To evaluate the long-term clinical effect of continuous subcutaneous insulin infusion (CSII) in adult type 1 diabetes mellitus (T1DM) patients in a regional public healthcare system real-world scenario.
MethodsAll adult T1DM patients on CSII for ≥10 years subjected to follow-up in the regional Castilla-La Mancha Public Health Service were included. The primary efficacy outcome was the variation in HbA1c during follow-up. Direct patient data were compiled through the web-based Spanish national registry on CSII therapy.
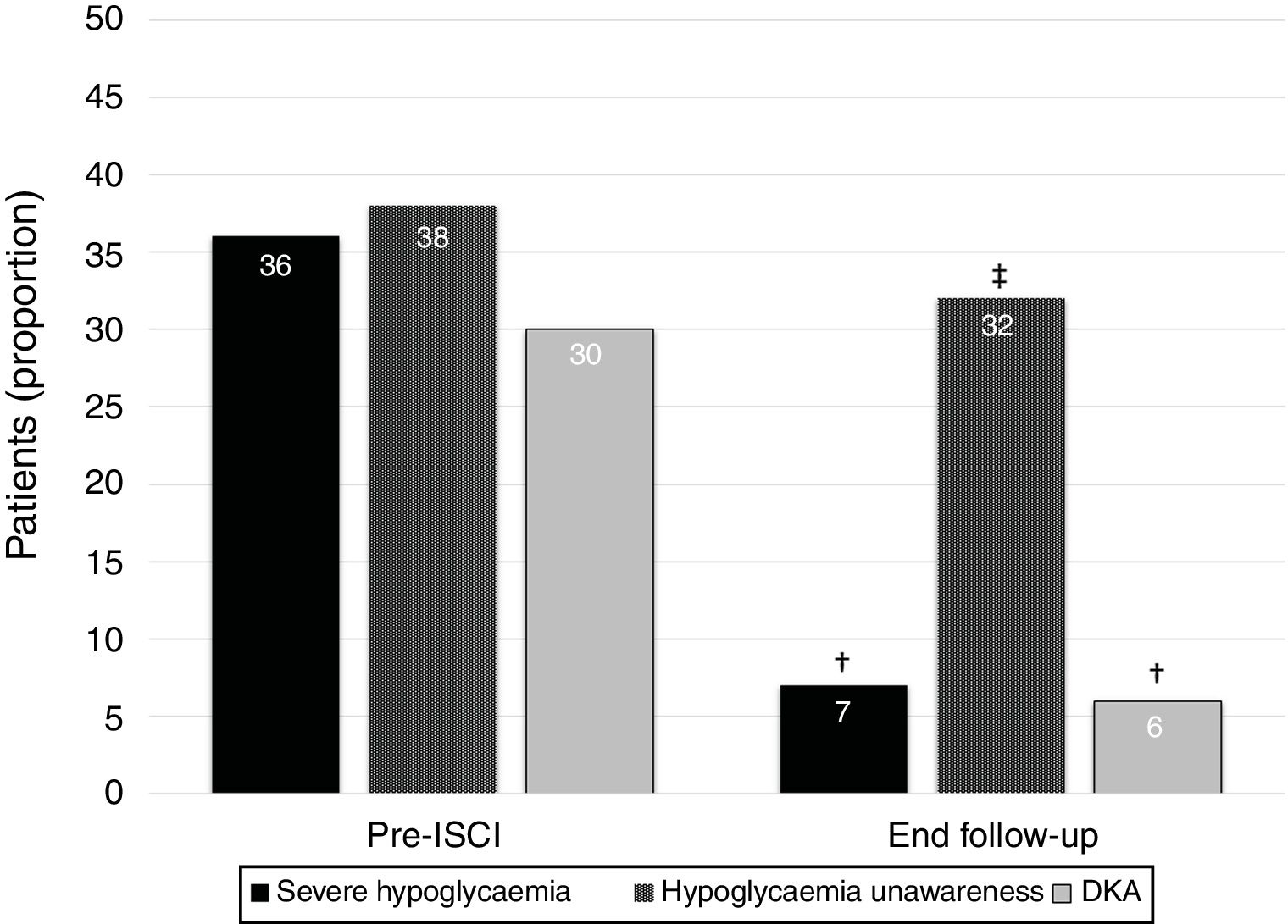
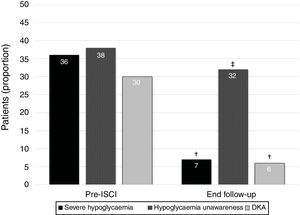
ResultsA total of 69 T1DM adult patients were treated with insulin pumps for ≥10 years in our region. The mean age was 45.0±10.5 years, with a T1DM duration of 13.9±8.5 years. The mean duration of CSII therapy was 11.4±2.1 years. The main indications for treatment were high glucose variability (39%), problematic hypoglycemia (26%), and HbA1c >53mmol/mol (7%) on multiple daily injections (20%). Sensor-augmented pump therapy was used by 31% of the patients. Glycosylated hemoglobin did not change during follow-up (58±11mmol/mol vs. 58±11mmol/mol; 7.5±1.0 vs. 7.5±1.0; p=0.66). However, the percentage of patients with at least one episode of severe hypoglycemia during the last year and unnoticed hypoglycemia decreased from 36% to 7% (p=0.006) and from 38% to 32% (p<0.001), respectively. The proportion of subjects with ≥1 episode of diabetic ketoacidosis in the last year decreased from 30% to 6% (p=0.045).
ConclusionsThe reduction of severe hypoglycemia without deterioration of glycemic control can be sustained over long-term CSII therapy.
Evaluar el efecto clínico a largo plazo de la infusión continua de insulina subcutánea (ICIS) en pacientes adultos con diabetes mellitus de tipo 1 (DMT1) en un contexto de la vida real de un sistema público de salud regional.
MétodosSe incluyó a todos los pacientes adultos con DMT1 tratados con ICIS durante ≥10 años que estaban en seguimiento en el servicio de salud pública regional de Castilla-La Mancha. El criterio de valoración principal de la eficacia fue la variación de la HbA1c durante el seguimiento. Se recopilaron datos directos de los pacientes a través del registro nacional de España sobre el tratamiento con ICIS por Internet.
ResultadosSe trató con bombas de insulina a un total de 69 pacientes adultos con DMT1 durante ≥10 años en nuestra región. La media de edad era de 45,0±10,5 años, y la duración de la DMT1 de 13,9±8,5 años. La duración media del tratamiento con ICIS era de 11,4±2,1 años. Las indicaciones principales para el tratamiento eran una variabilidad de la glucosa elevada (39%), hiperglucemia problemática (26%) y HbA1c>53 mmol/mol (7%) con múltiples inyecciones diarias (20%). El 31% de los pacientes utilizaban terapia con una bomba aumentada por sensor. La hemoglobina glucosilada no cambió durante el seguimiento (58±11 mmol/mol frente a 58±11mmol/mol; 7,5±1,0 frente a 7,5±1,0; p=0,66). No obstante, el porcentaje de pacientes que presentó al menos un episodio de hipoglucemia grave durante el año anterior e hipoglucemia no detectada disminuyó del 36 al 7% (p=0,006) y del 38 al 32% (p<0,001), respectivamente. La proporción de pacientes con ≥1 episodio de cetoacidosis diabética en el año anterior descendió del 30 al 6% (p=0,045).
ConclusionesLa disminución de la hipoglucemia grave sin deterioro del control de la glucemia puede mantenerse durante el tratamiento con ICIS a largo plazo.
The objectives of intensified insulin treatment in type 1 diabetes mellitus (T1DM) patients include to achieve strict glycemic control in order to minimize the risk of chronic diabetes complications, and to avoid severe hypoglycaemia and diabetes ketoacidosis (DKA) to attain the best possible quality of life.1–6 The benefit of continuous subcutaneous insulin infusion (CSII) compared with multiple daily injections (MDI) in patients with T1DM in the short term has been well established.7–11 An additional HbA1c reduction of 5mmol/mol (0.4%) together with a reduction of nocturnal hypoglycaemia are obtained with CSII use in T1DM patients compared with MDI treatment.12 CSII therapy is also associated with a reduction in the frequency of severe hypoglycaemia.11
Available knowledge about CSII long-term effects derives from a few descriptive single-center cohort studies.13–19 Information from complete health systems is even more scarce. The Czech National Register reported the clinical benefit and safety from 730 T1DM patients treated with CSII followed during a four-and-a-half-year period of time.20 Up to the moment, the wider perspective was shown by the Swedish National Diabetes Register including 2441 people treated with insulin pumps for 6.8yrs.21 Finally, the Spanish National Registry on CSII therapy launched by the Diabetes Technology Working Group of the Spanish Diabetes Association informed about safety and benefits of insulin pumps in 1275 T1DM patients followed during a median of 5 yrs. in a representative selection of Spanish centers.22
Recently, we described 313 patients from a complete public healthcare system on CSII therapy for 6.2yrs showing a significant reduction in HbA1c and in the percentage of patients with severe hypoglycaemia.23
The aim of the present subanalysis was to evaluate the sustainability at 10 years of the metabolic benefit of CSII therapy in routine clinical practice in an entire regional Spanish Public Health Service (Servicio de Salud de Castilla-La Mancha, SESCAM).
MethodsA cross-sectional multicenter observational study was designed in order to assess the situation of CSII-treated adult T1DM patients in a complete regional Public Healthcare Service in Spain in terms of prevalence, indications, benefits on glycemic control, hypoglycaemia frequency, rates of diabetes complications, presence of unaware (depending on the discretion of the diabetologist) and/or severe hypoglycaemia and/or DKA, and way of CSII use over time. The protocol was approved by the reference Castilla-La Mancha Public Healthcare Service (Servicio de Salud de Castilla-La Mancha, SESCAM) Ethic Committee. All participants provided written informed consent.
As previously reported,23 information was gathered by nine diabetologists from all SESCAM health care areas. Castilla-La Mancha Region (79.463km2) is the South-central Spanish region with a population size of 2.031.479 inhabitants (last formal national census held in 2017), with 14 hospitals treating adult T1DM patients distributed through eight health care areas (Albacete, Ciudad Real, Cuenca, Guadalajara, Mancha-Centro, Puertollano, Talavera de la Reina and Toledo). Data were entered through the web-based Spanish national registry on CSII therapy designed by the Diabetes Technology Working Group of the Spanish Diabetes Association.
Inclusion criteria for this subanalysis required treatment with CSII at least ≥10 years, age ≥18 years and T1DM diagnosed for >6 months.
The registry included 111 variables related to the patients’ clinical status before starting CSII and at the last follow-up visit during 2018. The main recorded variables included: demographic and anthropometric data, diabetes duration, CSII indications, HbA1c levels, acute and chronic diabetes complications, insulin doses and distribution, and CSII use. A detailed list of variable definitions and a full protocol can be found in our previously published article.23
Statistical analysisCross-sectional and longitudinal/retrospective analysis for patients meeting this subanalysis inclusion criteria were performed. Quantitative variables are expressed as means and standard deviation (SD) or median and range; qualitative variables are presented as total number of events and percentage. A paired Student's t-test or a Wilcoxon signed-rank test were used for the analysis of differences. Comparisons between proportions were analyzed using a chi-squared test. Fisher test was used when the proportion of patients analyzed was smaller than expected proportion. Mann–Whitney U and Wilcoxon signed-rank nonparametric tests were used to analyze statistical differences between groups and differences between baseline and study end, respectively. Between groups mean differences were analyzed through ANOVA test, post hoc analysis (Scheffé test) was perform if previous ANOVA was significant. Multivariable linear regression models were used to assess the association between HbA1c and participant characteristics. Significance was taken at p<0.05. Analyses were performed with IBM SPSS software version 21.0 for Windows (SPSS Inc., Chicago, IL).
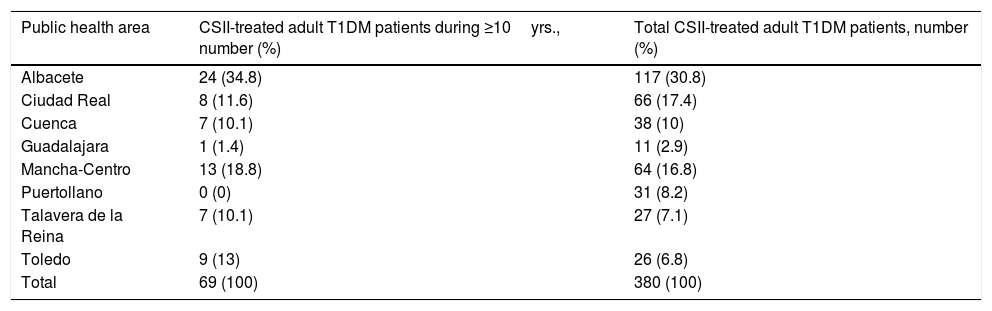
ResultsA total of 69 patients meeting inclusion criteria from the eight health care areas were analyzed. We detected a great variability in the number of T1DM adult patients on CSII therapy for ≥10yrs among health areas, from the highest proportion in Albacete (34.8%) to Puertollano with no patients. T1DM patients on CSII therapy for ≥10 years and total CSII-treated adult T1DM patients in each of the public health areas are shown in Table 1. Data completion for patients was 91.6% of possible data.
T1DM patients on CSII therapy for ≥10 years and total CSII-treated adult T1DM patients in each of the public health areas.
| Public health area | CSII-treated adult T1DM patients during ≥10yrs., number (%) | Total CSII-treated adult T1DM patients, number (%) |
|---|---|---|
| Albacete | 24 (34.8) | 117 (30.8) |
| Ciudad Real | 8 (11.6) | 66 (17.4) |
| Cuenca | 7 (10.1) | 38 (10) |
| Guadalajara | 1 (1.4) | 11 (2.9) |
| Mancha-Centro | 13 (18.8) | 64 (16.8) |
| Puertollano | 0 (0) | 31 (8.2) |
| Talavera de la Reina | 7 (10.1) | 27 (7.1) |
| Toledo | 9 (13) | 26 (6.8) |
| Total | 69 (100) | 380 (100) |
T1DM, type 1 diabetes mellitus; CSII, continuous subcutaneous insulin infusion.
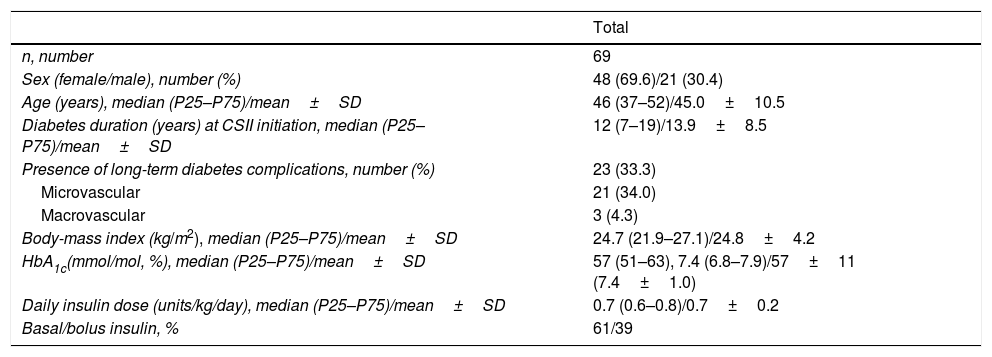
The patients showed a mean age of 45.0±10.1 years and T1DM duration at CSII initiation of 13.9±8.5 years. Mean duration of CSII therapy was 11.4±2.1 years (range 10–23 years). A total of 62 (90%) T1DM patients were treated with CSII for 10–14 years and 7 (10%) patients were on CSII for ≥15 years. Rest of demographics and baseline characteristics are shown in Table 2.
Baseline characteristics of the patients:.
| Total | |
|---|---|
| n, number | 69 |
| Sex (female/male), number (%) | 48 (69.6)/21 (30.4) |
| Age (years), median (P25–P75)/mean±SD | 46 (37–52)/45.0±10.5 |
| Diabetes duration (years) at CSII initiation, median (P25–P75)/mean±SD | 12 (7–19)/13.9±8.5 |
| Presence of long-term diabetes complications, number (%) | 23 (33.3) |
| Microvascular | 21 (34.0) |
| Macrovascular | 3 (4.3) |
| Body-mass index (kg/m2), median (P25–P75)/mean±SD | 24.7 (21.9–27.1)/24.8±4.2 |
| HbA1c(mmol/mol, %), median (P25–P75)/mean±SD | 57 (51–63), 7.4 (6.8–7.9)/57±11 (7.4±1.0) |
| Daily insulin dose (units/kg/day), median (P25–P75)/mean±SD | 0.7 (0.6–0.8)/0.7±0.2 |
| Basal/bolus insulin, % | 61/39 |
Baseline characteristics (age, diabetes duration, BMI, HbA1c and daily insulin dose) have been expressed through median followed by 25th and 75th percentiles and mean±standard deviation. P25, 25th percentile; P75, 75th percentile; SD, standard deviation; CSII, continuous subcutaneous insulin infusion.
The most frequent indications in the whole group of patients for CSII therapy were increased glycemic variability, defined by physician judgment, in 39% (n=27); problematic hypoglycaemia, defined as severe hypoglycaemia, nocturnal hypoglycaemia or impaired awareness of hypoglycaemia, in 26% (n=18); and suboptimal glycemic control, defined as HbA1c>53mmol/mol (7%) on MDI, in 20% of patients (n=14). Other causes of insulin pump initiation were on-going or planning pregnancy, in 12% of the patients (n=8), and lifestyle flexibilization or basal insulin allergy in 3% (n=2).
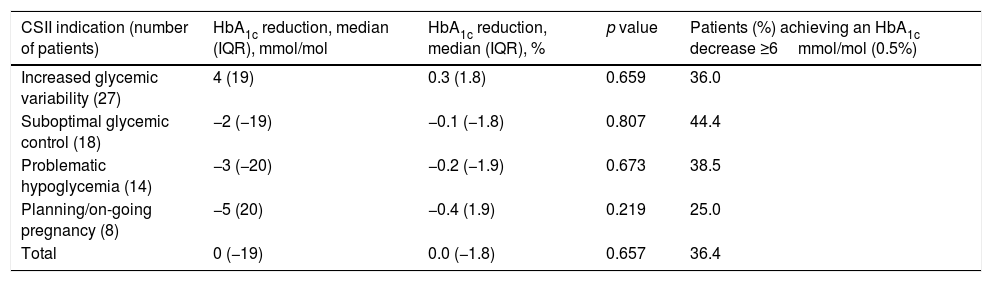
HbA1c levels were similar from the beginning of the study to the end of the follow-up (58±11mmol/mol vs. 58±11mmol/mol; 7.5±1.0 vs. 7.5±1.0; p=0.66). We did not find differences between indications for CSII and final HbA1c levels or proportion of patients achieving an HbA1c decrease ≥ 6mmol/mol (0.5%) (p=0.87) (Table 3).
Change in HbA1c based on indications for CSII.
| CSII indication (number of patients) | HbA1c reduction, median (IQR), mmol/mol | HbA1c reduction, median (IQR), % | p value | Patients (%) achieving an HbA1c decrease ≥6mmol/mol (0.5%) |
|---|---|---|---|---|
| Increased glycemic variability (27) | 4 (19) | 0.3 (1.8) | 0.659 | 36.0 |
| Suboptimal glycemic control (18) | −2 (−19) | −0.1 (−1.8) | 0.807 | 44.4 |
| Problematic hypoglycemia (14) | −3 (−20) | −0.2 (−1.9) | 0.673 | 38.5 |
| Planning/on-going pregnancy (8) | −5 (20) | −0.4 (1.9) | 0.219 | 25.0 |
| Total | 0 (−19) | 0.0 (−1.8) | 0.657 | 36.4 |
A1c reduction has been expressed in median (IQR). The number of subjects in each of the categories have been indicated. Patients with CSII indications different from expressed in Table 3 (n=2) had missing HbA1c data. CSII, continuous subcutaneous insulin infusion; IQR, interquartile range.
In multivariate analysis, final HbA1c was not associated with higher baseline HbA1c, sex, age, longer diabetes duration, body mass index, insulin dose per kilogram of body weight at baseline, proportion of basal/bolus insulin, acute or chronic diabetes complications or CSII indication.
The percentage of patients with at least one episode of severe hypoglycemia in the year before insulin pump decreased from 36% (n=25) to 7% (n=5) in the year before the final follow-up visit (p=0.006). Moreover, the proportion of subjects suffering from hypoglycaemia unawareness decreased from 38% (n=26) before CSII to 32% (n=22) at the end of the follow-up (p<0.001). Finally, the percentage of patients with at least one episode of DKA in the year before insulin pump showed a reduction from 30% (n=21) to 6% (n=4) in the year before the end of the follow-up (p=0.045) (Fig. 1). We did not find differences between indications for CSII and proportion of patients without severe hypoglycaemia, unaware hypoglycaemia or DKA at the end of the study.
During follow-up, insulin daily dose was reduced from the period before CSII to the end of the follow-up (0.71±0.2UI/kg/day vs. 0.66±0.2UI/kg/day; p=0.001). However, proportions of basal and bolus insulin remained stable from the beginning to the end of the study (61% vs. 57%, p=0.15; and 39% vs. 43%, p=0.11; respectively). Patient's body mass index scarcely increased during the follow up (25.0±3.9 vs. 25.3±4.1kg/m2; p<0.001). The ratio of insulin units per 10g of carbohydrates was 1.3±0.7, 1.3±0.6 and 1.3±0.6 for breakfast, lunch, and dinner, respectively. A total of 60.9% (n=42) of patients used different meal insulin to carbohydrate ratio. The mean number of daily boluses was 4.2±1.4; 70% (n=48) of patients used the bolus advisor for most of their boluses, and 41% (n=28) used different types of boluses. Patients used a mean of 5.5±2.2 basal rates per day and 1.7±1.1 basal patterns.
Sensor-augmented pump (SAP) therapy was used by 31% (n=21) of the patients. Frequency of daily boluses was higher in patients using continuous glucose monitoring systems compared with those treated only with CSII therapy (5.4±1.6 vs. 4.1±1.0bolus/day; p<0.001). However, HbA1c levels, percentage of patients suffering from at least one severe hypoglycaemia in the previous year, hypoglycaemia unawareness and DKA were similar among SAP user and non-users at the end of follow-up.
Eighty-one percent of the subjects received individual diabetes education for CSII-training, and 19% received group education. Regarding type of blood glucose self-monitoring before insulin pump initiation: 51% of patients used a self-monitoring notebook, 37% a digital software, and 12% both systems. Only 28% (n=19) of patients checked their ketone levels (blood and/or urinary) before CSII treatment was started.
CSII outcomes were evaluated in those using advanced functions versus those patients not using them or using them less frequently. We did not find any relation between final HbA1c values and insulin bolus frequency or bolus calculator use. However, a greater proportion of patients using five or more basal rates achieved HbA1c levels <53mmol/mol (7%) without DKA during the last year of follow-up (17% vs. 7%, p=0.037), and absence of hypoglycaemia unawareness (33% vs. 22%, p=0.045).
DiscussionThe novelty of our present results resides in the description of the long-term effects of CSII among adult T1DM patients from a Spanish Public Healthcare Service (Castilla-La Mancha, South-central Spanish region). Up to date, this is the longest view of CSII use from a public healthcare system. The main findings were a significant reduction in severe and unaware hypoglycaemia, along with a DKA decrease. This was seen without a negative effect on HbA1c. These finding suggest that one of the major long-term benefits of CSII in a real-world scenario is reduction in hypoglycaemia without a worsening of the rest of glycemic control.
Although several randomized controlled trials have demonstrated the beneficial impact of CSII on glycemic control, reports on long-term follow-up offer different variable outcomes. Up to the moment, the most recent meta-analysis and systematic review showed that insulin pump therapy reduced reduce both, HbA1c (−5mmol/mol, −0.4%) and rate of nocturnal hypoglycemia in T1DM patients.9 However, there are existing reports from a few hundred patients with variable long-term outcomes. CSII initial benefit was not sustained as soon as 3–5 years of follow-up,14,24,25 whereas benefit persist over this period in other studies.26–28 Longer follow-up showed a tendency to return to initial HbA1c after 10 years of insulin pump treatment.15,19 In fact, our data showed no HbA1c benefits over a 11.4 years period of time. Only Senn et al.17 reported long-term improvement in HbA1c during a mean follow-up of almost 9 years. In this study patients were provided with yearly updates and refreshing courses on flexible intensified insulin treatment plus continuous glucose monitoring. Structured programmed update diabetes educational sessions on insulin pumps were not available in any of the hospitals of our public health system. Therefore, cohort studies based on long-term real-world practice offered a different view from randomized controlled trials with a tendency to glycemic control deterioration over time, except in those cases where active diabetes educational programs were well established.17
As aforementioned, the use of insulin-pump therapy has previously demonstrated a reduction in nocturnal hypoglycemia effect without increasing HbA1c values. The most recent literature-summary meta-analyzed showed no difference in severe or minor hypoglycemia with CSII therapy compared with MDI treatment.9 Although the rate ratio for severe hypoglycemia in randomized controlled trials of MDI versus CSII in T1DM patients is 2.0 (1.08–3.69; p=0.027) for decision-making meta-analysis.29 Thus, observational studies report consistent reduction in severe hypoglycemia rates on the long-term.15,30 Moreover, Beato et al. found an improvement in hypoglycaemia awareness after 4.3 years of follow-up. Hypoglycaemia is recognized to reduce a sympathetic response to further hypoglycaemia, and therefore contribute to hypoglycemia unawareness, which in itself predicts hypoglycaemia. We also detected a reduction in the percentages of CSII-treated patients with severe hypoglycaemia and unaware hypoglycaemia. Therefore, our present data extrapolate previously results from randomized controlled trials showing insulin pumps as an effective tool to similarly reduce severe and unaware hypoglycaemia to a long-term real-word Spanish public healthcare system scenario.
The risk of DKA was higher with older insulin pumps, being the risk nowadays considered as lower than the risk of patients on MDI therapy.31 In fact, we described a reduction in the percentage of patients suffering from at least one episode de DKA along the study. Insulin pump therapy may have different outcomes especially as it relates to DKA depending on proper received diabetes educational programs. All of our subjects attended specific diabetes education training (81% individually) focus on insulin pumps and how to detect and correct early ketosis situations.
Continuous glucose monitoring funding is not regulated by Castilla-La Mancha Public Health Service, neither by the Spanish National Board of Health. Each health area decides to reimburse or not according to patient-health professionals requests and economic availability. Despite this, one out of every four CSII-treated T1DM patients used this tool in our region which was associated with a clinically significant HbA1c reduction of 6mmol/mol (0.5%), severe and unaware hypoglycemia at the end of follow-up.23 However, this benefit was not sustainable in the long-term and sensor-augmented pump users only showed higher frequency of daily boluses (5.4±1.6 vs. 4.1±1.0bolus/day; p<0.001). We did not analyzed factors affecting SAP results such as reason for indication, long-term adherence to interstitial glucose monitor systems and update diabetes educational received programs.
Our last set of analysis was aimed at assessing the influence of patients using advanced insulin pump functions. Previous reports have described that a greater use of automated bolus advisor, temporary basal rates and higher number of basal rates per day were associated with better glycemic control.23,32,33 Although we did not find this relation with the first two advanced functions, we detected a greater proportion of patients achieving the combined goals of HbA1c levels <53mmol/mol (7%) without DKA during the last year of follow-up (17% vs. 7%, p=0.037), and absence of hypoglycaemia unawareness (33% vs. 22%, p=0.045) when using a five or more daily basal rates.
Strengths of the study include its long follow-up duration (mean 11.4 years). This is also one of the few multicentre studies gathering direct information from patients which shows a whole view from a complete public healthcare service.
There are, nevertheless, some limitations inherent to this study. Owing to the absence of comparable data about patients treated with MDI regimens, we cannot present data from a control group and thus we can only build upon data from patients prior to the onset of the CSII treatment. Nonetheless, these extensive and long-term results sufficiently prove support the benefit of the CSII treatment, at least in visible improvement of patients who achieve insufficient results when treated with intensified insulin regimens MDI with insufficient results until the commencement of CSII treatment.
ConclusionsLong-term treatment with CSII was associated with a relevant reduction of severe hypoglycemia without deterioration in glycaemic control in a regional Spanish public health system real-word scenario.
Key messages- 1.
This article evaluates the long-term clinical effect of continuous subcutaneous insulin infusion (CSII) in adult type 1 diabetes mellitus (T1DM) patients in a public health system real-world scenario.
- 2.
Up to the moment, this is the wider and longest view of CSII long-term results.
- 3.
We showed that reduction of severe hypoglycemia without deterioration in glycaemic control can be sustained over long-term CSII therapy.
None.
FundingNone.
The authors are very grateful to José Ramón Muñoz (Investigation Support Unit, Ciudad Real General University Hospital, Ciudad Real, Spain) for advice and assistance in data analysis. The authors are also very grateful to Castilla-La Mancha Endocrine, Diabetes and Nutrition Society (SCAMEND) for their support in promoting this study.