding the volume of ethanol instilled in each PEIT.18,22,23 The most frequent approach is to introduce 50% of the liquid volume extracted with a maximum limit that ranges from 2ml8,24 to 10ml.11,15 However, greater amounts have been described in the literature.25 We use a maximum ethanol volume of less than 2ml in all cases to minimize pain and decrease the risk of ethanol leakage. In addition, the shrinkage of the cyst walls after liquid extraction allows a broad exposure to ethanol.
The main strengths of our study are its prospective nature, with a systematic and homogeneous procedure in all cases performed by the same experienced thyroid and ultrasound expert, and the overall 4-year follow-up, in which no cyst refilling was observed one month after the last treatment performed.
The official recommendations for the treatment of symptomatic thyroid cysts in the published guidelines10,11 propose that recurrent cystic thyroid nodules with benign cytology should be considered for surgical removal or percutaneous ethanol injection based on compressive symptoms and cosmetic concerns (weak recommendation, low-quality evidence). In addition, some authors note as a drawback of PEIT that the procedure may require several injections of large or multilocular thyroid cysts.11 Based on the results described in this and a previous study with more than a hundred patients treated, and with the evidence reported by others in the literature,13,14 we recommend that PEIT should be included as the treatment of choice after a failed drainage and confirmation of benignity. The repeated number of procedures, which in any case did not exceed three in 86% of the cases reported here, would not seem to be an impediment considering the tolerability, safety and efficacy of the technique.
In conclusion, the introduction of ultrasound-guided percutaneous techniques such as radiofrequency, microwaves, lasers and ablation with ethanol, among others, are changing the paradigm in the management of benign thyroid nodules by reducing the number of cases referred to surgery and thus decreasing the risk of major and invalidating complications. We have demonstrated that PEIT for the treatment of thyroid cysts is an effective and safe method for improving and eliminating symptoms over the long term that causes little pain and has minimal complications. We support the inclusion of PEIT as a first-line treatment for thyroid cysts prior to surgical removal in future guidelines. Finally, we suggest that the specific protocol we have described here could be used to create a consensual procedural model.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that they have no conflict of interest associated regarding the publication of this article.