Part I of this two-part article on the history of the teaching of qualitative analysis to undergraduate chemistry majors covers the origins of the course, its theoretical rationale, the impact of spot analysis and tailored organic reagents, and its transformation from a macro scheme to a semi-micro scheme.
La parte I de este artículo, que comprende dos partes sobre la historia de la enseñanza del análisis cualitativo en las carreras de química, cubre los orígenes del curso, su racionalidad teórica, el impacto de las reacciones a la gota y la búsqueda de reactivos orgánicos apropiados así su transformación de un esquema macro a un esquema semi-micro.
The teaching of a course in systematic inorganic qualitative analysis was a staple of the undergraduate chemistry laboratory for nearly 140 years. Though such courses began to gradually disappear from the curriculum – at least in the United States – starting in the 1970s, 2016 was the 175th anniversary of the publication of what is perhaps the single most influential textbook on the subject – Carl Remigius Fresenius’ Anleitung zur qualitativen chemischen Analyse – and it seems appropriate to celebrate this event with a review of the history of this rapidly disappearing laboratory technique.
Precipitants, reagents and test solutionsIt has long been known that, just as so-called “dry” or blowpipe analysis evolved from traditional metallurgical assaying techniques, so “wet” or solution analysis, in both its qualitative and quantitative forms, evolved from a traditional interest in the evaluation of the medicinal properties of mineral waters (Jensen, 1986; Kopp, 1844). As shown by Debus, water analysis prior to the 17th century relied almost exclusively on distillation and such physical properties as the color, taste, odor, and crystalline form of the resulting distillation residue (Debus, 1962). Only in the 17th century does one see an increasing realization that the analytical detection of a metal did not always require its reduction and isolation, as in a standard fire assay, but could also be done indirectly in aqueous solution through the use of characteristic color and precipitation reactions. Examples, such as the use of vegetable dyes to detect acids, copper compounds to detect ammonia, and nut gall to detect iron, can be found in the writings of several 17th-century chemists, including Libavius, Hoffmann, Glauber, Tachenius, and especially Robert Boyle (Szabadvàry, 1966).
However, it was not until the last quarter of the 18th century that an attempt was made by the Swedish chemist, Torbern Bergman (Fig. 1), to systematically collect and evaluate these tests in his 1778 essay “De analysi aquarum” (Bergman, 1779). In this essay Bergman reviewed in great detail the nature and preparation of 23 different aqueous test solutions commonly used in water analysis. Bergman referred to these solutions as “precipitants,” but in an editorial footnote to the 1784 English translation of his essay, the British chemist, Edmund Cullen, referred to them instead as “reagents,” a term which he attributed, in turn, to the French chemist Guyton de Morveau, though, more recently, de Menten has suggested even earlier French antecedents (de Menten, 2013; Jensen, 2012).
The etymological reasoning behind this term seems to have been that the unknown species in the analysis functioned as the active “agent” and the test solution employed to detect it as the passive “reagent” in the ensuing chemical interaction between the two. Likewise, whereas the unknown agent “acted” upon the reagent, the reagent itself merely “reacted” to this action. In other words, reaction was to action as reagent was to agent, where agent and action described the causative factors and reagent and reaction described the responding factors. Obviously both the terms chemical reaction and chemical reactant also evolved from this same usage.
To confuse matters further, for the first three decades or so of the 19th century British chemists frequently referred to reagents as “test solutions” or “tests” for short, as may be seen from the chests or cabinets of “chemical tests” offered for sale by such chemists as Accum, Henry, and Griffin during this period (Accum, 1816a). Thus, for example, Accum, in his 1816 Practical Essay on Chemical Re-agents or Tests, used the two words interchangeably and defined them as those (Accum, 1816b): ... substances which, when applied to other bodies, the nature or composition of which are unknown, quickly act upon them, and produce such changes as are sufficiently striking to the senses, and from which the quality or nature of the unknown body may be inferred... Most of the tests employed in the processes of chemistry indicate the component parts of bodies by occasioning either a precipitate, a sensible cloudiness, a change in color, an effervescence, or such other alterations of properties as experience has proved denote the presence or absence of certain bodies.
Though possibly a bit too wordy for the modern reader, most would nevertheless agree that this definition is as valid today as it was in 1816. However, while we still use the word test to describe either the act of analysis or the procedure used, we no longer use it as a synonym for reagent.
Group reagentsThe above authors presented the various reagents they discussed as specific tests for individual metals and acid radicals, but gave no advice on how to systematically combine them into a single sequential analytical scheme. The key to this development was actually the introduction of “group” reagents, rather than specific reagents, which could precipitate entire clusters of related metals. Application of these in the proper order allowed an analyst to separate the components of a complex mixture into ever smaller groups of related metals before applying the final confirmatory tests for each individual.
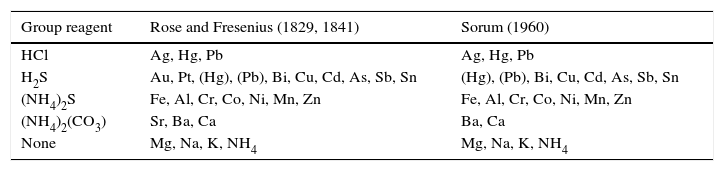
The first significant step in this direction was taken by the German chemist, Heinrich Rose (Fig. 2), in his 1829 monograph Handbuch der analytischen Chemie.1 Both the group reagents employed by Rose and the various metals in each group are summarized in Table 1, where they are compared with those used by Sorum in the 1960 edition of his qual manual (Sorum, 1949).2 As may be seen, save for the presence of Au, Pt, and Sr in Rose's scheme, the two are essentially identical, though there are, of course, many minor differences in how these groups were subsequently further separated and in some of the final confirmatory tests. The point, however, is that Rose established the basic outlines of systematic qualitative inorganic analysis as it would be practiced for the next 185 years. In making this table I have avoided using such labels as Group I, Group II, etc. for the simple reason that Rose arbitrarily chose to number his groups of metals in an order opposite that of their order of separation, such that the alkali metals were in Group I rather than Group V, the alkaline earth metals were in Group II rather than Group IV, etc. – a practice that was followed by subsequent qual manuals for much of the 19th century.
A comparison of the qual groups and reagents used by Rose and Fresenius with those used by Sorum.
| Group reagent | Rose and Fresenius (1829, 1841) | Sorum (1960) |
|---|---|---|
| HCl | Ag, Hg, Pb | Ag, Hg, Pb |
| H2S | Au, Pt, (Hg), (Pb), Bi, Cu, Cd, As, Sb, Sn | (Hg), (Pb), Bi, Cu, Cd, As, Sb, Sn |
| (NH4)2S | Fe, Al, Cr, Co, Ni, Mn, Zn | Fe, Al, Cr, Co, Ni, Mn, Zn |
| (NH4)2(CO3) | Sr, Ba, Ca | Ba, Ca |
| None | Mg, Na, K, NH4 | Mg, Na, K, NH4 |
As suggested by his use of the term Handbuch, Rose intended his monograph to be a reference work for practicing analytical chemists and not a textbook for beginning students. It was over 600 pages long and his pedantic organization of the contents was, in the words of Szabadvàry, “very dull” (Szabadvàry, 1966). Thus it was that the task of extracting the essence of Rose's insights and presenting them in a simplified format suitable for undergraduate instruction fell instead to a young German chemist by the name of Carl Remigius Fresenius (Fig. 3).
Fresenius first made his simplified abstract of Rose while still a student at the University of Bonn and in 1841, upon his transfer to Liebig's laboratory at Giessen, used it to teach an introductory course in qualitative inorganic analysis (Fresenius, 1841). The first edition of 1841 was quickly succeeded by a second in 1842, which carried a preface and endorsement by Liebig, by a third in 1844, and by a fourth in 1846, by which time Fresenius had transferred to Wiesbaden, where he eventually founded the Zeitschrift für analytische Chemie in 1862 and a family dynasty of analytical chemists that would include two of his sons and a grandson as well. By the time of his death in 1897 his textbook had passed through 16 editions and in 1919 his son, Theodor Wilhelm Fresenius, published yet a 17th edition (Fresenius, 1919). However, this was over 950 pages thick and had thus, rather ironically, degenerated into a reference work, not unlike the handbook of Rose that it had originally been intended to supplement 80 years earlier. Finally, in 1942, or slightly over a century after the appearance of the first edition, Fresenius’ grandson, Remigius Fresenius, coauthored a highly condensed (207 pages) introductory manual under a new title which recaptured the spirit and intent of the first edition (Fresenius and Gehring, 1942).
Within three years of its initial publication, the Fresenius manual was translated into English, French, Italian, Dutch, Spanish, Hungarian, Chinese, and eventually Russian. In this manner it rapidly spread the “Giessen method” of teaching introductory qualitative inorganic analysis throughout the civilized world. By the end of the 19th century essentially every university in the United States, Great Britain, and Continental Europe had its own in-house qualitative analysis manual whose contents were ultimately traceable to Fresenius, and by mid 20th century the number of books published on this subject probably numbered in the hundreds. Despite the many subsequent attempts, discussed below, to alter the original qual scheme outlined by Rose and Fresenius, it would prove to be remarkably resilient, as may be seen from the comparison given in Table 1.
There is, however, a curious puzzle connected with the Giessen origins of qualitative analysis. In 1845 a second manual of qualitative analysis, also containing a preface and endorsement by Liebig, was published by Heinrich Will under the title Anleitung zur chemischen Analyse zum Gebrauche im chemischen Laboratorium zu Giessen and which, as may be seen, explicitly stated in its title that it was based on the official course used at Giessen (Will, 1845). Will had been a student at Giessen since 1837 and a Privatdozent since 1839. Apparently he took over undergraduate instruction in qualitative analysis after Fresenius’ departure in 1845 and felt compelled to issue his own laboratory manual. This was in many ways a terse condensation of the manual by Fresenius, and it would be of some interest to know what the original author thought of this turn of events. Will would remain at Giessen his entire career and would succeed Liebig as Professor of Chemistry there after the latter's departure for Munich in 1852. Though Will's textbook would also undergo several editions and translations, it was never as influential as the volume by Fresenius.
Mention should also be made of yet a third condensation of Rose's analytical scheme for the beginning student. This was published in 1849 under the title of a Practical Introduction to H. Rose's Treatise on Chemical Analysis, and was the work of the British chemist, A. Normandy, who had first translated Rose's Handbuch into English several years earlier (Normandy, 1849). Rather curiously, in his introduction, Normandy made no mention of the books by either Fresenius or Will, though both had by this time been available for some years in English translation. Perhaps, because of this lack of awareness, his small manual appears to have had little or no impact on the subsequent development of qualitative analysis.
The first thing that strikes the modern reader of the pioneering books by Fresenius and Will, aside from the curious inversion in the numbering of the analytical groups commented on earlier, is that the laboratory instructions, especially in Will's manual, are not very specific. Unlike modern qual manuals, in which the student is told at each stage how many drops of reagent A or B to use, how much water to use when washing a precipitate, how many times to repeat the wash, or when to centrifuge, etc., nothing is said in these early manuals about what quantity or concentration of reagent to use or even about what equipment to use. Also conspicuously absent from both books are the flow charts used in later qual manuals to outline the overall separation scheme, though the manual by Will does contain several foldout tables designed to serve the same purpose.
What the two manuals have in common, however, is the so-called “100 Bottle Challenge.” In other words, each student was required to demonstrate his mastery of the qual scheme by successfully analyzing 100 unknowns of ever increasing complexity (Will, 1845): The contents of the bottles become more and more complex as one advances from 1 to 100. In the first ten solutions one seeks only an acid [i.e. anion]; in the second ten, perhaps only a base [i.e. cation]. The next twenty are solids, and both an acid and a base are to be sought in each bottle. Then occur bottles, each containing several bases, then others, each containing several acids; and thus increasing, till the last ten of the hundred bottles may be found to contain from ten to twenty ingredients.
It is very difficult to imagine modern-day freshmen surviving such a challenge.
TheoryThe above quote also calls attention to yet a third feature of these early manuals which will strike the modern reader as unfamiliar – namely their pervasive use of dualistic formulas and terminology. Based on the work of Lavoisier and Berzelius, the dualistic theory viewed ternary and other higher-order compounds as additive adducts of a basic metallic oxide and an acidic nonmetallic oxide. Thus calcium sulfate was thought of as an adduct of basic calcium oxide and acidic sulfur trioxide or as CaO·SO3. In keeping with this, basic solutions of calcium hydroxide were viewed as aqueous solutions of CaO, in which CaO, rather than hydroxide, was thought to be the source of the basic properties, and sulfuric acid as an aqueous solution of SO3, in which SO3, rather than hydrogen, was thought to be the source of the acidic properties – whence the use of the terms acid and base when referring to the nonmetallic and metallic components of salts.
In keeping with these usages, the manuals of both Fresenius and Will were organized around an analysis for the presence of various metallic oxides rather than for either simple metals or cations. In the companion field of quantitative analysis this practice of reporting the composition of a ternary salt in terms of its so-called component oxides, rather than its component elements, persisted well into the 1970s. Yet further complications arose from the fact that many atomic weight values used at the time were incorrect and thus the formulas of many compounds as well. For example, water was written as HO instead of as H2O and sodium carbonate as NaO·CO2 rather than as Na2O·CO2, etc.
These problems were only corrected in the 1860s as a result of Cannizzaro's famous pamphlet of 1858. This led not only to our current system of atomic weights, but also to our current method of writing the chemical formulas of salts, so that calcium sulfate now appeared as Ca(SO4) rather than as CaO·SO3. Yet, if one is to judge from various American editions of Fresenius, these changes did not begin to impact on his qual manual until the 1870s. Thus the 1871 reprint of the 1864 edition included an introductory essay by the book's translator, Samuel Johnson of Yale University, entitled “Chemical Notation and Nomenclature: Old and New,” but made no attempt to revise the older dualistic formulas within the body of the text itself (Fresenius, 1871). It was only with the edition of 1875 that this revision was finally made and the subtitle “New System” added to both the spine and title page (Fresenius, 1875).
The theoretical revisions required by the so-called “New System” of the 1860s were minor compared with the upheaval caused in the 1890s by the introduction of the theories of ionic dissociation and chemical equilibrium. These two theories were the main bulwark in the 1880s of the new and rising discipline of physical chemistry championed by Arrhenius, van’t Hoff, and especially by the Latvian/German chemist, Wilhelm Ostwald (Fig. 4). Indeed, it was Ostwald who first drew attention to their significance for the traditional practice of qualitative analysis in his classic 1894 monograph Die wissenschaftlichen Grundlagen der analytischen Chemie (Ostwald, 1894).
Ostwald's little book had an immediate impact. By the first decade of the 20th century numerous manuals of qualitative analysis were incorporating brief introductory sections on the theory of ionic dissociation and the laws of equilibrium and mass action, and were treating the subject as a procedure for the detection of ions rather than elements or oxides – though rather curiously the use of net ionic equations appears to have been uncommon before the 1930s. Some typical American examples include the manuals by Prescott and Sullivan (1902), Morgan (1906), Prescott and Johnson (1907), Medicus (1908), and Hinds (1910). In 1909 the French chemist, M. G. Chesneau, published an advanced monograph on the theory of chemical analysis emphasizing the same physico-chemical principles as Ostwald but from a uniquely French perspective (Chesneau, 1910), and in 1911 Julius Stieglitz of the University of Chicago published a two-volume introductory treatise on qualitative analysis, the first volume of which was devoted exclusively to theory (Stieglitz, 1911) – a pattern that would later be imitated by others as well (Reedy, 1924, 1938). With these events qualitative analysis acquired a theoretical structure that it has retained even to this day and one, as we will see below, that would have profound pedagogical consequences.
Spot reactionsIn the early decades of the 20th century a school of analytical chemistry arose in Austria dedicated to the scaling down of conventional analytical techniques. The first and most famous of its practitioners was the Austrian chemist, Fritz Pregl, who was awarded a Nobel Prize in 1923 for his development of organic micro-combustion analysis (Pregl, 1917). Starting around 1918 a second Austrian chemist, by the name of Fritz Feigl (Fig. 5), decided to do for qualitative analysis what Pregl had done for combustion analysis, the results of which were first summarized in his 1931 monograph Qualitative Analyse mit Hilfe von Tüpfelreaktionen (Feigl, 1931). Usually translated as either “spot” or “drop” reactions, Feigl replaced the conventional macro beaker and test-tube level precipitation and color reactions used in qual with semi-micro and/or micro equivalents that employed only a drop of both the unknown and the reagent, and which were usually performed either on a piece of filter paper or in a porcelain drop plate. In order to attain as much specificity as possible for a given test, Feigl also made heavy use of specially designed organic reagents, masking agents to eliminate interfering ions, and catalytic effects to amplify certain reactions. His monograph was soon translated and would go through numerous editions, the most recent of which appeared in 1983.3 Indeed, so great were the advances in this area that each new edition would lead to a virtual doubling of the book's size.
It is important to realize that Feigl's work was directed at the practicing analytical chemist and not at the teacher of undergraduate courses in qualitative analysis. Though spot tests soon proved to be a highly effective way of testing for known ions under well-defined conditions, such as the detection of trace contaminates, their open-ended application to the analysis of complex mixtures of unknowns was more problematic. While the ultimate goal was to develop spot tests that were each specific for one and only one ion, and thus eliminate the necessity for separations, practice always fell short of this ideal. Consequently, in each edition Feigl would summarize various imperfect proposals for a proper sequential application of the tests and would also organize his discussion of the various spot tests for individual metallic cations using the standard groups of the conventional qual scheme.
This strongly implied that one way to utilize the various spot tests was to first separate the ions in a complex mixture using the conventional qual scheme and reserve use of spot reactions for the final confirmatory tests. In fact in 1933 the Dutch analytical chemist, C. J. van Nieuwenburg, published an undergraduate lab manual based on this exact premise (Nieuwenburg and Dulfer, 1933), though it was not until 1940 that C. W. Davis finally brought van Niewenburg's proposals to the attention of American chemistry teachers (Davis, 1940).
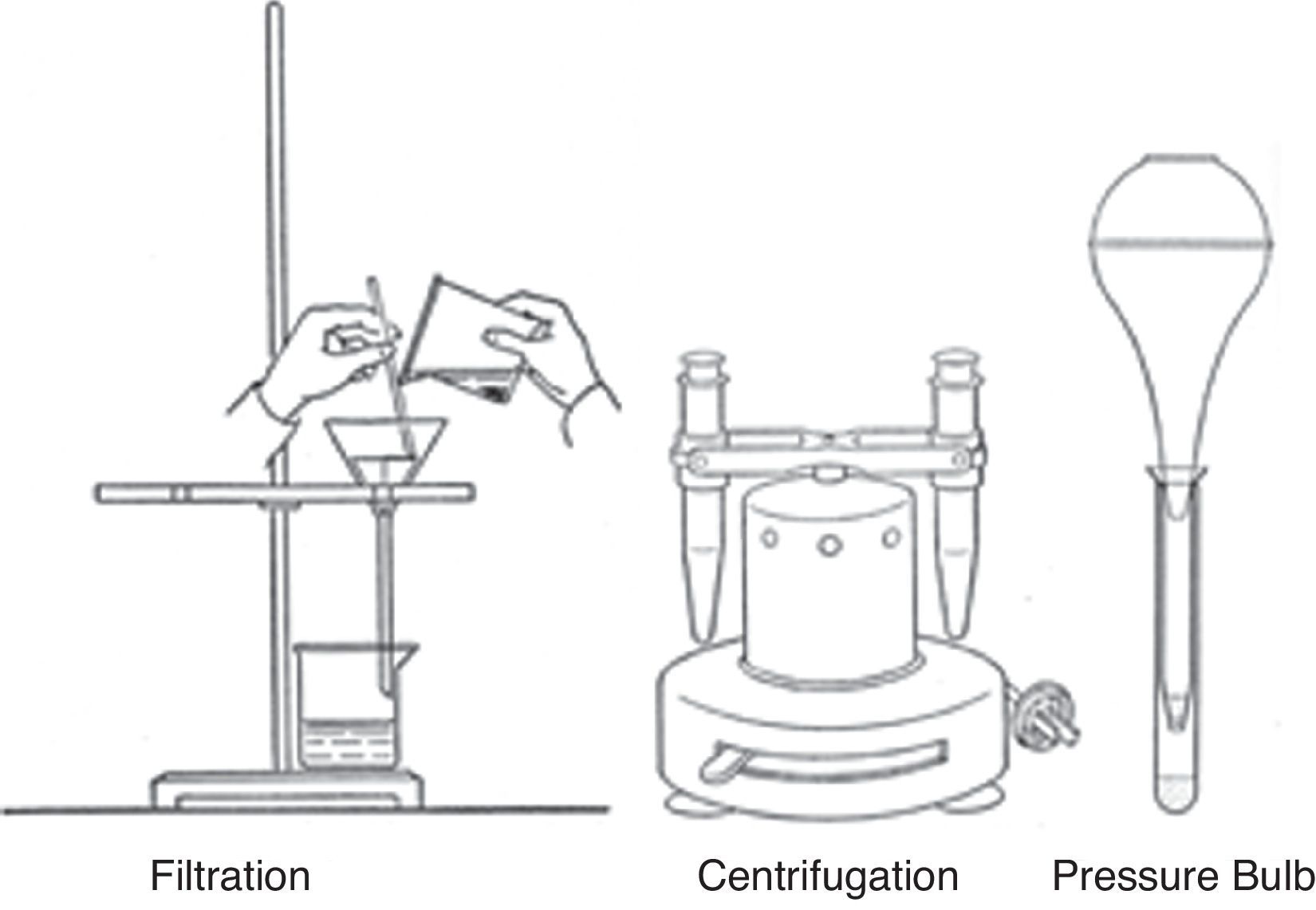
Scaling downAs events turned out, however, few American chemistry teachers would adopt van Niewenberg's suggestions concerning the use of spot reactions for confirmatory tests in the undergraduate qual course, largely for the reasons that will be outlined in Part II in the section on pedagogy. However, they would adopt yet another of his suggestions – namely that the entire conventional separation scheme be scaled down to the semi-micro level. Throughout the 19th century and the first four decades of the 20th century, macro filtration was the standard way to separate precipitates and every qual manual contained illustrations of filter stands, and instructions for the use of both a wash bottle and the proper way to fold filter paper (Fig. 6). Van Niewenburg's suggestion, made almost as a passing comment and with virtually no detail, was that separations at the semi-micro level could be carried out using a centrifuge rather than filtration.
This same suggestion was independently made about the same time by C. J. Engelder and W. J. Schiller of the University of Pittsburgh (Engelder and Schiller, 1932). Inspired, like van Niewenburg, by the work of Feigl, in 1936 these authors, in collaboration with T. H. Dunkelberger, published a detailed laboratory manual in which the term “semi-micro” was, for the first time, directly incorporated into the title and which not only used the centrifuge for all separations but also performed most of the precipitations and confirmatory tests in small centrifuge tubes rather than in conventional beakers or test tubes (Engelder, Dunkelberger and Schiller, 1936). If for no other reason than the economic savings that resulted from scaling down the conventional scheme, the proposals of these three authors quickly spread to other schools. Articles praising the semi-micro approach soon began appearing in the Journal of Chemical Education (Arthur, Burrows, Smith, & Adams, 1941; Smith, 1938; Wendt, 1940) and, with the publication of the second edition of their textbook in 1940, these authors were able to proudly proclaim that (Engelder, Dunkelberger and Schiller, 1940): The first edition of this book, which introduced the semi-micro technique into hundreds of institutional laboratories and, to a considerable degree, revolutionized the laboratory instruction in qualitative analysis, has more than fulfilled the authors’ hopes and expectations.
By the late 1940s and early 1950s the older macro approach to qualitative analysis, at least in the United States, had been almost totally displaced by the newer semi-micro approach. Not only were newer manuals based on this approach, but also the most recent revisions of older macro manuals. Thus the textbook by Baskerville and Curtman, which first appeared as a macro manual in 1916 (Baskerville & Curtman, 1916), would switch to the semi-micro approach in 1942 (Curtman, 1942), and that of Sorum, which evolved from a macro manual first published in 1911, would switch in 1949 (Sorum, 1949).
The introduction of the semi-micro approach also spelled the eventually demise of the glass-stoppered reagent bottle. These bottles, with their beveled and frosted glass labels, had been a standard feature, in one form or another, of the chemical laboratory for nearly 150 years (Fig. 7), but were now being rapidly replaced by small dropper bottles (Fig. 8). Once lining the shelves of undergraduate laboratories by the hundreds, glass-stoppered reagent bottles are now a rarity in most chemical laboratories and are no longer offered for sale by some laboratory supply houses.
Yet a third separation technique, also associated with the rise of semi-micro qual courses, was introduced in the 1940s by H. H. Barber of the University of Minnesota. Known as the “pressure bulb” method, it made use of a small centrifuge tube with a hole in the tip and a flared lip that allowed it to rest inside the top of a conventional test tube. A small plug of cotton was placed in the bottom of this tube and the liquid to be filtered was added and rapidly forced through the cotton plug using the air pressure from a small rubber bulb (Fig. 6). The pressure bulb method made the semi-micro approach even more economical by dispensing with costly centrifuges that often had short life-spans due to a combination of corrosive acids and sloppy undergraduate laboratory technique. This clever procedure and several others like it were described in great detail by Barber in his 1942 laboratory manual, but it is not known whether they were ever adopted beyond the confines of the University of Minnesota, where they originated (Barber & Taylor, 1942).
Finally, mention should be made of yet a fourth separation method, known as the “ring furnace technique,” developed by the Austrian chemist, Herbert Weisz, in the late 1950s (Weisz, 1961). However, this was specifically designed for micro-separations in conjunction with Feigl's spot methods and was never, to the best of my knowledge, adapted for use in an undergraduate qual course.
Organic reagentsAs already noted, one of the characteristic features of Feigl's spot analysis technique was the heavy use of specially designed organic reagents to improve the specificity of the various tests. Organic reagents had in fact been used since the beginnings of systematic qualitative analysis, but quite sparingly. Thus oxalate was used by both Rose and Fresenius to precipitate calcium. However, it wasn’t until 1905 that dimethylglyoxime was suggested by Tschugaeff as a confirmatory test for nickel (Tschugaeff, 1905), not until 1925 that aluminon was suggested as a specific reagent for aluminum (Hammett & Scottery, 1925), and not until 1977 that Sorum and Lagowski were willing to introduce the use of diphenylthiocarbazone as a confirmatory test for zinc in their well-known qual manual (Sorum & Lagowski, 1977).
Meanwhile the design and use of organic reagents, not only in the field of spot analysis, but also in the fields of colorimetric and volumetric analysis (recall the impact of EDTA) had become so pervasive that in 1941 Yoe and Sarver could devote a 339-page monograph to the subject in which the analytical uses of more than 600 organic reagents were reviewed (Yoe & Sarver, 1941). Yet, despite the efforts of White three years earlier to convince chemical educators that use of organic reagents could be rationalized as a logical extension of more traditional inorganic reagents (White, 1938), the chemical education community remained largely unconvinced and clung instead, with few changes, to the chemistry of the traditional qual scheme first introduced by Rose and Fresenius a century earlier.
Conflicts of interestThe author declares no conflicts of interest.
Szabadvàry mentions that the German chemist, C. H. Pfaff, had described many of the standard group reagents in 1821 but had failed to apply them systematically. See C. H, Pfaff, Handbuch der analytischen Chemie, Hammrich: Altona, 1821.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.
The reason Hg appears in both the first and second groups is because the first group separates Hg(I) whereas the second group separates Hg(II). Since PbCl2(s) is only marginally insoluble, some Pb(II) also carries over into the second group as well, especially if it is present in low concentrations.
This is Part I of a two-part article. Part II will appear in a future issue.
The practical section of the 2nd edition of 1935 was first translated as Feigl, F., Qualitative Analysis by Spot Tests, Inorganic and Organic Applications. Nordemann: New York, NY, 1937, and the theoretical section as Feigl, F., Specific and Special Reactions for Use in Qualitative Analysis with Special Reference to Spot Test Analysis. Nordemann: New York, NY, 1940.