The treatment guidelines for thyroid dysfunction recommend defining reference ranges for thyroid hormones in each area through assessment of local population data considering the iodine nutritional status. The aim of this study was to define the reference ranges of free thyroxine (FT4), TSH, and thyroglobulin levels in a general population from Jaen, an area of southern Spain with an adequate iodine nutritional status, and whether they were associated with urinary iodine levels.
Patients and methodsA cross-sectional study was conducted in 1003 subjects of the general population of the Jaen Health District. Levels of urinary iodine, FT4, TSH, thyroglobulin, and thyroid peroxidase (TPO) antibodies were measured according to age and sex.
ResultsMedian and mean urinary iodine levels were 110.59μg/l and 130.11μg/l respectively. Median TSH level was 1.83μIU/ml (p2.5=0.56μIU/ml, p97.5=4.66μIU/ml). Median FT4 level was 0.84ng/dl (p2.5=0.62ng/dl, p97.5=1.18ng/dl). TPO antibodies were detected in 5.7% of subjects. There was no correlation between urinary iodine levels and FT4, TSH or TPO antibodies. Subjects with positive TPO antibodies had higher TSH levels (3.34μIU/l versus 2.14μIU/ml, p=0.001; odds ratio=2.42).
ConclusionsUrinary iodine levels in Jaen are optimal according to World Health Organization standards. Reference ranges of FT4, TSH, and thyroglobulin do not differ from those reported in the literature and are no associated to urinary iodine levels. The prevalence of positive TPO antibodies was similar to that reported in other Spanish areas.
Las guías de tratamiento de disfunción tiroidea recomiendan definir los intervalos de referencia de las hormonas tiroideas de cada área mediante la evaluación de datos de población local considerando el grado de nutrición yódica de la misma. El objetivo de este estudio fue definir los rangos de referencia de la tiroxina libre (T4L), TSH y tiroglobulina en población general de Jaén, área con un nivel de nutrición yódica adecuado, y si estos estaban afectados por la yoduria.
Material y métodosEstudio descriptivo transversal realizado en 1.003 sujetos de población general en el Distrito Sanitario de Jaén. El yodo urinario, T4L, TSH, tiroglobulina y los anticuerpos antitiroperoxidasa (anti-TPO) fueron analizados en función de la edad y el sexo.
ResultadosLa mediana de yoduria fue 110,59 μg/l y la media 130,11 μg/l. La mediana de TSH fue 1,83μUI/ml (p2,5 = 0,56 μUI/ml, p97,5 = 4,66 μUI/ml). La mediana de T4L fue 0,84ng/dl (p2,5 = 0,62ng/dl, p97,5 = 1,18 ng/dl). El 5,7% de los sujetos tenían anticuerpos anti-TPO positivos. No existía correlación entre los valores de T4L, TSH ni los anticuerpos anti-TPO con los niveles de yoduria. Los sujetos con anticuerpos anti-TPO positivos tenían una TSH más elevada (3,34 μUI/ml frente 2,14 μUI/ml; p = 0,001; odds ratio = 2,42).
ConclusionesEl yodo urinario en Jaén está dentro de los valores recomendados por la Organización Mundial de la Salud. Los rangos de referencia de T4L, TSH y tiroglobulina no son diferentes a lo descrito en la literatura y no difieren según la yoduria. La prevalencia de anticuerpos anti-TPO positivos es semejante a la descrita en otras poblaciones de España.
In the last few decades thyroid gland dysfunction has become a highly relevant issue, with interest focusing particularly on aspects related to iodine nutritional status (IN) in the population and subclinical thyroid dysfunction. In a first study conducted in the province of Jaén (Spain), median ioduria in school children was found to be 90μg/l. The concentrations were subsequently seen to have improved in both pregnant women—with median ioduria 105.89μg/l—and in the general population, with median ioduria 110.59μg/l.1,2 This improved IN is probably the result of so-called silent iodoprophylaxis.
On the other hand, both subclinical hyperthyroidism (HyperSC) and subclinical hypothyroidism (HypoSC) have been implicated in different clinical conditions. Current studies have evidenced the susceptibility of patients with HypoSC to suffer vascular endothelial dysfunction, which constitutes an early sign of arteriosclerosis.3 The explanation for this relationship is based on the fact that patients with HypoSC also suffer alterations referred to the lipid profile, oxidative stress and insulin resistance. Other studies suggest that the increased risk of endothelial dysfunction in these patients is related to TSH receptor hyperstimulation produced by TSH. While not without controversy, particularly in elderly patients, L-thyroxine therapy appears to partially improve the cardiovascular risk described in patients with subclinical thyroid conditions—though further data are needed to conform this.4 Likewise, HyperSC has been implicated in worsened quality of life among the affected patients, with a tendency toward depression and anxiety. It can also produce alterations in cardiac rhythm ranging from sinus tachycardia to arrhythmias such as atrial fibrillation. On the other hand, excessive thyroid hormone (TH) levels have been associated to increased bone remodeling, which in many cases implies the development of severe osteopenia and an increased risk of fractures.5,6 These associations between THs and clinical dysfunction point to the need to perform TH measurements particularly in situations with increased risk, adjusting the reference values to the characteristics inherent to the study population, since many variables are able to influence the hormone standards.
Likewise, IN has a special correlation to TH levels, since the free T4 (FT4) concentrations are clearly affected in very iodine-deficient regions. Iodine nutritional status has changed naturally in our setting. Thus, the main objective of this study was to establish the reference values (RVs) for TSH, FT4 and thyroglobulin in our health care area according to gender and age, using the same measurement method in all cases, and involving a single reference hospital.
Material and methodsA multicenter, cross-sectional descriptive study was conducted in rural and urban health care areas. The study involved individuals of either gender and of any age and race assisted in the Jaén Health District (comprising 11 different primary care centers). The patients were not institutionalized and required care in the primary care setting, with the indication of laboratory tests due to reasons unrelated to possible thyroid disease. Present or past thyroid or endocrine-metabolic disorders were discarded among the included patients, along with any disease capable of altering the thyroid function parameters, and people requiring treatments containing iodine, amiodarone or other drugs capable of altering thyroid function (bexarotene, lithium, interferon, interleukin, phenobarbital, rifampicin). We also excluded pregnant women and individuals subjected to any diagnostic studies involving the use of iodine contrast media in the previous 12 months.
The sample size was calculated proportional to the number of inhabitants covered by each of the selected primary care centers. Based on previous studies, and in order to ensure a precision of 1% in estimating a proportion by means of a normal asymptotic two-tailed 95% confidence interval with correction for finite populations, taking the expected proportion to be 4%, and considering that the Jaén Health district has a recruitment population of 202,933 inhabitants (99,991 males and 102,942 females), we found that 1465 experimental units would have to be included in the study.
Sampling was stratified according to age and gender. Sampling was performed on a consecutive basis, selecting the patients from the centers until the required sample size was obtained. All the participants signed the corresponding informed consent document, and the study was approved by the Research Ethics Committee of Complejo Hospitalario de Jaén.
VariablesThe study variables were gender, defined as a dichotomic variable (male or female); age classified as a continuous variable and as a dichotomic variable with four categories: <15 years of age, 16–40 years of age, 41–65 years of age, and >65 years of age; and race.
Laboratory methodsThe blood samples were collected by vein puncture under fasting conditions and were refrigerated at 4°C until transfer to the laboratory of Complejo Hospitalario de Jaén for analysis. Chemiluminescence immunoassay (Beckman Access) was used to determine TSH (reference range 0.63–4.19μIU/ml), FT4 (reference range 0.8–1.33ng/dl), thyroglobulin (reference range 0–43ng/ml), anti-thyroid peroxidase antibodies (anti-TPO Ab) and TSH receptor antibodies (anti-TSHR Ab). Anti-TPO antibody positivity was considered for >65IU/ml, while anti-TSHR antibody positivity was defined as >2IU/ml.
Ioduria was determined using the colorimetric technique of Benotti and Benotti.7 The urine samples consisted of first morning urine and were frozen at −20°C until shipment to the laboratory of the Biomedical Research Institute of Malaga for processing.
Statistical analysisThe variables were entered in a validated software support for statistical analysis. In defining the normal range for FT4, TSH and thyroglobulin, we used percentile 2.5 (p2.5) as the lower limit of normal and percentile 97.5 (p97.5) as the upper limit of normal—excluding from the statistical analysis those subjects with positive autoimmunity and/or very high ioduria concentrations (>1000μg/l), or with extreme FT4 and/or TSH values. The Kolmogorov–Smirnov test applied to THs, TSH and thyroglobulin proved significant (p<0.05)—thus demonstrating that the hormone values did not follow a normal distribution. Nonparametric techniques were used to compare the variables TSH, FT4 and thyroglobulin: the Mann–Whitney U-test for establishing comparisons according to gender, and the Kruskal–Wallis test for establishing comparisons according to age. A descriptive analysis was made of the main parameters, with calculation of the minimum and maximum values, median and interquartile range (IQR) in the case of quantitative variables.
The association between FT4, TSH and ioduria and the correlation of these variables with gender, age and the presence of anti-TPO Ab was explored using the Spearman correlation test and liner regression analysis. Multiple regression models were designed to calculate the risk of elevated TSH in the presence of positive anti-TPO Ab and of low TSH in the presence of positive anti-TSHR Ab. Statistical significance was considered for p<0.05.
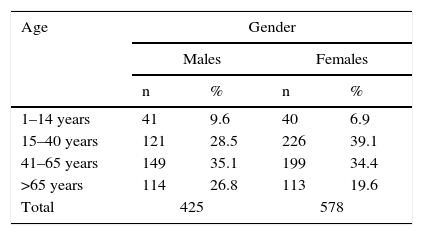
ResultsSample descriptionA total of 1230 subjects were initially recruited. Of these, 227 were excluded due to the impossibility of recording the basic variables, the existence of exclusion criteria, or missing data in the case histories and which a priori were not available to us. The final study sample therefore consisted of 1003 subjects: 425 males and 578 females with a mean age of 45.64 years (range, 1–94 years). The age and gender distribution is shown in Table 1.
A total of 97.5% of the participants were Caucasians. Eighteen were of Latin American origin, three were Chinese, and one came from Morocco.
Laboratory test variablesThe median ioduria value of the sample was 110.59μg/l (p25=79.90μg/l, p50=110.59μg/l, p75=161.76μg/l, p97.5=342.84μg/l). The median TSH value of the sample was 1.83μIU/ml (p2.5=0.56μIU/ml, p25=1.34μIU/ml, p50=1.83μIU/ml, p75=2.48μIU/ml, p97.5=4.66μIU/ml).
There were no significant differences in TSH concentration according to gender (p=0.081).
With regard to age, those subjects under 15 year of age showed significantly higher TSH levels than the rest of the groups, with a median value of 2.13μIU/ml versus a median of 1.87μIU/ml between 15 and 40 years of age (p=0.002); 1.76μIU/ml between 41 and 65 years of age (p=0.001); and 1.83μIU/ml in those over 65 years of age (p=0.007). On stratifying the study sample according to gender and age ranges, significant differences were observed in the group of males under 15 years of age with respect to the rest, with values of 2.21μIU/ml versus 1.85μIU/ml; 1.72μIU/ml (p=0.004); and 1.80μIU/ml (p=0.006), respectively. There were no differences according to age in the case of the females (Table 2).
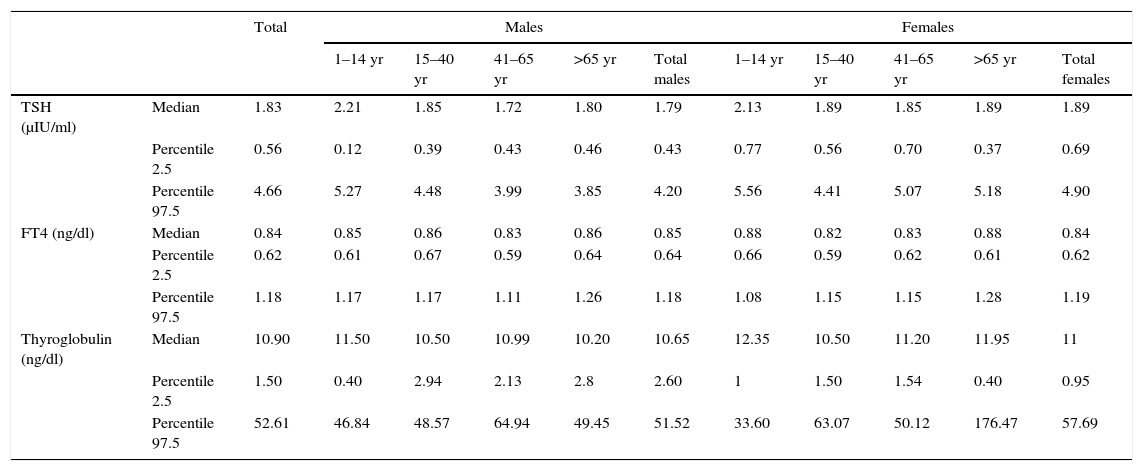
Median and percentiles 2.5–97.5 corresponding to TSH, FT4 and thyroglobulin in the population according to age and gender.
| Total | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–14 yr | 15–40 yr | 41–65 yr | >65 yr | Total males | 1–14 yr | 15–40 yr | 41–65 yr | >65 yr | Total females | |||
| TSH (μIU/ml) | Median | 1.83 | 2.21 | 1.85 | 1.72 | 1.80 | 1.79 | 2.13 | 1.89 | 1.85 | 1.89 | 1.89 |
| Percentile 2.5 | 0.56 | 0.12 | 0.39 | 0.43 | 0.46 | 0.43 | 0.77 | 0.56 | 0.70 | 0.37 | 0.69 | |
| Percentile 97.5 | 4.66 | 5.27 | 4.48 | 3.99 | 3.85 | 4.20 | 5.56 | 4.41 | 5.07 | 5.18 | 4.90 | |
| FT4 (ng/dl) | Median | 0.84 | 0.85 | 0.86 | 0.83 | 0.86 | 0.85 | 0.88 | 0.82 | 0.83 | 0.88 | 0.84 |
| Percentile 2.5 | 0.62 | 0.61 | 0.67 | 0.59 | 0.64 | 0.64 | 0.66 | 0.59 | 0.62 | 0.61 | 0.62 | |
| Percentile 97.5 | 1.18 | 1.17 | 1.17 | 1.11 | 1.26 | 1.18 | 1.08 | 1.15 | 1.15 | 1.28 | 1.19 | |
| Thyroglobulin (ng/dl) | Median | 10.90 | 11.50 | 10.50 | 10.99 | 10.20 | 10.65 | 12.35 | 10.50 | 11.20 | 11.95 | 11 |
| Percentile 2.5 | 1.50 | 0.40 | 2.94 | 2.13 | 2.8 | 2.60 | 1 | 1.50 | 1.54 | 0.40 | 0.95 | |
| Percentile 97.5 | 52.61 | 46.84 | 48.57 | 64.94 | 49.45 | 51.52 | 33.60 | 63.07 | 50.12 | 176.47 | 57.69 | |
yr: years.
No statistically significant correlation was found between ioduria and the TSH concentrations, though TSH tended to be lower in the presence of higher ioduria values.
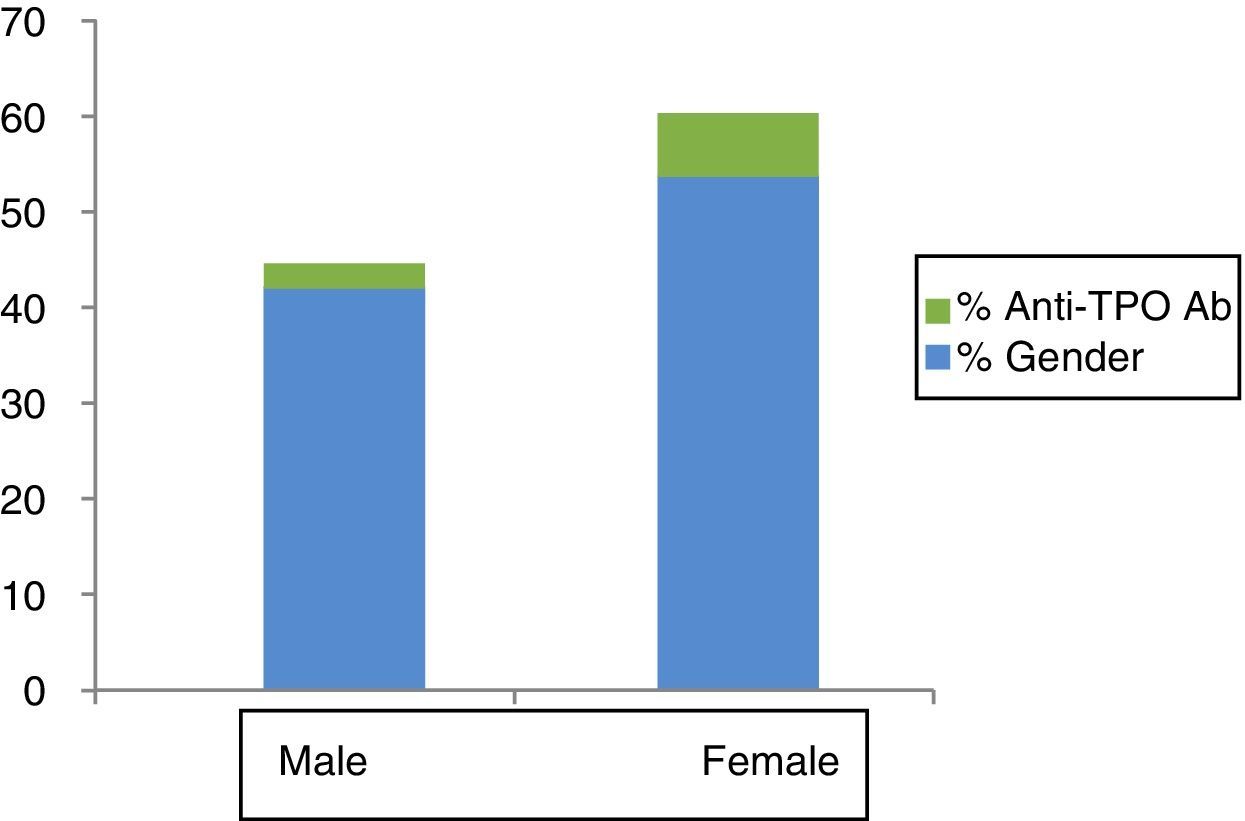
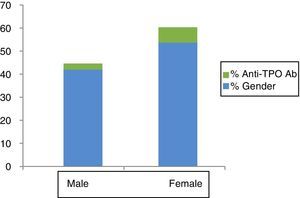
Anti-TPO positivity was recorded in 5.7% of the subjects—the percentage being significantly greater in females than in males (7.9% versus 2.8%; p=0.001) (Fig. 1).
There were no differences according to gender or age ranges for the subjects with positive anti-TSHR Ab, the prevalence in the global sample being 5.2%. The subjects with positive anti-TPO Ab showed significantly higher mean TSH values than those with negative anti-TPO Ab (3.34μIU/ml versus 2.14μIU/ml; p=0.001; odds ratio [OR] 2.42).
On the other hand, on considering >4.66μIU/ml as the upper limit of normal for TSH, the percentage of individuals with HypoSC in the global sample was 5.1%. Only 2.3% of the participants had TSH values below the lower limit of normal in relation to the reference value obtained (0.56μIU/ml).
The median FT4 concentration was 0.84ng/dl (p2.5=0.62ng/dl, p25=0.76ng/dl, p50=0.84ng/dl, p75=0.95ng/dl, p97.5=1.18ng/dl). There were statistically significant gender differences in FT4 concentration, with a higher median value in males (0.87ng/dl versus 0.84ng/dl in females; p=0.003). In relation to age ranges, statistically significant differences were observed between the 1 and 14 years of age group versus the subjects between 15 and 40 years of age and between 41 and 65 years of age, with a median of 0.87ng/dl in the youngest group versus 0.84ng/dl (p=0.022) in the second group and 0.83ng/dl (p=0.006) in the third group. The analysis of mean thyroxine concentration according to gender and age ranges revealed statistically significant differences between the females aged 1–14 years of age and those over 65 years of age versus the 15–40 and 41–65 years of age groups (0.88ng/dl in the extreme groups versus 0.82ng/dl and 0.83ng/dl in the intermediate groups) p<0.005 (Table 2). No correlation was found between the FT4 values and ioduria, or between FT4 and the presence of thyroid gland autoimmunity.
The median thyroglobulin concentration was 10.90ng/dl (standard deviation [SD] 24.26ng/dl). There were no significant thyroglobulin differences between genders or the different age ranges (Table 2). No correlation was found between thyroglobulin concentration and ioduria, though the former tended to be lower in the presence of higher ioduria values.
DiscussionThere has been an improvement in iodine nutritional status in our population over the last decade—median ioduria increasing among school children from 90μg/l to a current median of 160μg/l.1,2 In the general population the concentration is 110μg/l, which is consistent with the recommendations of the World Health Organization.8 In this context, the objective of the present study can be regarded as clearly justified on intuiting that the variations in TH levels will not be affected by iodine deficiency.
On the other hand, it is increasingly common to request thyroid function tests. This implies that the diagnosis of subclinical thyroid gland dysfunction has become very prevalent, with HypoSC rates of 4–8.5% and slightly lower rates for HyperSC—the latter increasing in elderly individuals.9 In many cases such a diagnosis results in “overtreatment”, affording fewer benefits and with possible adverse effects.
The potential negative effects of THs upon the cardiovascular system and bone metabolism, and in terms of mortality and psychological disorders, makes it a priority issue to corroborate the reliability of the HT determinations and results in order to ensure a correct diagnosis of thyroid gland dysfunction.4,10,11 This situation leads us to establish more precise and specific reference ranges for each laboratory method, as recommended by the American Thyroid Association (ATA) in relation to pregnant women.12 The principal objective of this study was to determine the reference ranges for THs, TSH and thyroglobulin in the general population according to age and gender. In this regard, children and women of childbearing potential constitute priority population groups, in view of the potential damage which thyroid gland dysfunction may cause in terms of growth and maturation among the former, and in intellectual development of the offspring and obstetric problems during pregnancy.13,14
The interpretation of laboratory test results is normally based on reference ranges determined by the manufacturer of the laboratory kit employed. As a result, the values obtained in a given individual may be normal or abnormal according to whether they fall within or outside the reference range, respectively.15 Thyroid stimulating hormone is the most sensitive marker for diagnosing thyroid disorders, and is moreover inversely related to FT4 concentration.16,17 However, the laboratory of our reference center proposes an upper limit of normal for FT4 of 1.33ng/dl, which differs to a degree from the value of 1.18ng/dl recorded in our study—this concentration being similar to that contemplated in the documentation of the laboratory kit used to determine the parameter. This discrepancy between the result obtained in a large general population sample and the specification used by the laboratory to indicate normality points to the need to recertify the reference ranges we use in routine clinical practice. This situation is not seen in the case of TSH, where the results obtained are consistent with the values proposed by the reference laboratory.
With regard to the method for determining THs and TSH, the standard used by many laboratories can give rise to falsely elevated TSH levels, due to the presence of heterophilic antibodies targeted to thyroid antigens. This aspect is important in the detection of hyperthyroidism, since patients can present non-suppressed TSH, which complicates the diagnosis, even in the presence of symptoms of thyrotoxicosis.18 Nevertheless, simple immunoassay determination of TSH affords the most sensitive indicator for detecting abnormal thyroid function. However, due consideration is always required of the possible existence of variability between and within individuals, which is not only dependent upon ethnicity or age but also on the presence of other factors that can alter the results obtained.19 This fact makes it difficult to establish specific reference ranges for each population, and causes us to continuously question whether the measurements made are truly adequate. Likewise, differences between laboratories can be found, despite the use of one same method, when the manufacturer of the method differs. This is referred to as analytical sensitivity, and explains the distinct results obtained between different reference laboratories despite the use of one same analytical technique.18
In our study the reliability of the results is fundamented upon the fact that IN of the population was adequate according to the criteria of the World Health Organization (mean 130.11μg/l); the population was homogeneous; and those subjects with positive autoimmunity or iodine overload were excluded from the statistical analysis. In any case, the results referred to the TSH levels were similar to those found in other publications and for the same age ranges—though in some studies the ethnic composition of the patients differed.20,21 In most published studies the TSH values are seen to increase with age, and are moreover higher in females than in males.22 This was not observed in our study, however, since the TSH levels among patients over 65 years of age were seen to be similar to those in the rest of the age groups. Although a negative correlation was found between TSH and ioduria, statistical significance was not reached. Furthermore, in contrast to a recent study in populations subjected to active iodoprophylaxis, we did not record an increase in TSH among those individuals with elevated ioduria.23,24
In women of childbearing potential (20–40 years of age), the TSH values were found to be similar to those recorded in males, with no differences versus the data published by other authors. This observation questions the correct cut-off point for the diagnosis of primary hypothyroidism in pregnant women or women of childbearing potential, since the limit specified by the ATA12 (2.5μIU/ml) for the first trimester or for the diagnosis of HypoSC might be too strict, as most studies report a TSH value in percentile 97.5 in women of this age above that value.25,26 A previous study by our group established the reference ranges for TSH corresponding to each trimester of pregnancy. In this regard, the upper limit of normal in the first trimester was found to be 4.18μIU/ml.2 Similar studies in Spain report greater agreement with our reference ranges than with those proposed by the ATA.12
In the case of children, our results corresponding to THs are consistent with the reference ranges of our laboratory and are similar to the values described by other authors in this same age interval.20,21,27 With regard to the FT4 values, many studies describe a decrease with advancing age. This appears to be conditioned by thyroid gland autoregulation, implying a lesser need for available free thyroid hormone in elderly individuals. In our study there were significant differences in the FT4 values in girls between 1 and 14 years of age and women between 15 and 65 years of age—the concentrations being higher among the former. We likewise observed no correlation between FT4 and ioduria. It should be mentioned that the values calculated for the 1–15 years age interval might not clearly constitute reference values, since the difficulty of securing an adequate sample size may have caused the study sample to not be fully representative.
The prevalence of HypoSC was similar to that described in other national and international publications. In turn, less than 1% of the subjects had HyperSC, defined as TSH below the lower limit of normal for our reference range.28,29
With regard to hypothyroxinemia—a condition that is currently regarded as important particularly in relation to neuromotor development of the offspring of women with this problem30—the prevalence was found to be 2.3%. Only three of our subjects presented biochemical hyperthyroidism.
In relation to the strengths of our study, it is the first to be carried out in Spain in a population covering the entire age range, in a concrete region with adequate IN, and using the analytical method of the reference laboratory. This made it possible to reliably define the reference ranges for this population according to gender and age groups—thereby fulfilling the primary objective of the study. Likewise, the ethnic homogeneity of the sample; the fact that the laboratory tests were performed by the same laboratory; and the conduction of follow-up by a single investigator caused the reliability of the results to be even greater. The study also has some weaknesses, however. Firstly, screening bias was introduced by the way in which the healthy individuals were recruited, since these subjects were not selected on a random basis but were included from among those people seen in primary care. As a result, they represent “pathological” subjects to some extent—though we attempted to minimize the risk of including individuals that might influence the laboratory test results, with the definition of precise exclusion criteria.
In conclusion, we have established the reference ranges for TSH and FT4 corresponding to each gender and age group in a population with adequate iodine levels, adopting p2.5 as the lower limit of normal and p97.5 as the upper limit of normal (Table 2). It also can be concluded that in this case the HT, TSH and thyroglobulin values are not influenced by ioduria.
Authors’ contributionPablo Olmedo-Carrillo: Principal Investigator. Field work.
Piedad Santiago-Fernández: Study design and guidance.
Eduardo García-Fuentes: Determination of ioduria.
Tomás Ureña-Fernández: Coordinator in the primary care centers.
Carmen Gutiérrez-Alcántara: Preparation and discussion of the article.
Carolina Sánchez-Malo: Preparation and discussion of the article.
Manuela Gassó-Campos: Preparation of the analytical techniques for the determination of thyroid hormones and antithyroid antibodies.
María José Martínez-Ramírez: Study design. Preparation and discussion of the article.
Conflict of interestThe authors state that they have no conflicts of interest.
Thanks are due to Dr. Piedad Santiago, Jaén Health District, Complejo Hospitalario de Jaén.
Please cite this article as: Olmedo Carrillo P, Santiago Fernández P, García Fuentes E, Ureña Fernández T, Gutiérrez Alcántara C, Sánchez-Malo C, et al. Definición de los rangos de referencia de T4 libre, TSH y tiroglobulina en sujetos sanos del Distrito Sanitario de Jaén. Endocrinol Diabetes Nutr. 2017;64:417–423.