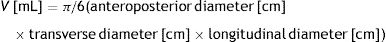Several retrospective and cross-sectional studies have revealed a higher prevalence of autoimmune thyroid diseases (AITD) with a predominance of autoimmune hypothyroidism in prolactinoma patients compared to the general population. To date, we have no data on the clinical course of AITD in these patients. The aim of this prospective study was to assess the clinical course of AITD in female patients with prolactinomas compared to an age- and thyroid-risk factors-matched control group.
Materials and methodsThe study population consisted of 144 females (71 patients/73 controls) who underwent approximately a 6-year follow-up. Physical examination, thyroid ultrasound and laboratory testing (measurement of antibodies to thyroglobulin, thyroid peroxidase, TSH-receptor; serum TSH and FT4 levels) were performed twice – at the baseline and at the follow-up visits.
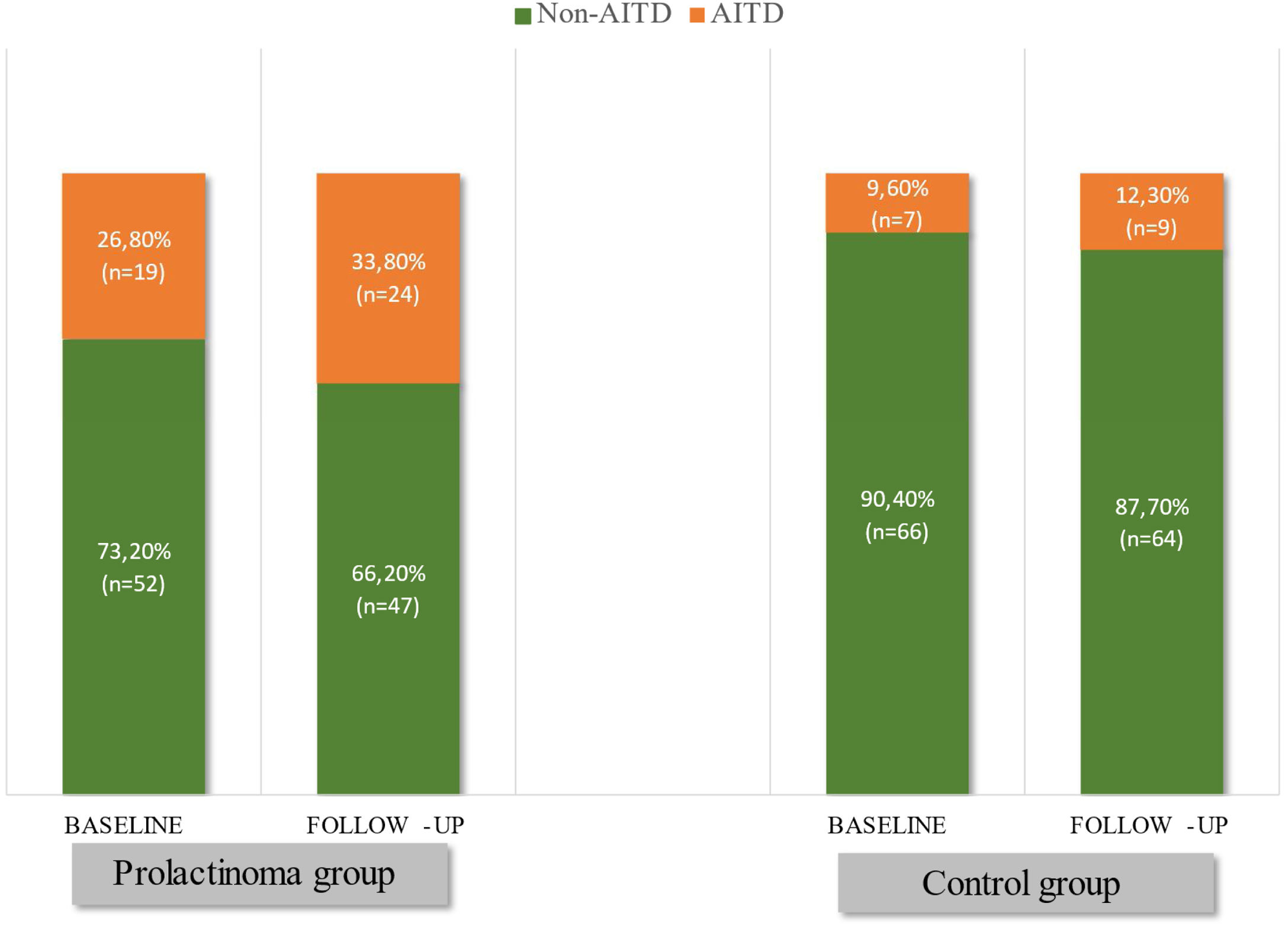
ResultsAITD were diagnosed in 26.8% (n=19) of the patients and 9.6% (n=7) of the controls (p=0.007) at baseline visit. At the end of the follow-up (FU), these percentages increased to 33.8% (n=24) among the patients versus 12.3% (n=9) in the control group (p=0.002). Hypothyroidism was significantly more frequent in prolactinoma patients than in controls at the end of the study (19.7% vs. 4.1%; p=0.003). Two prolactinoma patients had hyperthyroidism at the baseline visit and restored euthyroid state with negative TSH-receptor antibodies during the follow-up. We did not observe hyperthyroidism in the control group. Among the hypothyroid subsets, the average daily levothyroxine dose at FU visit varied from 25 to 200mcg in the prolactinoma group compared to 25 to 50mcg in the control group.
ConclusionsFemale patients with prolactinomas seem to be prone to autoimmune hypothyroidism. As a pathogenetic mechanism, we could suggest the selective immunomodulatory action of PRL predominantly on cell autoimmunity, complement activation and antibody-dependent cytotoxicity, resulting in earlier and more rapid progression of Hashimoto's thyroiditis towards hypothyroid state in genetically predisposed individuals.
Varios estudios retrospectivos y transversales revelaron una mayor prevalencia de enfermedades tiroideas autoinmunes (ETAI) con predominio de hipotiroidismo autoinmune en pacientes con prolactinoma en comparación con la población general. Hasta la fecha, aún no disponemos de datos sobre la evolución clínica de la ETAI en estos pacientes. El objetivo de este estudio prospectivo fue evaluar el curso clínico de las ETAI en mujeres con prolactinomas en comparación con un grupo de control emparejado por edad y factores de riesgo de ETAI.
Materiales y métodosLa población de estudio consistió en 144 mujeres (71 pacientes/73 controles), que se sometieron a un seguimiento de aproximadamente 6 años. El examen físico, la ecografía tiroidea y las pruebas de laboratorio (medición de anticuerpos contra la tiroglobulina, la peroxidasa tiroidea, el receptor de TSH, los niveles séricos de TSH y FT4) se realizaron dos veces: al inicio y en las visitas de seguimiento.
ResultadosETAI se diagnosticó en el 26,8% (n=19) de los pacientes y el 9,6% (n=7) de los controles (p=0,007) en la visita inicial. Al final del seguimiento (S) estos porcentajes aumentaron hasta el 33,8% (n=24) entre los pacientes frente al 12,3% (n=9) en el grupo control (p=0,002). El hipotiroidismo fue significativamente más frecuente en pacientes con prolactinoma que en los controles al final del estudio (19,7% frente a 4,1%; p=0,003). Dos pacientes con prolactinoma tenían hipertiroidismo en la visita inicial y recuperaron el estado eutiroideo con anticuerpos contra el receptor de TSH negativos durante el seguimiento. No observamos hipertiroidismo en el grupo control. Entre los subgrupos de hipotiroidismo, la dosis diaria promedio de levotiroxina en la visita de S varió de 25 a 200 mcg en el grupo de prolactinoma en comparación con 25 a 50 mcg en el grupo de control.
ConclusionesLas mujeres con prolactinomas parecen ser propensas al hipotiroidismo autoinmune. Como mecanismo patogénico, podríamos sugerir la acción inmunomoduladora selectiva de la PRL predominantemente sobre la autoinmunidad celular, la activación del complemento y la citotoxicidad dependiente de anticuerpos, lo que daría como resultado una progresión más temprana y rápida de la tiroiditis de Hashimoto hacia el estado hipotiroideo en individuos genéticamente predispuestos.
The role of prolactin as a powerful immunomodulator, influencing both humoral and cell-mediated immune response, has been demonstrated in numerous experimental in vitro and in vivo studies. Hyperprolactinemia has been documented in the active phase of different systemic and organ-specific autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, Sjogren's syndrome, scleroderma, psoriasis and multiple sclerosis.1–5 A direct correlation between prolactin levels and disease activity has been established in some of these diseases.5–7 Furthermore, some investigators report a significant correlation between the degree of hyperprolactinemia and the severity of some systemic complications in SLE, such as anaemic syndrome, serositis, glomerulonephritis and central nervous system manifestations.8,9 These results suggest a possible role of prolactin in the pathogenesis, clinical course and prognosis in these patients. In light of this data, there is surprisingly little research on the immune status of patients with hyperprolactinemia. Undoubtedly, autoimmune thyroid diseases (AITD) are the most common autoimmune pathology. The first reports of an increased incidence of anti-thyroid antibodies in hyperprolactinaemic subjects appear in the 1990s.10,11 The first clinical studies that found an increased frequency of AITD in patients with hyperprolactinemia were published more than two decades later.12,13 Our retrospective studies in Bulgarian and Belgian prolactinoma patients contributed further to this field of knowledge, as we not only established the expected higher incidence of AITD compared to the general population, but also demonstrated a higher prevalence of subclinical hypothyroidism.14,15 These data were confirmed in our cross-sectional case-control study that compared prolactinoma female patients to age-matched and thyroid-associated risk factors-matched control group of women.16
The main aim of this study was to follow up the clinical course of autoimmune thyroid diseases (AITD) in female patients diagnosed with prolactinoma, compared to age and risk factor-matched control group of women.
Study design and definitionsWe conducted a prospective comparative study in 144 female subjects (71 prolactinoma patients and 73 controls), approved by the Institutional Ethics Committee. All study procedures were performed after a specific informed consent was signed by each participant and a researcher in two identical copies (one for the participant and one for the patient's file). The diagnosis of prolactinoma, based on current guidelines, was established in cases with typical hyperprolactinemia-related clinical symptoms; presence of pituitary adenoma on imaging (CT or MRI) and corresponding to tumour size elevated prolactin levels.17,18The diagnosis of autoimmune thyroid disease (AITD) was based on the presence of at least two of the following criteria: (1) typical thyroid ultrasound characteristics (hypoechoic non-homogenous structure); (2) positive anti-thyroid auto-antibodies; (3) impaired thyroid function. Subclinical hyperthyroidism was defined as suppressed serum TSH below 0.1mIU/l accompanied by normal FT4 and FT3 levels. Overt hyperthyroidism – laboratory constellation of a suppressed TSH below 0.1 0.1 mIU/l and increased FT4 and/or FT3 levels above the upper reference range. Subclinical primary hypothyroidism – all cases of normal serum FT4 levels in combination with increased above the upper reference range TSH levels (above 4.0mIU/l). Overt primary hypothyroidism was defined as permanently reduced thyroid function with TSH levels above the upper reference range (typically above 10.0mIU/l) and decreased FT4 levels below the lower reference range with or without clinical symptoms. We enrolled only female subjects because of a significantly higher incidence of both prolactinomas and AITD in women. Exclusion criteria: 1. Functional hyperprolactinemia; 2. Co-secretion of other adenohypophyseal hormones; 3. Negative pituitary imaging (CT, MRI); 4. Known thyroid disease prior to study entry (AITD, nodular goitre, etc.); 5. Past history of thyroid disease (acute/subacute/postpartum thyroiditis, etc.); 6. Thyroid surgery; 7. Radiotherapy of the head/neck region; 8. Radio-iodine therapy; 9. Intake of immunomodulators, iodine-containing drugs, lithium and other medications with a possible impact on thyroid function; 10. Pregnancy at study entry;
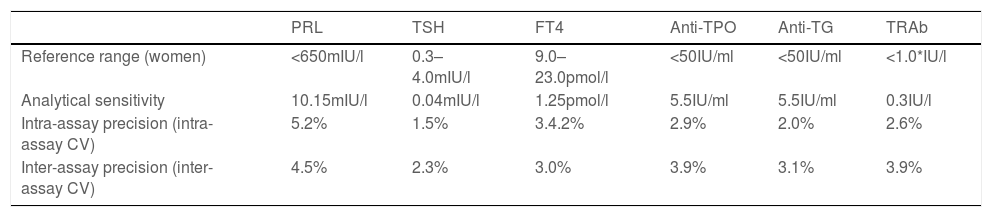
Materials and methodsA semi-structured interview was used in history-taking in all patients and healthy controls enrolled in the study. Special attention was paid to past history (information about past and concomitant thyroid disease, thyroid surgery, intake of drugs with a potential impact on thyroid function, radiotherapy of the neck region etc.), family history, noxious agents (substances with potential impact on thyroid function, smoking, alcohol, ionising radiation etc.), gynaecological history during follow-up (pregnancies, deliveries). A physical examination focusing on the thyroid gland was performed in all study subjects. The hormonal and immunological tests of the study subjects were performed in our hospital's certified centralised laboratory. Blood samples were collected by venipuncture in a test tube with a clot activator, at room temperature, at approximately 8 o’clock in the morning after a 30-min rest period in the sitting position in order to avoid stress-induced increase in prolactin levels. The serum was separated by centrifugation at room temperature. The samples were stored frozen at −80°C until the hormonal analysis was performed. The hormonal analysis consisted of immunoradiometric determination of serum PRL levels (IRMA); TSH (IRMA) and radioimmunological measurement of FT4 levels (RIA). FT3 levels were measured only in patients with low TSH and normal FT4 levels in order to identify the subjects with overt/subclinical hyperthyroidism and those with secondary hypothyroidism after transsphenoidal surgery. Because of their low number (n=5), the FT3 values were excluded from the statistical analysis and were not presented in the tables. The radioimmunological analysis (RIA) included the determination of serum concentrations of anti-thyroid peroxidase antibodies (anti-TPO-Ab); anti-thyroglobulin antibodies (anti-TG-Ab); anti-TSH-receptor antibodies (TSH-R-Ab). The main characteristics of the commercial kits we used are presented in Table 1.
Main characteristics of the commercial kits used for hormonal analysis and immunological diagnostics.
| PRL | TSH | FT4 | Anti-TPO | Anti-TG | TRAb | |
|---|---|---|---|---|---|---|
| Reference range (women) | <650mIU/l | 0.3–4.0mIU/l | 9.0–23.0рmol/l | <50IU/ml | <50IU/ml | <1.0*IU/l |
| Analytical sensitivity | 10.15mIU/l | 0.04mIU/l | 1.25рmol/l | 5.5IU/ml | 5.5IU/ml | 0.3IU/l |
| Intra-assay precision (intra-assay CV) | 5.2% | 1.5% | 3.4.2% | 2.9% | 2.0% | 2.6% |
| Inter-assay precision (inter-assay CV) | 4.5% | 2.3% | 3.0% | 3.9% | 3.1% | 3.9% |
Legend: *TRAb – anti-TSH-receptor antibodies; below 1.0 – negative; from 1.0 to 1.5 - borderline; above 1.5 – positive; anti-TPO – anti-thyroid peroxidase antibodies; anti-TG – anti-thyroglobulin antibodies.
The ultrasound examination was performed by two highly-experienced endocrinology specialists certified in thyroid ultrasound, using Toshiba Eccocee (SSA-340A) and ESAOTE Caris plus ultrasound machines. Mathematical models: based on the dimensions determined by ultrasound, we calculated the thyroid gland volume as the sum of the volumes of both thyroid lobes. The volume of each lobe (V) was calculated using Brunn's formula, approved by the WHO for the calculation of the volume of an ellipsoid with a correction factor 0.524 (π/6):
Statistical analysis: The data analysis was performed using SPSS 18.0 (SPSS Inc, Chicago, Il., USA). We used the following statistical methods for data description and statistical conclusions: Descriptive methods: The quantitative variables were described by: N (number of observations), arithmetic mean or median depending on the distribution, standard deviation, minimal and maximal value. The normality of distribution of the sample was verified using the Shapiro–Wilk test. Qualitative variables were described by: N (number of observations) and relative frequency distribution (as percentages). Variation analysis was used to calculate the mean values of the main indicators. The comparative analysis of the metric variables between two groups was based on the Student's t-test (in cases of normal distribution of the sample) or the Mann–Whitney test (when distribution was not normal). Correlation analysis was used to determine the relationships between the variables studied (Pearson's or Spearman's correlation coefficient depending on the sample characteristics). Hypothesis testing for qualitative variables was performed by χ2 test. The null hypothesis was rejected when the p-value was observed to be less than 0.05 (the initially set level of significance).
ResultsWe included 144 women in the study: 71 prolactinoma patients and 73 healthy controls, prospectively followed up for at least 36 months. We excluded 3 prolactinoma patients with secondary hypothyroidism due to postoperative partial hypopituitarism after transsphenoidal surgery in order not to affect the thyroid function analysis. All the participants with a short follow-up (less than 3 years) were also excluded from this study.
In the patient group, 59 (83.1%) had microprolactinomas, the remaining 12 women (16.9%) had macroprolactinomas. Thirteen patients (18.3%) had residual adenomas after neurosurgery performed prior to study entry. The majority of patients were treated with cabergoline (n=65; 91.6%), 5 patients received bromocriptine (7.0%), and 1 patient (1.4%) was treated consecutively with bromocriptine, cabergoline and quinagolide. Although all the patients had been treated with dopamine agonists (DA), 25.3% (n=18) of them had persisting hyperprolactinemia, only 3 (4.2%) of whom were categorised as resistant to DA (2 macroadenoma and 1 microadenoma). In 21.1% of the cases, hyperprolactinemia was a result of poor compliance.
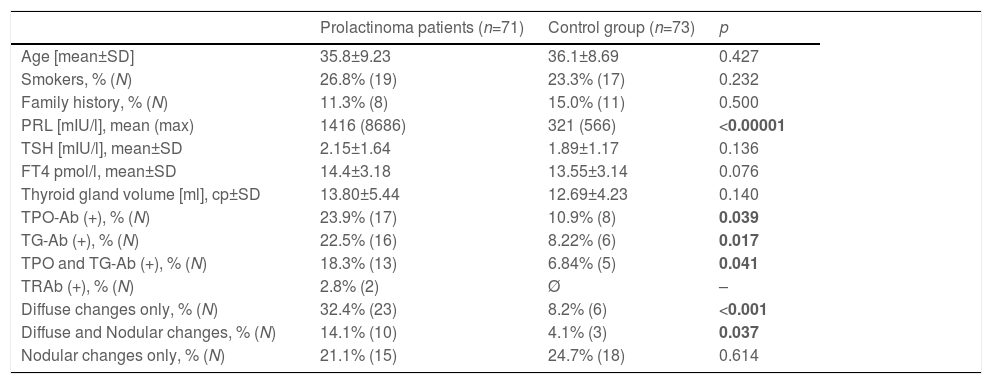
The main basal demographic, hormonal, immunological and ultrasound characteristics of the investigated subjects are presented in Table 2. Pregnancies during the observation period occurred in 23.2% (n=17) of the controls and in 18.3% (n=13) of the prolactinoma patients (p=0.462).
Main baseline characteristics of the study groups.
| Prolactinoma patients (n=71) | Control group (n=73) | р | |
|---|---|---|---|
| Age [mean±SD] | 35.8±9.23 | 36.1±8.69 | 0.427 |
| Smokers, % (N) | 26.8% (19) | 23.3% (17) | 0.232 |
| Family history, % (N) | 11.3% (8) | 15.0% (11) | 0.500 |
| PRL [mIU/l], mean (max) | 1416 (8686) | 321 (566) | <0.00001 |
| TSH [mIU/l], mean±SD | 2.15±1.64 | 1.89±1.17 | 0.136 |
| FT4 pmol/l, mean±SD | 14.4±3.18 | 13.55±3.14 | 0.076 |
| Thyroid gland volume [ml], ср±SD | 13.80±5.44 | 12.69±4.23 | 0.140 |
| TPO-Ab (+), % (N) | 23.9% (17) | 10.9% (8) | 0.039 |
| TG-Ab (+), % (N) | 22.5% (16) | 8.22% (6) | 0.017 |
| TPO and TG-Ab (+), % (N) | 18.3% (13) | 6.84% (5) | 0.041 |
| TRAb (+), % (N) | 2.8% (2) | Ø | – |
| Diffuse changes only, % (N) | 32.4% (23) | 8.2% (6) | <0.001 |
| Diffuse and Nodular changes, % (N) | 14.1% (10) | 4.1% (3) | 0.037 |
| Nodular changes only, % (N) | 21.1% (15) | 24.7% (18) | 0.614 |
Legend: PRL – prolactin; TSH – thyroid-stimulating hormone; FT4 – free thyroxin; TPO-Ab – anti-thyroid peroxidase antibodies; TG-Ab – anti-thyroglobulin antibodies; TRAb – anti-TSH-receptor antibodies.
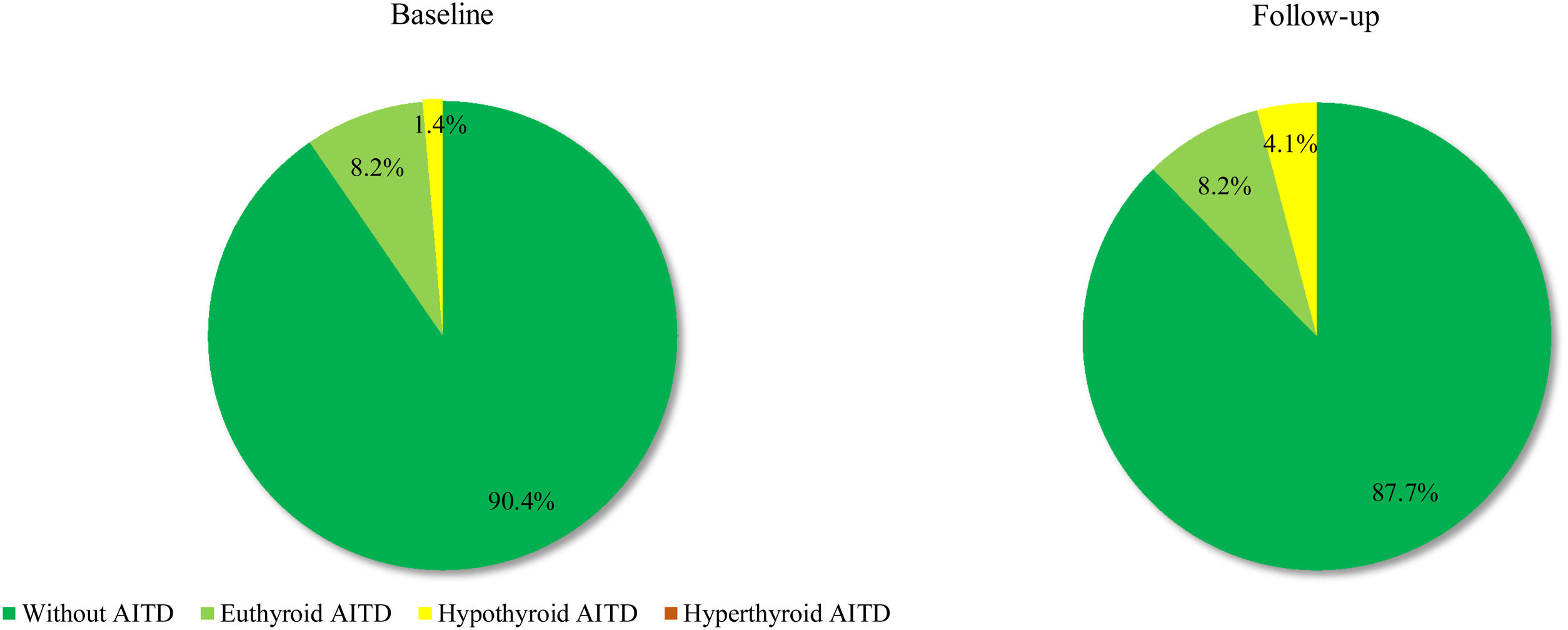
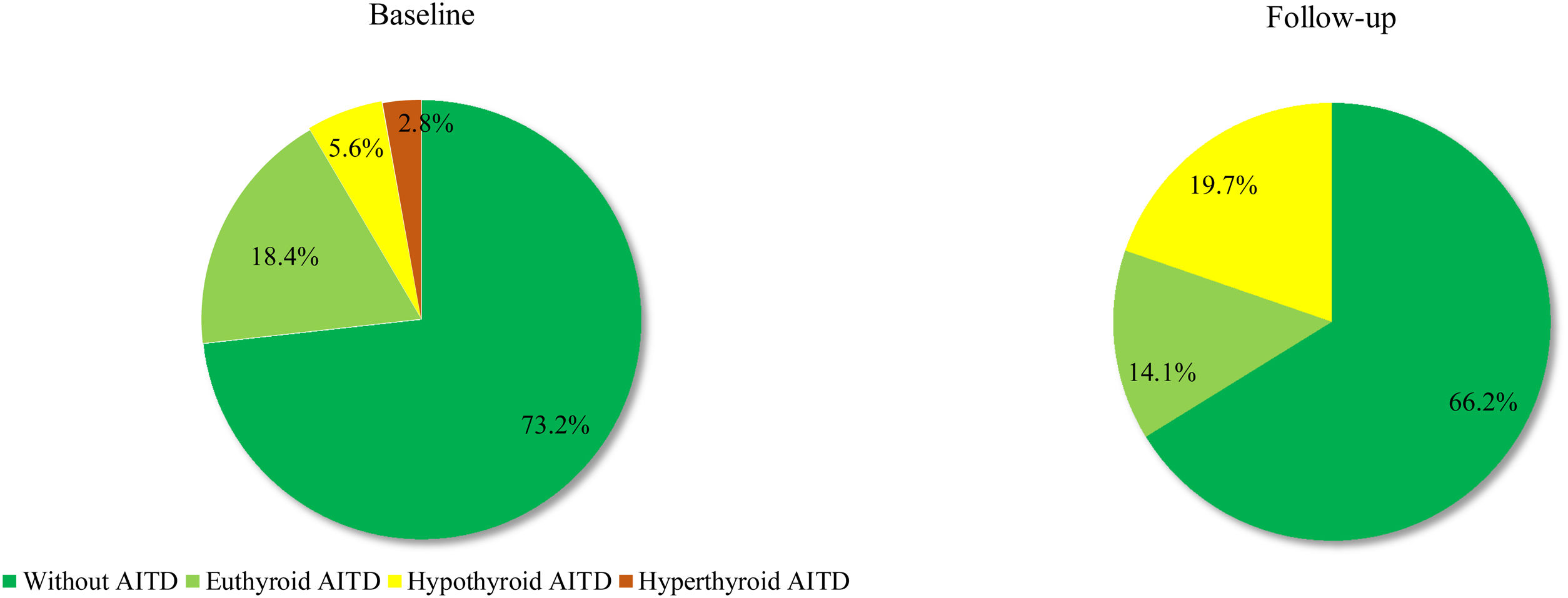
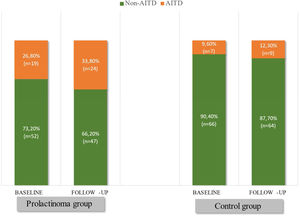
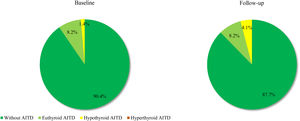
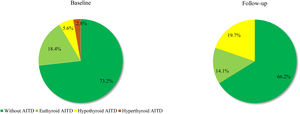
Based on the above-mentioned criteria, AITD was recorded in 9.6% (n=7) of the controls and in 26.8% (n=19) of the prolactinoma patients at study entry (p=0.007). Mean follow-up duration for both groups was around 6 years (6.68 vs. 6.25 years; p=0.107). The two groups did not differ significantly in mean age (42.3±9.97 vs. 42.6±8.58; p=0.408) or in mean levels of TSH (2.04±0.99 vs. 2.16±0.95; p=0.241) and FT4 (14.21±3.03 vs. 14.06±2.86; p=0.787). At the time of the follow-up visit, 33.8% (n=24) of the patients and 12.3% (n=9) of the controls had AITD (p=0.002) (Fig. 1). The subgroup analysis of thyroid function revealed a significant difference regarding the frequency and severity of primary hypothyroidism between both groups. At the beginning of the study, in the control group, 6 (8.2%) of the 7 (9.6%) patients with AITD were euthyroid and only 1 (1.4%) woman had subclinical hypothyroidism, which was well controlled on 50 mcg levothyroxine daily. In the prolactinoma group 18.4% (n=13) of the 19 (26.8%) patients with AITD were euthyroid, 5.6% (n=4) were hypothyroid and 2 (2.8%) patients had hyperthyroidism. Subclinical hypothyroidism was four times more common in the patient group in comparison to the control group (5.6% vs. 1.4%; p=0.162) (Figs. 2 and 3). At the end of the study, in the control group, 6 (8.2%) of the 9 (12.3%) women with AITD were euthyroid and 3 (4.1%) had subclinical hypothyroidism, controlled with mean daily doses of levothyroxine from 37.5 to 50mcg. In the patient group 10 (14.1%) of the 24 (33.8%) women with AITD were in euthyroid state and 14 (19.7%) women were in hypothyroid phase, undergoing substitution therapy with daily doses of levothyroxine varying from 25mcg to 200mcg. During the follow-up period, 9 prolactinoma patients went from euthyroid to hypothyroid state, compared to 2 women in the control group (p=0.025). At the end of the follow-up, hypothyroidism was almost five times more common in the patients (19.7%, n=14) in comparison to the controls (4.1%, n=3) (p=0.003) (Figs. 2 and 3). The higher substitution doses in the patient group should be noted, although a comparative statistical analysis was not possible due to the small number of women with hypothyroidism in the control group. There were no hyperthyroid subjects in either group at the end of the study. The two prolactinoma patients diagnosed with hyperthyroidism at the baseline visit achieved stable clinical, hormonal and immunological remission under thyrostatic and dopamine agonist therapy.
Spearman's correlation analysis in hyperprolactinaemic-naive patients with positive anti-thyroid antibodies at study entry revealed a moderate positive correlation between prolactin levels and anti-TPO-Ab concentrations (Rs=0.421; p=0.045). In contrast, we did not find such a correlation between PRL and anti-TG-Ab levels (Rs=0.267; p=0.334). Neither did we observe any significant relationship between anti-thyroid antibodies and PRL in normoprolactinaemic subjects (patients under dopamine agonist treatment and control group).
Among euthyroid/hypothyroid prolactinoma patients with AITD, the normalisation of PRL levels on DA treatment resulted in a significant decrease in anti-TPO-Ab in 11 of them (50%); in 10 subjects, there was no change of anti-TPO-Ab levels despite the significant reduction in PRL. Inverse association (increased anti-TPO-Ab/decreased PRL) was only seen in one patient. Correlation was not statistically significant (r=0.196; p=0.382), most probably due to the small sample size.
DiscussionThis prospective study examined the clinical course and progression of AITD in patients diagnosed with prolactinoma and in a control group of women matched for age and thyroid risk factors, diagnosed and followed up in a single tertiary clinical centre. We found an almost three times higher frequency of AITD in prolactinoma patients compared to the control subjects. In contrast, nodular goitre appeared with the same frequency in both study groups. In addition, for the 6-year average follow-up period of the participants, the tendency towards a higher incidence of new cases of AITD among the prolactinoma patients was maintained. Similar to our previous studies, there was a significant difference in the frequency of hypothyroidism, but not of hyperthyroidism. Last but not least, the severity of hypothyroidism, estimated on the basis of the average daily dose of levothyroxine required to achieve euthyroid state, was higher in the prolactinoma patients compared to the controls.
Autoimmune thyroiditis is the most common organ-specific autoimmune disease. Its clinical manifestation is the result of the interaction of predisposing genetic and exogenous factors. Genetic factors include certain polymorphisms in the major histocompatibility complex (MHC) genes, some immunomodulatory genes such as the cytotoxic T lymphocyte antigen-4 (CTLA-4), CD40 and CD226, the protein tyrosine phosphatase-22 (PTPN22), as well as thyroid-specific genes (genes encoding ТSH-receptor and thyroglobulin).19,20 Multiple environmental factors, such as iodine intake, stress, infectious agents, some drugs, are considered to be inductors of the autoimmune process in genetically predisposed subjects. As a rule, the most common spontaneous evolution in patients with AITD is a progressive decrease in thyroid function over time. However, the time of transition from the euthyroid to the hypothyroid phase and the progression of hypothyroidism is individually determined and results from the interaction of underlying genetically-determined thyroid autoimmune predisposition and case-specific unique exogenous factors. Prolactin may be regarded as one of these factors. With its dualistic action as a hormone and a cytokine, it is an experimentally-proven powerful immunomodulator with a complex effect on cellular and humoral immunity. Prolactin modulates the early phases of T-cell activation, stimulates lymphocyte secretion of TNF-α, γIFN and IL-2 and triggers IL-2 stimulated proliferation; it increases macrophage phagocytic activity and the production of IL-1, γIFN and NO; it stimulates B-lymphocyte clonal expansion, maturation and differentiation and immunoglobulin production; it inhibits the negative selection of auto-reactive B-lymphocytes; it activates antigen-presenting dendritic cells as it transforms their secretion profile to proinflammatory.21–25 The immunological profile of our patients suggests a selective effect of PRL on these processes, leading to an increased risk of autoimmune thyroiditis and a faster progression of the autoimmune process towards hypothyroidism in these prolactinoma patients who are genetically predisposed to AITD.26 Arguments that support this hypothesis are the positive correlation we found between the levels of anti-TPO antibodies and the degree of hyperprolactinemia and the higher substitution daily doses of levothyroxine in the prolactinoma patients compared to the control group. Our attempts to compare the results of this prospective follow-up of our patients to literature data were unsuccessful. To date, only a few retrospective and cross-sectional case-control comparative studies have been published with comparable results to our previous studies with a similar design. Three published studies compare the incidence of AITD in patients with prolactinoma and other adenohypophyseal tumours. The retrospective study by Dogansen et al. in 78 patients with acromegaly and 83 patients with prolactinoma demonstrates a two-times higher incidence of Hashimoto's thyroiditis with a significant prevalence of autoimmune hypothyroidism in patients with prolactinoma.27 In an Italian study by Pilli et al., the incidence of AITD was twice as high in patients with prolactinoma compared to a control group of non-functioning pituitary tumours (NFPA) or empty sella syndrome (13.4% vs. 6.3%; p=0.042). Furthermore, multivariate analysis points to serum prolactin as the only independent predictor of AITD. The lower incidence of AITD in this Italian cohort compared to our data could be explained by the difference in iodine status in these countries. Like us, the authors did not find a significant difference in the frequency of nodular goitre in the two groups.28 Another retrospective study with a similar design, conducted at the McGill University Health Center, also confirms more frequent AITD in patients with prolactinoma compared to NFPA.29
Dopamine agonist treatment is the first-line treatment in almost all patients diagnosed with prolactin-secreting pituitary adenomas, and cabergoline is the drug of choice due to its high efficacy, safety profile and low resistance rate.17,18 Thus, the favourable impact of DA therapy on autoimmunity could be expected during long-term follow-up. A positive correlation between prolactin levels during DA treatment and disease activity has been demonstrated in patients with SLE and rheumatoid arthritis.2,6 One half of our patients with euthyroid and hypothyroid AITD presented with a significant decrease in anti-TPO-Ab levels after achieving normal PRL levels. We could hypothesise that DA-induced stable normoprolactinaemia may positively affect the autoimmune process. Larger studies and meta-analyses are needed to confirm this observation.
The main advantage of our study is the prospective follow-up of prolactinoma patients and a control group of individuals matched for sex, age and thyroid risk factors. It is the first study designed to monitor not only the new cases of AITD, but also their progression over time. The most significant limitation of this study is that the design did not include examination of cellular immunity and complement factors in these patients. The investigation of a wider range of autoantibodies in patients with hyperprolactinemia would also be of interest. A recently published study found not only a high prevalence of various autoantibodies in a quarter of the patients with hyperprolactinaemia, significantly higher serum levels of IL-4 and IL-6, but also an abnormal distribution of peripheral B-lymphocyte subclasses compared to age-matched healthy women.30 This data suggests a possible role of hyperprolactinemia in the clinical manifestation of autoimmune polyglandular syndromes.
ConclusionsProlactinoma patients appear to be predisposed to autoimmune hypothyroidism. The selective immunomodulatory effect of prolactin primarily on cell immunity, activation of complement and antibody-dependent cytotoxicity, leading to earlier and faster progression of Hashimoto's thyroiditis to hypothyroid state in genetically predisposed subjects might be considered to be an additional pathogenetic mechanism. The degree of hyperprolactinemia at diagnosis may be a predictor of a more active autoimmune process in this patient cohort.
FundingThis work is supported by the Bulgarian Ministry of Education and Science under the “Young Scientists and Postdoctoral Students” National Research Programme.
Authors’ contributionAll the authors equally contributed to the design of the study, statistical analysis and interpretation of data as well as to drafting and revising the paper. All the authors read and approved the final version of the manuscript.
Conflicts of interestThe authors certify that there is no conflict of interest with any financial organisation regarding the material discussed in the manuscript.
We would like to express our gratitude to Dr Emil Nachev and Dr Ralitsa Ivanova, who performed the ultrasound examination of the study participants.