Oral hormonal contraceptives (OHC) are a classical cause of elevated plasma copper (Cu) levels (hypercupremia).1 We describe two cases of hypercupremia secondary to OHC and discuss the potential implications in relation to its differential diagnosis.
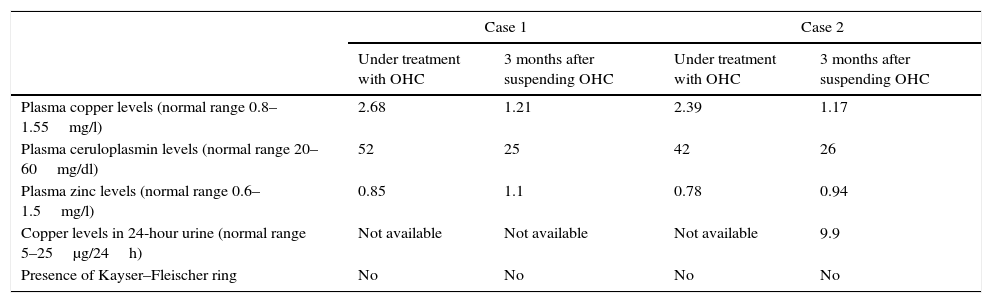
The first case was a 21-year-old woman referred to the Nutrition clinic with denutrition secondary to Crohn's disease (CD), with severe colon involvement and extradigestive symptoms (single-joint osteoarthritis and bilateral anterior uveitis) for the previous two years. At the time of consultation she was enrolled in a clinical trial with Mongersen (antisense oligonucleotide, anti-Smad7) for her CD, and had been receiving OHC (0.03mg ethinylestradiol/0.15mg levonorgestrel) for over a year. The patient reported a usual body weight of 64kg, with a height of 1.8m, and had lost 6kg in the previous month as a result of a CD flare-up. Nutritional support was started with oral supplements until resolution of the flare-up and the recovery of body weight. In the absence of data indicative of CD activity, control nutritional tests revealed normal albumin and ferritin levels, with no folic acid, vitamin B12 or liposoluble vitamin (A, E, D) deficiencies. Hypercupremia (plasma Cu 2.68mg/l [normal range 0.8–1.55mg/l]) was detected, with normal ceruloplasmin and zinc levels. Since the patient was taking OHC, which could possibly increase Cu levels, oral contraceptive suspension was decided upon, with a re-evaluation three months later, following the normalization of plasma Cu (Table 1).
Evolution of the biochemical parameters of cases 1 and 2.
| Case 1 | Case 2 | |||
|---|---|---|---|---|
| Under treatment with OHC | 3 months after suspending OHC | Under treatment with OHC | 3 months after suspending OHC | |
| Plasma copper levels (normal range 0.8–1.55mg/l) | 2.68 | 1.21 | 2.39 | 1.17 |
| Plasma ceruloplasmin levels (normal range 20–60mg/dl) | 52 | 25 | 42 | 26 |
| Plasma zinc levels (normal range 0.6–1.5mg/l) | 0.85 | 1.1 | 0.78 | 0.94 |
| Copper levels in 24-hour urine (normal range 5–25μg/24h) | Not available | Not available | Not available | 9.9 |
| Presence of Kayser–Fleischer ring | No | No | No | No |
OHC: oral hormonal contraceptives.
The second case corresponded to a 19-year-old female with polycystic ovary syndrome receiving treatment with OHC (0.03mg ethinylestradiol/3mg drospirenone) for the previous four years. She was referred to the Nutrition clinic due to grade 2 overweight (weight 75kg, height 1.59m and a body mass index [BMI] 29.7kg/m2), and lifestyle modifications were prescribed (diet and physical exercise). Under asymptomatic conditions, control laboratory testing revealed Cu 2.39mg/l (range 0.8–1.55mg/l), with normal ceruloplasmin levels. After the results were confirmed, OHC was suspended, with a re-evaluation three months later, following the normalization of plasma Cu (Table 1).
In both cases the Cu measurements were made by the same laboratory. Cupremia was measured by atomic absorption spectrophotometry. This technique was used for all the Cu measurements (both basal and after the suspension of OHC); the normalization of plasma Cu therefore cannot be attributed to any change in the measurement method.
Copper is the third most abundant oligoelement in the body, after zinc and iron.2 It is mainly bound to ceruloplasmin, with lesser amounts bound to albumin, or circulating free in plasma.
There is evidence of the influence of estrogens upon copper metabolism.3 In this regard, there are differences between men and women,4 with cyclic variations in the course of the menstrual cycle1,5 or during pregnancy.1 Although not stated in the Summary of Product Characteristics of most OHC, these drugs have been shown to increase plasma Cu by 0.57mg/l on average. The mechanism underlying hypercupremia fundamentally comprises an increase in plasma ceruloplasmin as a consequence of estrogen action,6 in a non-dose dependent manner.7 The type of progestogen present in the OHC probably modulates the effect upon Cu metabolism, with a greater increase in cupremia among those patients receiving OHC containing progestogens with antiandrogenic actions.8 The Cu transporters ATP7A and ATP7B are possibly implicated in OHC-mediated hypercupremia.9 There is, therefore, an individual susceptibility to develop this side effect.
The clinical repercussion of hypercupremia secondary to OHC is not clear. Toxicity symptoms secondary to hypercupremia (usually of a digestive nature) are considered to appear when plasma levels exceed 3mg/l,2 with possibly fatal consequences on their exceeding 5mg/l. According to a meta-analysis published in 2013, hypercupremia due to OHC usually does not exceed 2mg/l.6 The question is whether hypercupremia induced by prolonged OHC use can give rise to Cu deposits in the tissues and induce disease over the long term, particularly cardiovascular disorders. Epidemiological studies suggest that there is an association between Cu levels and an increase in mortality due to coronary disease.10 However, it is not clear whether this association is attributable to a direct effect of Cu upon arteriosclerosis, or whether it constitutes an inflammation marker. It should be noted that there have been three reports of corneal Cu deposits secondary to OHC-mediated hypercupremia.11,12 It is not known whether there is any factor predisposing to the development of this complication in women with hypercupremia secondary to OHC.
There are a number of causes of hypercupremia, and establishing the corresponding differential diagnosis sometimes requires multiple and expensive tests. In this respect, the identification of OHC as a cause of Cu elevation can simplify the study of certain patients. The duration of exposure to OHC required to produce hypercupremia has not been clearly established, though according to some studies this is not a decisive factor in the appearance of the disorder.6 Likewise, it is not clear how much time should be allowed to elapse after treatment suspension before Cu levels are re-evaluated, though there have been reports of Cu normalization 3–4 weeks after OHC withdrawal.11 No markers have been established to date allowing us to predict which women will present hypercupremia secondary to OHC and which will not.
In our two cases hypercupremia can be attributed to contraceptive use, in view of the normalization of Cu levels after OHC was suspended and the absence of other possible causes justifying Cu elevation (liver function, normal levels of ferritin and other laboratory test parameters, no evidence of neoplastic disease or other disorders associated with hypercupremia). There was no evidence of clinical repercussions or the formation of corneal Cu deposits in either patient. Nevertheless, OHC suspension was advised in view of the potential deleterious effects of chronic exposure.
There is insufficient evidence to recommend systematic cupremia and ceruloplasmin testing in all patients receiving OHC. However, such tests should be requested in patients presenting clinical or laboratory test evidence of excess Cu levels (liver disease, digestive or neurological symptoms, etc.) after starting treatment.
In patients under study due to hypercupremia and who are taking such contraceptives, these should be regarded as a possible cause. In the event of hypercupremia attributable to an OHC-mediated pharmacological effect, such treatment should be suspended in the presence of symptoms attributable to hypercupremia and/or plasma Cu levels of >3mg/l. In asymptomatic patients with plasma levels <3mg/l, the decision to continue treatment should take into account the few data available on the long-term effects and the potential risk associated with sustained hypercupremia.
Please cite this article as: Araujo Castro M, Prieto Coca L, Izquierdo Álvarez C, Oliván Palacios B, Campos del Portillo R. Hipercupremia secundaria a anticonceptivos orales: a propósito de 2 casos. Endocrinol Diabetes Nutr. 2017;64:509–511.