Ghrelin is a gastrointestinal peptide involved in regulation of body weight and energy balance. However, its behavior after bariatric surgery and its relationship to insulin resistance are still controversial. A simultaneous assessment was made of the association between changes in ghrelin levels and different variables after three types of bariatric surgery.
Patients and methodsGhrelin levels were measured in 103 morbidly obese subjects before and 6 months after bariatric surgery (Roux-en-Y gastric bypass (RYGB), biliopancreatic diversion of Scopinaro (BPD), and sleeve gastrectomy (SG)), and in 21 non-obese subjects.
ResultsGhrelin levels increased after RYGB (p<0.05), were unchanged after BPD, and decreased after SG (p<0.05). The percent change in ghrelin levels (Δ-ghrelin) was associated to the type of surgery in a multiple linear regression model (p=0.017). When the same analysis was only performed in subjects in whom the gastric fundus was maintained (RYGB and BPD), Δ-ghrelin was negatively associated to Δ-HOMA-IR (p=0.001). In morbidly obese subjects who underwent RYGB and BPD, the odds ratio of a lower Δ-HOMA-IR in patients with Δ-ghrelin in the Q1 quartile versus those with Δ-ghrelin in the Q4 quartile was 8.74 (1.73–44.06) (p=0.009).
ConclusionsChanges in ghrelin levels after bariatric surgery are associated to the presence or absence of the gastric fundus. After bariatric surgery, the decrease in insulin resistance was associated to increased ghrelin levels in procedures in which the fundus is not excluded.
La ghrelina es un péptido gastrointestinal que interviene en la regulación del peso corporal y del equilibrio energético. Sin embargo, su comportamiento después de la cirugía bariátrica y su relación con la resistencia a la insulina todavía está en discusión. Nosotros hemos realizado una evaluación simultánea de la asociación entre los cambios en los niveles de ghrelina y diferentes variables después de tres tipos de cirugía bariátrica.
Pacientes y métodosSe analizaron los niveles de ghrelina en 103 obesos mórbidos, antes y 6 meses después de la cirugía bariátrica (baipás gástrico en Y de Roux [RYGB], derivación biliopancreática de Scopinaro [BPD] y gastrectomía tubular), y en 21 sujetos no obesos.
ResultadosLa ghrelina sérica aumentó tras el RYGB (p<0,05), no se modificó tras la BPD y disminuyó tras gastrectomía tubular (p<0,05). El porcentaje de cambio en los niveles de ghrelina (Δ-ghrelina) se asoció con el tipo de cirugía en un modelo de regresión lineal múltiple (p=0,017). Cuando se realizó el mismo análisis solo con aquellos sujetos en los que se mantiene el fundus gástrico (RYGB y BPD), Δ-ghrelina se asoció negativamente con el Δ-HOMA-IR (p=0,001). En los sujetos obesos mórbidos sometidos a RYGB y BPD, la odss ratio de tener un Δ-HOMA-IR más bajo de las personas con Δ-ghrelina en el cuartil Q1 frente a aquellos con Δ-ghrelina en el cuartil Q4 fue de 8,74 (1,73-44,06) (p=0,009).
ConclusionesLos cambios en los niveles de ghrelina después de la cirugía bariátrica están asociados con la presencia/ausencia del fundus gástrico. Después de la cirugía bariátrica, la disminución de la resistencia a la insulina se asoció con el aumento de los niveles de ghrelina en aquellas técnicas en las que el fundus no está excluido.
Bariatric surgery is the best available therapy to achieve significant and sustainable weight loss with the greatest chances of improving obesity-associated complications, but results vary widely depending on the procedure used.1–3 Biliopancreatic diversion of Scopinaro (BPD) has been shown to be superior to Roux-en-Y gastric bypass (RYGB) with regard to weight loss, although the results were based on a short follow-up period.2,3 However, BPD is not frequently used nowadays because of the complications presented.1 RYGB is considered the standard reference in terms of safety and efficient weight loss. But laparoscopic sleeve gastrectomy (SG) is gaining more interest, not only as a first step in the treatment of high-risk subjects, but also because it is a simpler technique and a faster procedure than RYGB, and has shown excellent results in short-term excess weight loss compared to RYGB.4
It is clearly established that gut hormones change after bariatric surgery,5–8 although inconsistent data have been reported.6 Nonetheless, to what extent this is a beneficial effect or simply a marker of the anatomical changes is still debatable. Ghrelin is a gastrointestinal peptide involved in the regulation of body weight and energy balance and produced mainly by the X/A cells of the gastric fundus. It is involved in appetite and food regulation, increases food intake and promotes fat accumulation.9,10 However, data are contradictory regarding the association between ghrelin and insulin resistance, with negative or no significant associations.5,11–14 The regulation of insulin resistance is complex and involves many pathways that are not fully understood and could probably also be controlled by neuroendocrine hormones, such as ghrelin.14
With this background, the aim of the present study was to undertake an evaluation of the serum changes in ghrelin levels 6 months after three types of bariatric surgery (RYGB, BPD and SG), and their relationship with different anthropometric and biochemical variables.
Materials and methodsSubjectsThe study was performed in 103 morbidly obese subjects before and 6 months after bariatric surgery and in 21 healthy, non-obese subjects. The non-obese subjects underwent laparoscopic surgery for cholelithiasis. Morbidly obese subjects underwent bariatric surgery by either laparoscopic RYGB (n=30) at the Regional University Hospital in Malaga (Spain), open BPD (n=47) or laparoscopic SG (n=26) at Virgen de la Victoria Clinical Hospital in Malaga (Spain). In RYGB and BPD, the fundus is not excluded, while in SG the fundus is excluded. The characteristics of these techniques have been shown in previous studies.5,15 Subjects were excluded if they were receiving insulin or hypoglycemic agents, had cardiovascular disease, arthritis, acute inflammatory disease and infectious disease. All participants gave their informed consent, and the study was reviewed and approved by the Ethics and Research Committee.
Laboratory measurementsBlood samples were collected after a 12-h fast. The serum was separated and frozen at −80°C. Serum biochemical variables were measured in duplicate.16 Total ghrelin was analyzed by enzyme immunoassay kits (DRG Instruments GmbH, Marburg, Germany). The Homeostasis Model Assessment of insulin resistance (HOMA-IR) was calculated. The percent change (Δ) in the variables 6 months after bariatric surgery was calculated as (variable at sixth month−baseline variable)×100/baseline variable.17
Statistical analysisThe statistical analysis was done with SPSS (Version 11.5 for Windows; SPSS, Chicago, IL, USA). Comparison between different groups was made with the one-way ANOVA, and the post hoc analysis was done with Duncan's multiple range test. The differences within the same group, before and after bariatric surgery, were compared with the Student's t-test for paired samples. The Pearson's correlation coefficient was calculated to estimate the linear correlations. Multiple regressions were used to determine the association between serum concentrations of ghrelin and other biomarkers. In the logistic regression model, the dependent variable was to have the Δ-HOMA-IR above or below of 50th percentile. Values were considered to be statistically significant when p≤0.05. The results are given as the mean±SD, and as the mean±SEM in the figures.
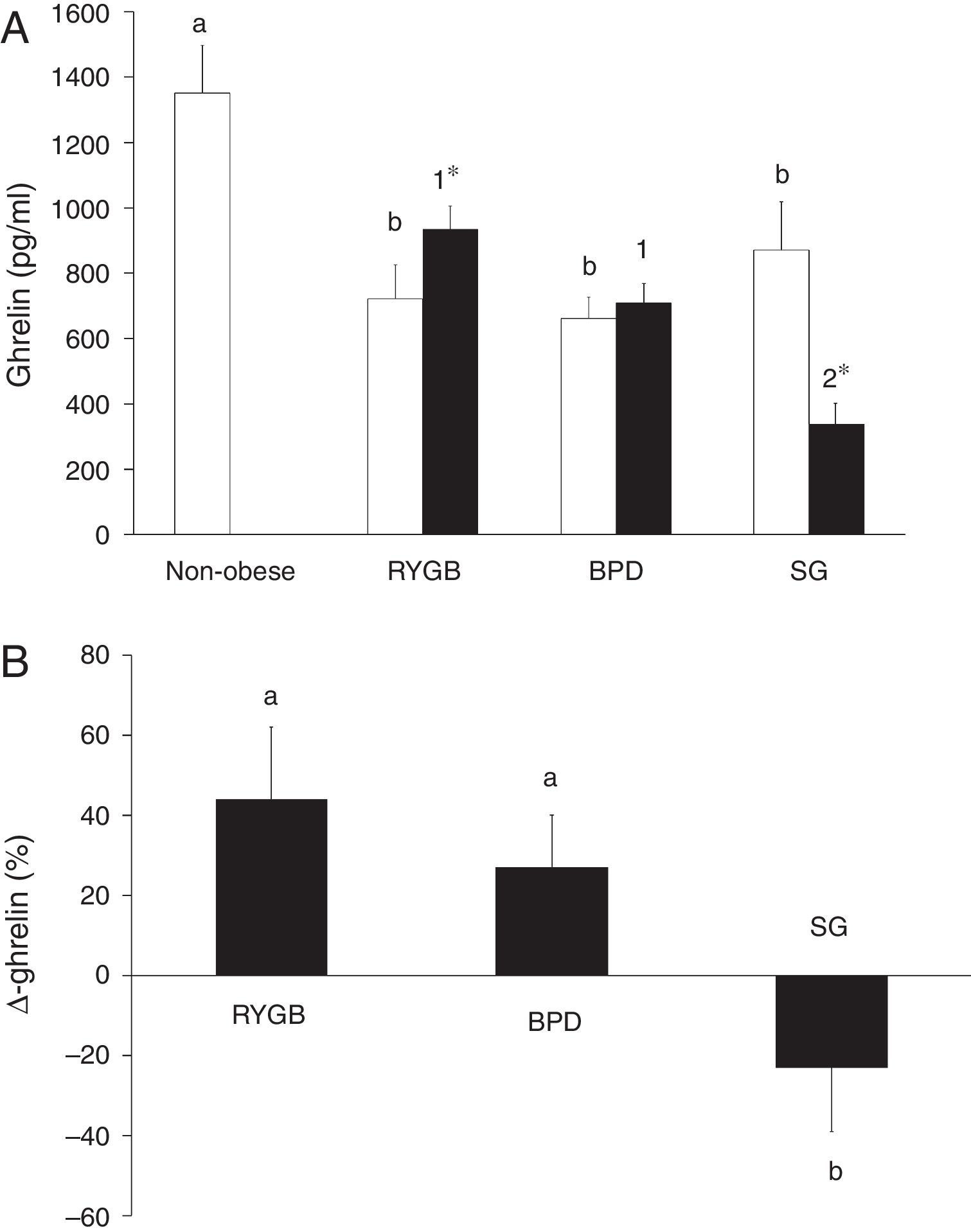
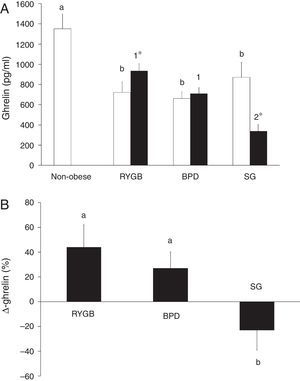
ResultsGhrelin levels are decreased in morbidly obese subjects and are associated with different variables before bariatric surgeryTable 1 summarizes the characteristics of the non-obese and the morbidly obese subjects before and after bariatric surgery. Ghrelin levels in the morbidly obese subjects before surgery were significantly lower than in the non-obese subjects, with no significant differences according to the type of surgery (Fig. 1A).
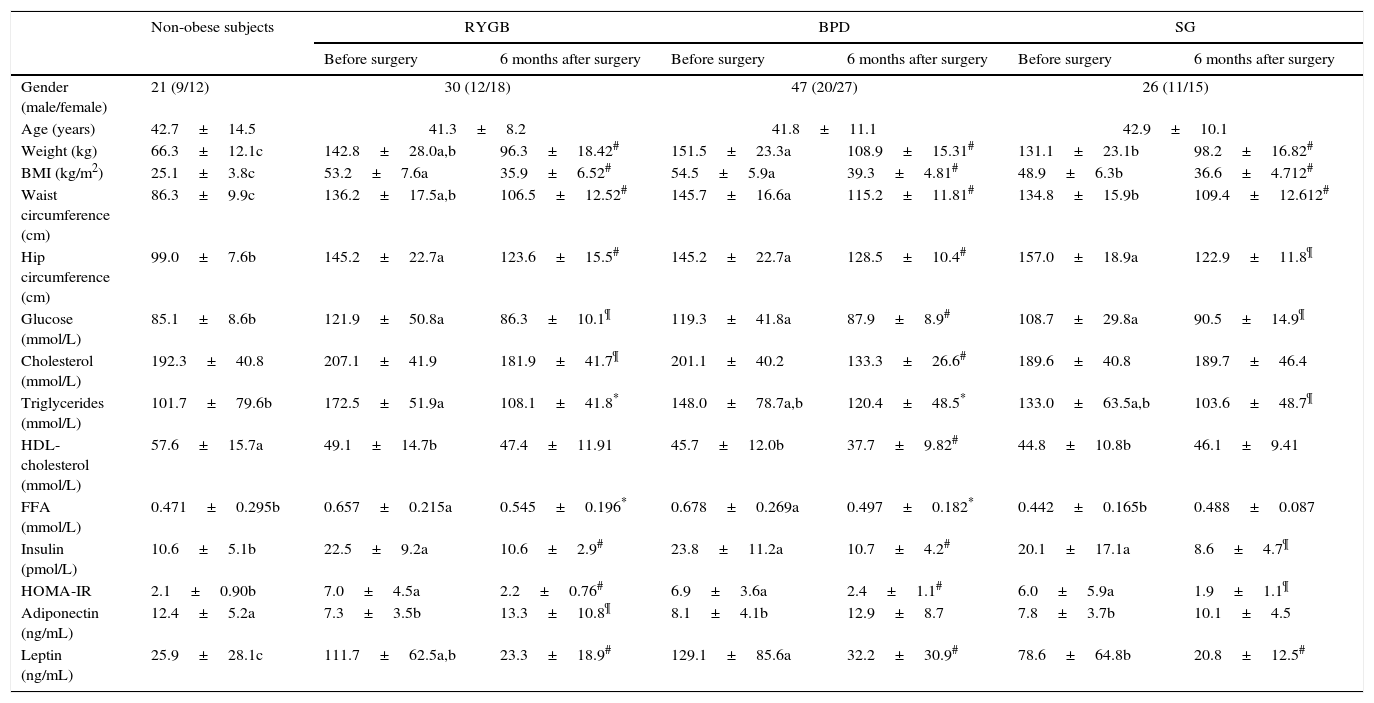
Clinical and biochemical variables in non-obese and morbidly obese subjects before and 6 months after bariatric surgery.
| Non-obese subjects | RYGB | BPD | SG | ||||
|---|---|---|---|---|---|---|---|
| Before surgery | 6 months after surgery | Before surgery | 6 months after surgery | Before surgery | 6 months after surgery | ||
| Gender (male/female) | 21 (9/12) | 30 (12/18) | 47 (20/27) | 26 (11/15) | |||
| Age (years) | 42.7±14.5 | 41.3±8.2 | 41.8±11.1 | 42.9±10.1 | |||
| Weight (kg) | 66.3±12.1c | 142.8±28.0a,b | 96.3±18.42# | 151.5±23.3a | 108.9±15.31# | 131.1±23.1b | 98.2±16.82# |
| BMI (kg/m2) | 25.1±3.8c | 53.2±7.6a | 35.9±6.52# | 54.5±5.9a | 39.3±4.81# | 48.9±6.3b | 36.6±4.712# |
| Waist circumference (cm) | 86.3±9.9c | 136.2±17.5a,b | 106.5±12.52# | 145.7±16.6a | 115.2±11.81# | 134.8±15.9b | 109.4±12.612# |
| Hip circumference (cm) | 99.0±7.6b | 145.2±22.7a | 123.6±15.5# | 145.2±22.7a | 128.5±10.4# | 157.0±18.9a | 122.9±11.8¶ |
| Glucose (mmol/L) | 85.1±8.6b | 121.9±50.8a | 86.3±10.1¶ | 119.3±41.8a | 87.9±8.9# | 108.7±29.8a | 90.5±14.9¶ |
| Cholesterol (mmol/L) | 192.3±40.8 | 207.1±41.9 | 181.9±41.7¶ | 201.1±40.2 | 133.3±26.6# | 189.6±40.8 | 189.7±46.4 |
| Triglycerides (mmol/L) | 101.7±79.6b | 172.5±51.9a | 108.1±41.8* | 148.0±78.7a,b | 120.4±48.5* | 133.0±63.5a,b | 103.6±48.7¶ |
| HDL-cholesterol (mmol/L) | 57.6±15.7a | 49.1±14.7b | 47.4±11.91 | 45.7±12.0b | 37.7±9.82# | 44.8±10.8b | 46.1±9.41 |
| FFA (mmol/L) | 0.471±0.295b | 0.657±0.215a | 0.545±0.196* | 0.678±0.269a | 0.497±0.182* | 0.442±0.165b | 0.488±0.087 |
| Insulin (pmol/L) | 10.6±5.1b | 22.5±9.2a | 10.6±2.9# | 23.8±11.2a | 10.7±4.2# | 20.1±17.1a | 8.6±4.7¶ |
| HOMA-IR | 2.1±0.90b | 7.0±4.5a | 2.2±0.76# | 6.9±3.6a | 2.4±1.1# | 6.0±5.9a | 1.9±1.1¶ |
| Adiponectin (ng/mL) | 12.4±5.2a | 7.3±3.5b | 13.3±10.8¶ | 8.1±4.1b | 12.9±8.7 | 7.8±3.7b | 10.1±4.5 |
| Leptin (ng/mL) | 25.9±28.1c | 111.7±62.5a,b | 23.3±18.9# | 129.1±85.6a | 32.2±30.9# | 78.6±64.8b | 20.8±12.5# |
The results are given as the mean±SD. Different letters indicate significant differences between the means of the baseline variables of morbidly obese subjects before surgery and non-obese subjects (a, b and c) (p<0.05). Different numbers indicate significant differences between the means of the three types of surgery in the variables analyzed 6 months after the bariatric surgery (1 and 2) (p<0.05).
Significant differences between before and after bariatric surgery in each type of bariatric surgery.
(A) Serum ghrelin levels in non-obese and morbidly obese subjects before (□) and after bariatric surgery (■), according to the type of surgery undergone. Different letters indicate significant differences between the means of the baseline ghrelin of morbidly obese subjects before surgery and non-obese subjects (a and b) (p<0.05). Different numbers indicate significant differences between the means of the three types of surgery in ghrelin levels 6 months after bariatric surgery (1 and 2) (p<0.05). Significant differences between before and after bariatric surgery in each type of bariatric surgery (*p<0.05). (B) Percentage change in serum ghrelin levels (Δ-ghrelin) in morbidly obese subjects according to the type of bariatric surgery undergone. Different letters indicate significant differences between the means (a and b) (p<0.05). Roux-en-Y gastric bypass (RYGB), biliopancreatic diversion of Scopinaro (BPD) and sleeve gastrectomy (SG). Data are presented as mean±SEM.
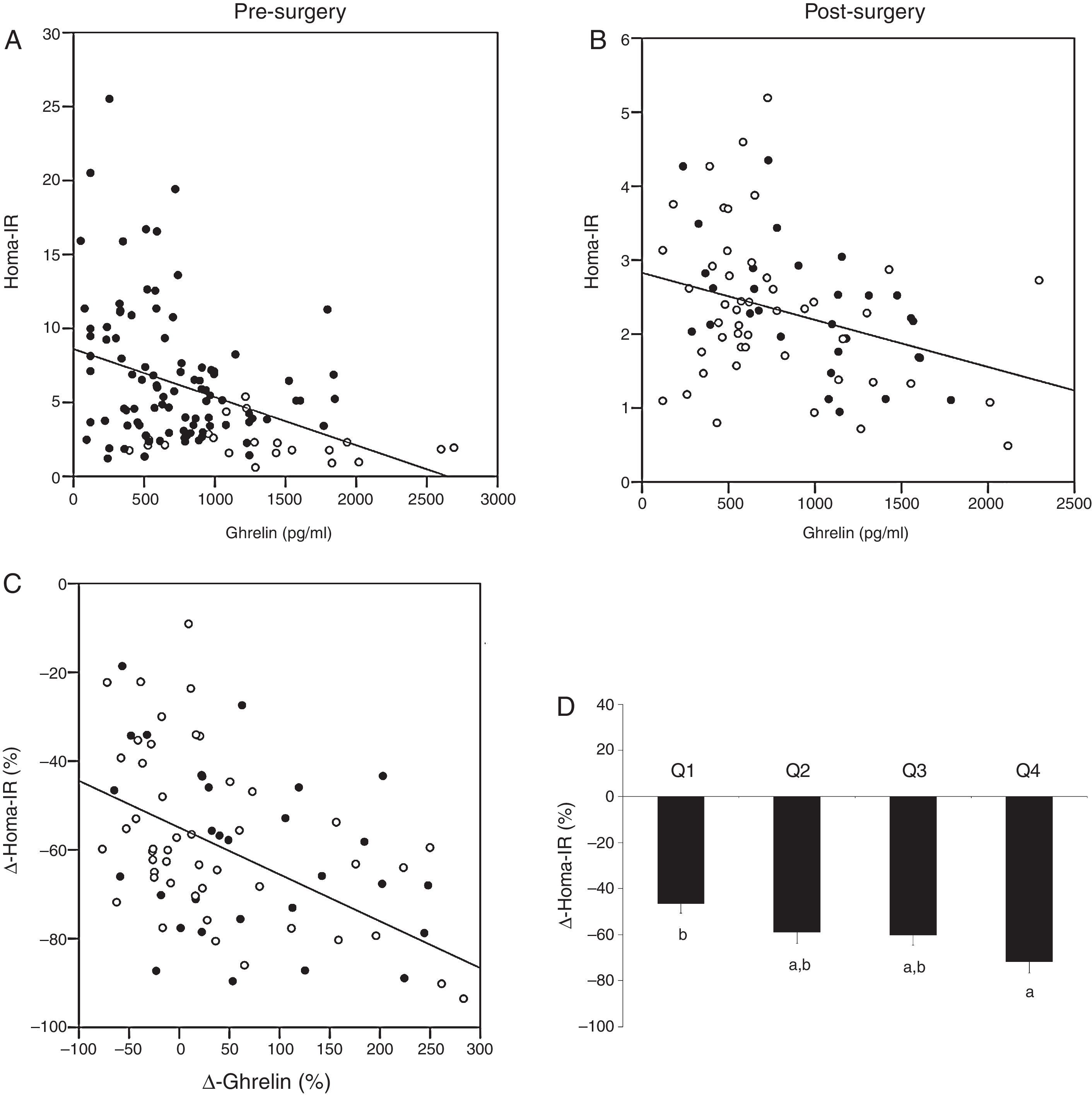
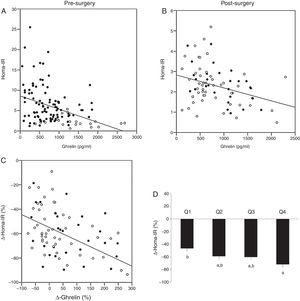
Baseline levels of ghrelin correlated positively with HDL-cholesterol (r=0.194, p=0.036) and adiponectin (r=0.280, p=0.002), and negatively with weight (r=−0.363, p<0.001), BMI (r=−0.350, p<0.001), waist circumference (r=−0.328, p<0.001), hip circumference (r=−0.379, p=0.001), glucose (r=−0.180, p=0.047), insulin (r=−0.341, p<0.001) and HOMA-IR (r=−0.330, p<0.001) (Fig. 2A). The variable associated with ghrelin levels in a multiple linear regression model was HDL-cholesterol (p=0.037) (R2=0.333). This association remained after adjustment for gender, age, BMI, waist and hip circumferences, triglycerides, HOMA-IR, leptin and adiponectin.
Associations between serum ghrelin levels and HOMA-IR. (A) Before bariatric surgery. Non-obese (□) and all morbidly obese subjects before bariatric surgery (■). (B) After bariatric surgery. Roux-en-Y gastric bypass (RYGB) (■) and biliopancreatic diversion of Scopinaro (BPD) (□). (C) Association between the change in serum ghrelin levels (Δ-ghrelin) and the change in HOMA-IR (Δ-HOMA-IR) as a result of bariatric surgery (RYGB (■) and BPD (□)). (D) Changes in the HOMA-IR level (Δ-HOMA-IR) 6 months after those techniques in which the gastric fundus is maintained (RYGB and BPD) according to Δ-ghrelin quartiles (Q1: ≤25th percentile; Q2: >25th and ≤50th percentile; Q3: >50th and ≤75th percentile; Q4: >75th percentile). Data are presented as mean±SEM. Different letters indicate significant differences between the means (p<0.05).
Significant improvement occurred in most of the variables after bariatric surgery, with significant differences depending on the type of intervention (Table 1). Fig. 1 shows that ghrelin levels increased after RYGB. BPD did not modify ghrelin levels and SG decreased ghrelin levels. The Δ-ghrelin was different between SG and the other two types of surgery, with a decrease after SG and an increase after RYGB and BPD (Fig. 1B).
Ghrelin changes are associated with the type of bariatric surgery and insulin resistance levelsWhen we considered all subjects (RYGB, BPD and SG groups) 6 months after bariatric surgery, ghrelin correlated negatively with weight (r=−0.299, p=0.001), BMI (r=−0.276, p=0.003), waist circumference (r=−0.323, p=0.001), hip circumference (r=−0.276, p=0.003), glucose (r=−0.301, p=0.001), triglycerides (r=−0.259, p=0.006), and positively with HDL-cholesterol (r=0.306, p=0.001) and adiponectin (r=0.327, p=0.001). No other significant associations were found (data not shown). The Δ-ghrelin correlated negatively with the Δ-weight (r=−0.213, p=0.035), Δ-BMI (r=−0.213, p=0.035), Δ-cholesterol (r=−0.228, p=0.023), Δ-insulin (r=−0.219, p=0.022), Δ-HOMA-IR (r=−0.283, p=0.004) and Δ-leptin (r=−0.200, p=0.052). No other significant associations were found (data not shown). The variable associated with Δ-ghrelin in a multiple linear regression model was the type of surgery (p=0.017) (R2=0.479). This association remained after adjusting for gender, age, Δ-BMI, Δ-cholesterol, Δ-HDL, Δ-HOMA-IR and Δ-leptin.
As the gastric fundus was not excluded in those morbidly obese subjects who underwent RYGP or BPD, while it was excluded in those who underwent SG, we performed the same statistical analysis only with those morbidly obese subjects who underwent RYGB and BPD. Ghrelin levels correlated negatively with waist circumference (r=−0.278, p=0.018), triglycerides (r=−0.310, p=0.009), insulin (r=−0.248, p=0.037) and HOMA-IR (r=−0.247, p=0.040) (Fig. 2B). The Δ-ghrelin correlated negatively with the Δ-HOMA-IR (r=−0.413, p<0.001) (Fig. 2C). No other significant associations were found (data not shown). The variable associated with the Δ-ghrelin in a multiple linear regression model was the Δ-HOMA-IR (p=0.001) (R2=0.249). This association remained after adjusting for gender, age, Δ-BMI, Δ-waist, Δ-HDL, Δ-triglycerides and type of surgery (RYGP or BPD). No interaction was found between Δ-HOMA-IR and type of surgery (RYGP or BPD) with a univariate general linear model (data not shown). When those morbidly obese subjects underwent RYGB and BPD were categorized according to Δ-ghrelin quartiles (Q1: ≤25th percentile; Q2: >25th and ≤50th percentile; Q3: >50th and ≤75th percentile; Q4:>75th percentile), those in the Q4 quartile of Δ-ghrelin had a significant higher Δ-HOMA-IR than those in Q1 percentile (p=0.004) (Fig. 2D). Using a logistic regression model, the OR of having a lower Δ-HOMA-IR (>−60.3% (50th percentile)), adjusted for age and sex, of the persons with Δ-ghrelin in Q1 quartile (Q1: ≤25th percentile) versus those with Δ-ghrelin in Q4 quartile (Q4: >75th percentile) was 8.74 (95% confidence interval: 1.73–44.06) (p=0.009). No significant differences were found in the other variables studied according to Δ-ghrelin quartiles (data not shown).
No significant correlations were found between ghrelin levels and insulin and HOMA-IR values in those subjects underwent SG (data not shown). Also, no significant correlations were found between Δ-ghrelin and Δ HOMA-IR and Δ-insulin (data not shown).
DiscussionThere is consensus that ghrelin levels are diminished in obesity.5,18 As in other studies, our results show low ghrelin levels in morbidly obese subjects, being its levels negatively associated with BMI, weight and waist and hip circumferences.
The effect of different types of bariatric surgery on ghrelin levels has been analyzed in a large number of studies, with contradictory results.6,7,19 We found an increase in ghrelin levels after RYGB, with no significant changes after BPD, and a slight but significant decrease after SG. Also, in a study performed in subjects who underwent SG, ghrelin levels decreased significantly after 12 months and were maintained for several years after.20 However, further studies failed to observe consistent changes in ghrelin after RYGB or BPD, as has been summarized in several reviews.21,22 Low levels of ghrelin after RYGB have also been reported, in some classic studies,8 other recently published23 or even initially declining that normalizes at 12 months.24 This can be due to various reasons, such as the large variability in the type of studies, inherent differences in the surgical procedures, or diabetes status of the patient population. In our case, the study was carried out at the same time at two different centers and, although the assignment of the morbidly obese subjects to one or other of the techniques was not random, no differences were found between RYGB and BPD in the variables analyzed. Although the three groups of subjects (RYGB, BPD and SG) showed similar levels in the variables related to diabetes status (glucose, insulin and HOMA-IR), the subjects who underwent SG had a slightly better metabolic profile than those who underwent RYGB or BPD. To avoid this bias, the multiple regression models were adjusted for different variables associated with this improved metabolic profile.
Our results show that changes in ghrelin levels 6 months after bariatric surgery depend on the type of surgery performed. Differences in surgical techniques might contribute to the previously inconsistent results found in different studies. One hypothesis to explain this is that surgeries that conserve the contact of nutrients with the fundus do not result in ghrelin decreases, while those that do not conserve the fundus lead to a decrease in ghrelin levels.8 Also, the technique used in the measurement of ghrelin could be involved in the differences found. This hypothesis would agree with the results of our study; the fundus is excluded in SG, and these subjects were those who experienced a decrease in their ghrelin levels. However, in another prospective longitudinal two-year study comparing subjects who underwent one of two surgical procedures, RYGB (which preserves food contact with the fundus) and another procedure that does not preserve food contact with the fundus, there was no significant difference between groups, indicating that the ghrelin increase after bariatric surgery does not depend exclusively on contact with the fundus.25 We cannot exclude that other factors, either unknown or not considered in this study, may be affecting the changes that occur in ghrelin levels after bariatric surgery.
Our results also show that after those techniques that do not exclude the gastric fundus (RYGB and BPD), the changes in ghrelin levels were associated with the improvement of insulin resistance: those morbidly obese subjects with higher ghrelin increase (Δ-ghrelin) after RYGB and BPD are those subjects with higher HOMA-IR decrease (Δ-HOMA-IR). This association was also found when we considered the ghrelin levels before surgery. Different studies show a negative association between insulin resistance and ghrelin.11,26 Also, Pöykkö et al. showed that type 2 diabetes subjects had lower ghrelin levels compared to non-diabetic subjects independently of age, gender and BMI.27 However, in a prospective follow-up study no significant difference was found between the ghrelin levels of subjects who had normal glucose tolerance and those who developed impaired fasting glucose, impaired glucose tolerance or type 2 diabetes.13 However, it is unclear if these low ghrelin levels are a risk factor or a compensatory response.28 Ghrelin inhibits insulin secretion in most animal studies,29 and the secretion of ghrelin was progressively inhibited by increasing concentrations of insulin in newborn rat X/A stomach cells.30 Ghrelin achieves its functions through binding the ghrelin receptor GHS-R in peripheral metabolic organs including the endocrine pancreas, via a Ca2+-mediated pathway.14 However, this effect is eliminated by higher insulin resistance.30 We cannot conclude that ghrelin has an effect on insulin secretion or insulin resistance, or vice versa, but it could be possible. We only demonstrate an inverse association between ghrelin levels and insulin resistance. On the other hand, Vestegard et al. reported increased insulin resistance after acute administration of ghrelin.31 Similarly, administration of ghrelin in subjects with total gastrectomy and truncal vagotomy reduced insulin-mediated glucose disposal rate.32 But some studies did not find any association between ghrelin level and insulin sensitivity.12
In conclusion, the results seem to suggest that changes in ghrelin levels after bariatric surgery are associated with the presence/absence of the gastric fundus. On the other hand, the improvement in the insulin resistance after RYGB and BPD is associated with the increase of ghrelin levels, although other undetermined features must be involved in the differences found in ghrelin levels between after RYGB and BPD. Further studies are needed to relate the changes in ghrelin levels after bariatric surgery to the remission of diabetes.
FundingThis work was supported in part by a grant from the Instituto de Salud Carlos III (Spain) (PI12/00338) and the Consejería de Economia, Innovacion, Ciencia y Empresa de la Junta de Andalucía (Spain) (CTS-8081). This study has been co-funded by FEDER funds.
Authors contributionsLGS, FJT and EGF designed the research; CSF and SGS collected the serum samples and analyzed the samples; MT and SV collected the anthropometric data; LOW and ARC performed the bariatric surgery; LGS and EGF analyzed the data; LGS and EGF wrote the paper; LGS had primary responsibility for the final content. All authors read, contributed and approved the final manuscript.
Conflict of interestThe authors declare that they have no conflict of interest.
CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and CIBER on Diabetes and Metabolic Diseases (CIBERDEM) are projects ISCIII. L. Garrido-Sánchez is supported by a fellowship from the Fondo de Investigación Sanitaria (FIS) “Miguel Servet I” CP 13/00188.