Postsurgical hypoparathyroidism is the most common form of hypoparathyroidism.1 The first guidelines for the long-term management of hypoparathyroidism in adults appeared in 2015,2 and a following set in 2016.3 The American Thyroid Association published a statement on the diagnosis, prevention, and management of postoperative hypoparathyroidism in 2018.4 These reports, as well a recent consensus statement published in this journal5 recommend oral calcium and calcitriol supplements (OCCS) or other active vitamin D analogues as the standard treatment of chronic hypoparathyroidism. Such therapy can alleviate the symptoms of hypocalcemia, but its renal complications due to hypercalciuria are the most serious long-term risks for these patients.1,3,6,7 Alternative management with subcutaneous injections of human recombinant parathyroid hormone has not been reported so far as causing a significant sustained reduction in urinary calcium excretion.8
Since the major therapeutic challenge in patients with hypoparathyroidism is to achieve an effective management of hypocalcemia while avoiding hypercalciuria, the current standard of care is based on keeping serum calcium in the low-normal range or slightly below the lower limit of the reference range, 24-h urine calcium excretion lower than 300mg, and calcium-phosphate product below 55mg2/dl2.1–3,9,10 Once a stable regimen of treatment is established, follow-up with routine biochemical monitoring of serum calcium levels, phosphate, magnesium, and renal function, as well as the assessment of symptoms of hypocalcemia and hypercalcemia, are recommended at regular time intervals.1 In order to prevent the toxic renal effects of hypercalciuria, measurements of 24-h urinary calcium excretion every year2,10 or twice yearly9 play a key role in the current management of chronic hypoparathyroidism. The measurement of 1,25-dihydroxyvitamin D has only been contemplated in special situations where compliance and/or absorption might be a concern and the parenteral administration of calcitriol is being considered.9
In the absence of other reliable alternative indicators of hypercalciuria, 24-h urine collections have been implemented despite volume omissions that can produce false negative results.1,11,12 Urinations after bedtime occur frequently and it is probably these specimens that are most often ‘forgotten’, giving rise to erroneously low solute excretions in supposedly 24-h urine.13 The misplacement of samples, the lack of container capacity, the erroneous inclusion of urine from the first void, and the loss of urine during defecation also contribute to incomplete urine collections. In addition, it may be inconvenient to carry containers of urine around for a full day.12 Furthermore, 24-h urine collections are unpopular with both patients and laboratory staff, even more so if they need to be done twice yearly over a lifetime.11 Consequently, it is not surprising that 24-h urine collection in the setting of chronic hypoparathyroidism has been reported as a procedure with a low compliance rate.6 In fact it is assumed that the typical percentage of incomplete 24-h urine samples in non-hospitalized individuals is 15–20% and that missed volumes are around 30%.12 All these inconveniences imply that the necessary diagnosis of hypercalciuria by means of 24-h urine collections may often be unreliable.
In a recent study, we have searched for biomarkers that could be useful for predicting hypercalciuria in patients with permanent postsurgical hypoparathyroidism whose condition had been stabilized with OCCS.14 We found that urinary calcium excretion correlated significantly with the serum level of 1,25-dihydroxyvitamin D (r=0.467; p<0.001). In the multiple linear regression analysis the serum level of 1,25-dihydroxyvitamin D remained independently and significantly associated with urinary calcium excretion (B=6.248±1.423; 95% CI 3.391–9.160; p<0.001). The area under the ROC curve for serum 1,25-dihydroxyvitamin D to discriminate between patients with and without hypercalciuria was 0.797 (95% CI, 0.679–0.916). A cut-off of 33.5pg/ml for serum 1,25-dihydroxyvitamin D had 100% sensitivity and 63.6% specificity to predict hypercalciuria. No patient with a serum 1,25-dihydroxyvitamin D level equal to or less than 33.5pg/ml had hypercalciuria, regardless of the level of serum calcium. To the best of our knowledge, this is the first evidence of a reliable serum biomarker for identifying patients who are at risk of hypercalciuria after chronic hypoparathyroidism treated with OCCS.
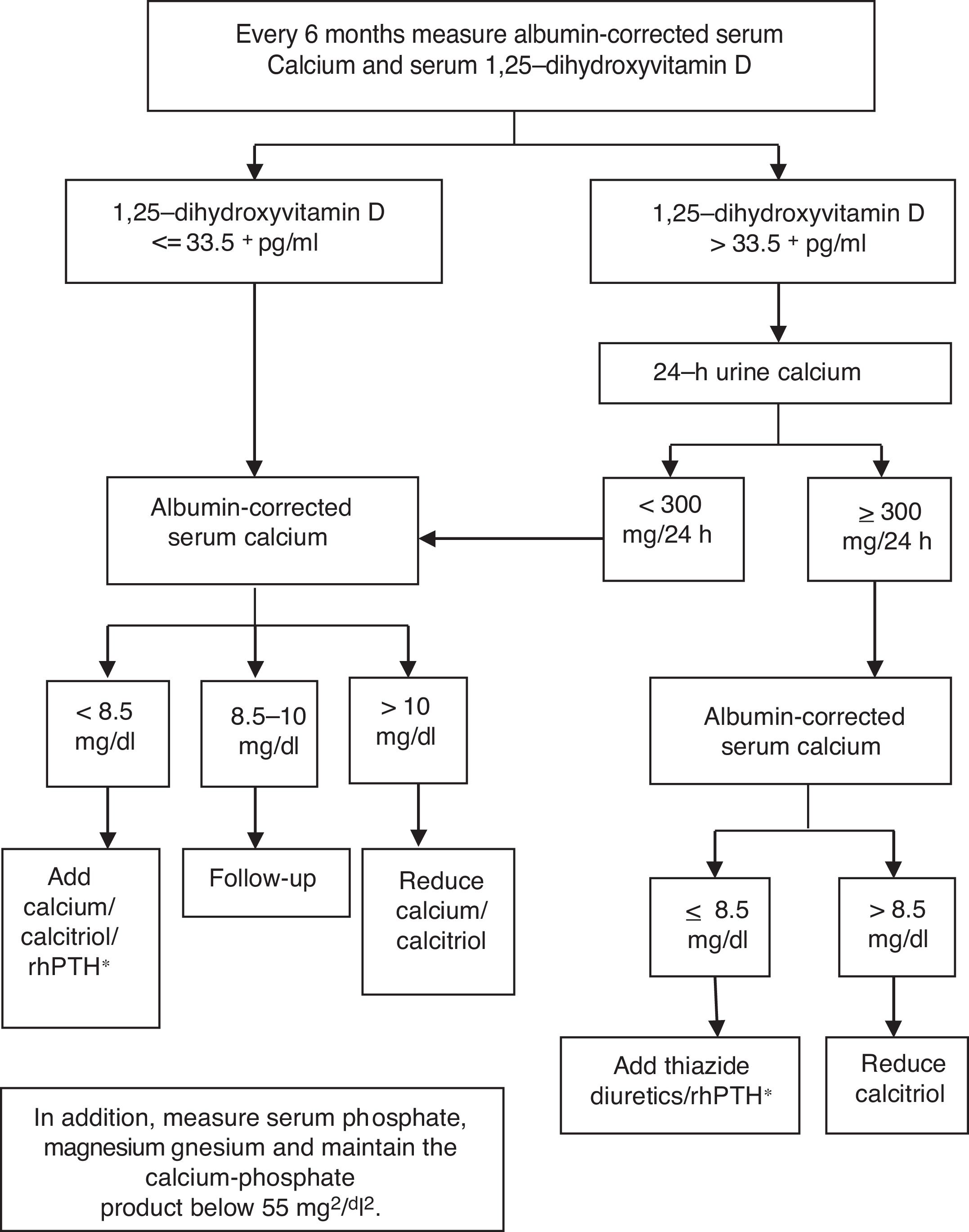
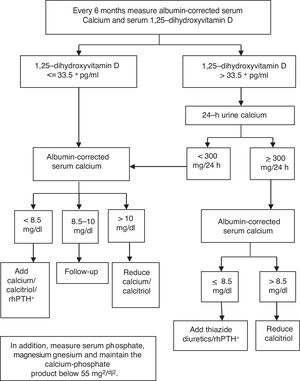
The results of our study suggest that monitoring patients with chronic hypoparathyroidism should include serum 1,25-dihydroxyvitamin D assessment. The measurement of 24-h urine calcium excretion should be limited to confirming hypercalciuria in those patients with serum levels of 1,25-dihydroxyvitamin D above 33.5pg/ml. This approach seems more reliable and less cumbersome for patients than routine 24-h urine collections. We propose an algorithm (see Fig. 1) for the management of patients with chronic hypoparathyroidism receiving treatment with OCCS. It should be noted that the measurement of serum levels of 1,25-dihydroxyvitamin D is available in almost all the clinical laboratories of our country, and its cost is around 10 euros. Although specific cost–benefit studies are necessary it can be anticipated that this approach is feasible and could reduce the economic burden associated with chronic hypoparathyroidism.
Regardless of the evidence that serum calcium is of limited value in predicting hypercalciuria in the setting of hypoparathyroidism,6 a policy of keeping the fasting serum calcium concentration around or below the normal limit has been recommended for many years.1–3,6,9,15 Our data show that patients with chronic hypoparathyroidism receiving treatment with OCCS are at risk of hypercalciuria above a specific level of serum 1,25-dihydroxyvitamin D (33.5pg/ml), regardless of their serum calcium concentrations.14 Because serum 1,25-dihydroxyvitamin D is more closely related to the absence of hypercalciuria than is serum calcium, it seems reasonable to suggest that when 1,25-dihydroxyvitamin D is lower than 33.5pg/ml, the optimal goal of therapy in patients with chronic hypoparathyroidism receiving treatment with OCCS should be to achieve normal serum calcium values, instead of the currently recommended low-normal levels.
In terms of clinical practice, no patients receiving treatment with OCCS, without other conditions that could modify the urinary calcium excretion (drugs, idiopathic hypercalciuria), present hypercalciuria when the serum 1,25-dihydroxyvitamin D level is equal to or lower than 33.5pg/ml, regardless of the level of serum calcium. In the event of 1,25-dihydroxyvitamin D above 33.5pg/ml and confirmation of hypercalciuria by 24-h urine collection, the dosage of calcitriol supplements should be reduced in order to achieve a serum level of 1,25-dihydroxyvitamin D below 33.5pg/ml.
In summary, the assessment of circulating 1,25-dihydroxyvitamin D can be used as a reliable biomarker for identifying patients at risk of hypercalciuria after chronic hypoparathyroidism treated with OCCS. The strategy based on 1,25-dihydroxyvitamin D assessment for monitoring these patients will reduce, not only hypercalciuria and its adverse renal effects but also the high frequency of visits to emergency departments as well as hospital admissions, for symptomatic hypocalcemia as a consequence of maintaining low-normal serum calcium. However, further studies to confirm this approach in other cohorts are required before changing the current guideline on this issue. We encourage other working groups to reproduce our findings (although the cut-off point of the 1,25-dihydroxyvitamin D serum level may be different in each laboratory) in order to improve the management of chronic hypoparathyroidism.
Conflict of interestThe authors declare that they have no conflict of interest related to this paper.