Electrochemistry is an important topic in chemistry due to its wide application in everyday life. Although it is found in most high school curricula throughout the world, it is commonly regarded as an abstract and difficult topic to teach. This paper explores the teacher knowledge possessed by a diverse group of South African grade 12 teachers for teaching this topic. Two types of teacher knowledge were explored — knowledge of the content to be taught and topic specific pedagogical content knowledge. Topic specific content knowledge is the ability to reason about teaching the topic through 5 components regarded as important for the transformation of content knowledge, viz. Learner Prior Knowledge, Curricular Saliency, What is difficult to teach, Representations and Conceptual Teaching Strategies. 64 teachers responded to two instruments, measuring the two teacher knowledge bases. Results reflected the socio-diversity of the teachers with overall moderately high scores obtained on content knowledge which were not necessarily matched by good tspck. Reasons for this are explored.
La electroquímica es un tema importante de la química debido a su amplia aplicación en la vida diaria. Aunque se le encuentra en la mayoría de los currículos de bachillerato por todo el mundo, es visto comúnmente como un tópico abstracto y difícil de enseñar. Este artículo explora el conocimiento que posee para enseñarlo un grupo diverso de profesores de grado 12° en Sudáfrica. Se exploraron dos tipos de conocimiento de los profesores —conocimiento del contenido a enseñar y conocimiento pedagógico del contenido del tópico específico (cpcte). El conocimiento del contenido del tópico específico es la capacidad de razonar acerca de la enseñanza de las cinco componentes consideradas como importantes para la transformación del conocimiento del contenido, esto es, Conocimiento Previo del Aprendiz, Preponderancia Curricular, Qué es Difícil de Enseñar, Representaciones y Estrategias Conceptuales de Enseñanza. 64 profesores respondieron dos instrumentos, para medir sus dos bases de conocimientos. Los resultados reflejaron la diversidad social de los profesores con calificaciones ligeramente superiores en el conocimiento del contenido, que no necesariamente casaban con un buen cpcte. Las razones de lo anterior son exploradas.
The knowledge that teachers develop with experience is personal and therefore also difficult to measure. However there is some commonality in the way that expert teachers transform content knowledge for teaching, known as pedagogical content knowledge (pck). This kind of knowledge is a unique from other as it talks selectively to the practice of teaching (Shulman, 1986). When applied to a given topic, e.g. electricity, electrochemistry, etc. it assumes the specificity of the topic and thus differs from the general application within the discipline. We have called it Topic Specific pck (Mavhunga and Rollnick, 2013). Topic Specific pck (tspck) assists teachers to consider the specific information about the content knowledge of the topic in relation to prior learner knowledge, structure of the topic in terms of most important core concepts distinguished from subordinate concepts as well as pre-concepts needed to teach each of the core concepts. We have argued previously (Mavhunga and Rollnick, 2013), that when teachers reason about the teaching of a topic by considering the aspects of content knowledge listed above, they transform the content knowledge and thereby develop the unique knowledge for teaching the topic (tspck) in the process. In this study we determine the teacher knowledge bases associated with the ability to transform content knowledge in the topic of electrochemistry for effective learning. The need for the study follows the observed poor national performance of learners in chemistry in the final school National Examination in South Africa, specifically in questions on electrochemistry. We explored measurement of the ability of groups of teachers in the Johannesburg area of the Gauteng province from different socio-economic secondary schools in South Africa to transform concepts in this topic in planning for teaching. We therefore ask specifically:
What is the quality of the ck and tspck of a diverse group of South African teachers in the topic of electrochemistry and how do these two forms of knowledge relate to each other?
Literature ReviewTo answer the above question we review learning difficulties in electrochemistry and the meaning of ck. We then examine the literature on pck to come up with our framework of topic specific pck, and review work on the measurement of pck.
Learning difficulties in electrochemistryElectrochemistry has been regarded as one of the most difficult chemistry topics in which both students and teachers have difficulties (Nakhleh, 1992; Ogude and Bradley, 1994; Sanger and Greeenbowe, 1997). Although much work has been done in identifying learning difficulties, learners continue to experience the same difficulties in today’s classrooms.
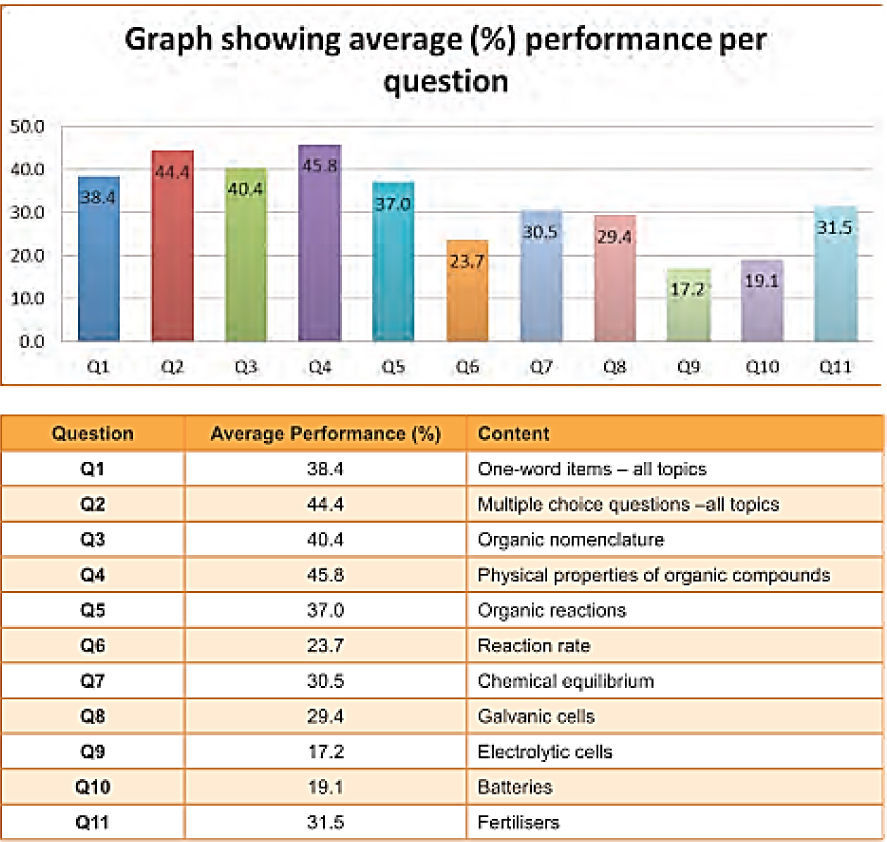
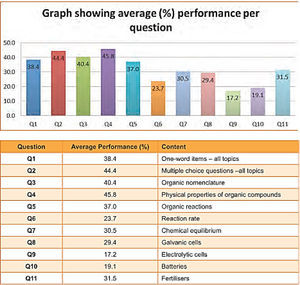
Electrochemistry is an important topic in chemistry as it has many applications from battery development to neuroscience and brain research (Miller, 2014). It also underpins later topics in the curriculum and consolidates earlier ones, having links to thermodynamics, rate of reaction and chemical equilibrium. The work of Ogude and Bradley (1994) shows that even college students have difficulties with the qualitative interpretation of the microscopic processes that take place in operating chemical cells. Also Sanger and Greenbowe (1997) report that students find this topic difficult and have beliefs about the complexity of this section that influence their performance and learning. There are four specific areas that appear to present the greatest difficulties and these are classified by Ogude and Bradley (1994) as conduction in the electrolyte, electrical neutrality, electrode processes and terminology, aspects relating to the cell components, current and EMF. The authors point to the inconsistent use of language in the textbooks and in the classrooms by teachers as one of the sources of misconceptions. Misconceptions in the above listed four areas include among others the notion that water is not reactive in the electrolysis of aqueous solutions, the belief that electrons flow through the electrolyte and salt bridge to complete a circuit and the negative and the positive signs which are assigned to electrodes represent net electron charges. There is consensus in the literature that these learning difficulties are common in learners across different countries including learners in the final year (Grade 12) of the South African Secondary Schools system. According to the National Curriculum in South Africa, electrochemistry is a major topic in the grade 12 chemistry curriculum. Similar to the findings in the literature, learners at this grade are reported to perform poorly in questions on this topic. The report on the national examinations (Department of Basic Education, 2014) shows that electrochemistry is one of the most poorly answered questions as shown in Figure 1.
Reasons for the poor performance have been attributed among other things to poor school management, but more prevalently to poor teacher preparation and thus poor understanding of content concepts by teachers. A construct that explicitly considers knowledge for teaching specific topics, exploring common misconceptions as part of learner prior knowledge is tspck. Thus in this study, we explore the quality of tspck in electrochemistry in four groups of teachers who are teaching the topic. In the discussion below we define and address issues of measurement of tspck.
Defining tspckGood teaching is not only about knowledge but also the capability to reason soundly about teaching. Sound reasoning by teachers requires both a process of thinking about their actions and a sufficient repertoire of content, principles and experience from which to reason Shulman (1987). The pedagogical reasoning and action framework, developed by Shulman (1987) provides a process of reasoning about teaching. It consists of aspects of reasoning, starting with comprehension of content knowledge, followed by its transformation, then the actual instruction, evaluation, reflection and finally new comprehension. Key to Shulman’s framework is the element of transformation of content knowledge. According to Shulman “[teacher’s]…. comprehended ideas must be transformed in some manner if they are to be taught” (Shulman, 1987, p. 16). We have expanded on the idea of reasoning leading to transformation of content by identifying five knowledge components which when used to reason through concepts of a topic, transformation of content emerges (Mavhunga and Rollnick, 2013). These five knowledge components are explained in detail in Geddis (1993) as: (i) Students’ Prior Knowledge, (ii) Curricular Saliency, (iii) What is difficult to teach, (iv) Representations and (v) Conceptual teaching strategies. The responses generated from reasoning through concepts using the listed knowledge components as a collective, reflect the extent of understanding knowledge for teaching the topic, which is tspck. We thus defined tspck as the capacity to transform content knowledge in a specific topic. This description of tspck is in line with the consensus definition of professional teaching knowledge presenting the construct as ‘the knowledge of, reasoning behind, planning for, and enactment of teaching a particular topic in a particular way for a particular reason to a particular students for enhanced student outcomes’(GessNewsome and Carlson, 2013). The knowledge generated in reasoning a topic through these five components differs from topic to topic. The idea of topic specific rather than generic pck has been confirmed empirically in the literature from a number of studies. For example, in a Turkish study with two experienced chemistry teachers in the topics of electrochemistry and radioactivity Aydin (2012) discovered two different types of pck one for each topic, named pck A for teaching electrochemistry and pck B for teaching radioactivity. Luft, Hill, Weeks, Raven, and Nixon (2013) found that life science teachers displayed more limited pck when teaching topics out of their field than when teaching in field. This further illustrates the topic specificity of pck.
Measuring tspckLike general pck, the measurement of the quality of tspck remains challenging as the knowledge measured is abstract and embedded in the minds and experiences of teachers. Previous efforts in the literature include capturing of the teacher’s thinking through the use of a template with specific teacher prompts called Content Representations (CoRe) accompanied by Professional-experience Repertoires (PaPeRs) which provide qualitative reflections and further insight into the teacher’s planning of a lesson (Loughran, Mulhall, and Berry, 2004). Both the CoRe and the PaP-ers are qualitative in nature thus are more suitable for capturing than measurement. There has been a steady growth of pck tools in the literature that attempt to measure pck for example Rohaan, Taconis, and Jochems (2009) in science subjects, Riese and Reinhold (2009) in Physics, Tepner and Witner (2011) in chemistry and Jüttner, Boone, Park, and Neuhaus (2013) in Biology. Veal and MaKinster (1999) refer to models describing pck at the discipline level using taxonomies. These models are therefore epistemologically unsuitable for measurement at a topic specific level.
In this study we used a tspck instrument in electrochemistry developed by Ndlovu (2014) Similar tools have been designed and used by Mavhunga and Rollnick in chemical equilibrium (2013) and Davidowitz and Vokwana in organic chemistry (2014).
Defining content knowledge (ck)Content knowledge of teachers is less often unpacked than pck suggesting that most authors consider knowledge of content to be self-explanatory. Differing terms are used in both mathematics and science education literature. In his first account of pck, Shulman talks of three kinds of content knowledge — “subject matter content knowledge (sic), pedagogical content knowledge and curricular knowledge” (Shulman, 1986, p. 9). Later in a more comprehensive account, he lists content knowledge rather than “subject matter content knowledge” as one of seven categories for a teacher’s knowledge base again alongside pck and curriculum knowledge. In mathematics education, Ball, Thames, and Phelps (2008) interpret content knowledge as knowledge of the subject and its organising structure. Their interpretation includes Schwab’s distinction of syntactic and substantive knowledge structures (Schwab, 1978 in Shulman, 1986) as well as later work by Grossman, Wilson, and Shulman (1989) which brings in a further two categories — knowledge of content and beliefs about a discipline. Ball et al. (2008) consider it difficult to untangle the kind of mathematics used in teaching from mathematics content per se (Shulman, 1986) and evolve a special brand of content knowledge, termed mathematical knowledge for teaching, which they refer to as specialised content knowledge. Their concept is closer to topic specific pck, discussed above.
In science education, a useful breakdown emerges from Cochran and Jones (1998, p. 708) who reviewed research on subject matter knowledge or pre-service teachers. They suggest an umbrella conception of subject matter knowledge (SMK) which includes content knowledge (considered as the facts and concepts of SMK), substantive knowledge (explanatory structures or paradigms of the field), syntactic knowledge (methods and processes of generating new knowledge in the field) and beliefs about subject matter. This distinction is also favoured by Kind and Kind (2011) while Abell (2007) chooses to combine substantive and content knowledge.
In this paper, syntactic knowledge and beliefs about science are not under consideration, as the focus is on teachers’ understanding of “central ideas, relationships, elaborated knowledge and reasoning ability” (Abell, 2007, p. 1110), so the term “content knowledge” is used collectively for content knowledge and substantive knowledge for the topic of electrochemistry using Abell’s description above.
MethodThe research design was based on mixed-methods (MM). Mixed-Methods includes aspects of both qualitative and quantitative methods research and has over the years become more common in studies of individual behaviours and more social phenomena (Teddlie and Tashakkori, 2009). We employed case study as a research strategy as it allows targeted in-depth explorations of interactions within the groups of the sample.
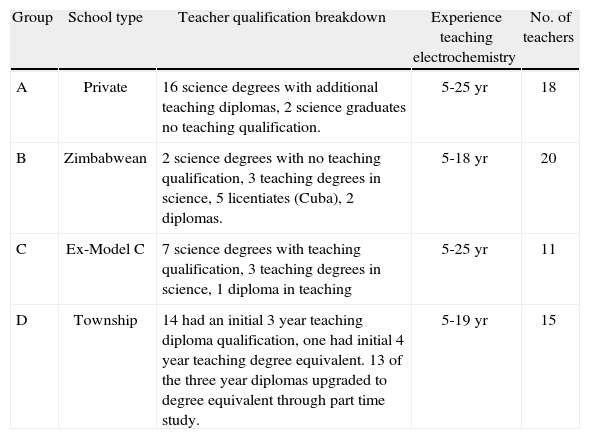
SampleThe sample consisted of four distinct groups of teachers, making a total of n = 64. They were all drawn from the Province of Gauteng, which is the most economically active province in South Africa, including the cities of Johannesburg and Pretoria. The province is continually increasing in population due to influx of people from the rural provinces and neighbouring countries. The teachers were all experienced secondary school chemistry teachers who have been teaching the topic of electrochemistry for at least five years. An important reason for the requirement of experienced teachers is the understanding that teachers’ pck improves with practice (Loughran et al., 2004). Therefore the sample would reflect the quality of tspck in electrochemistry in teachers across the Gauteng Province with reasonable validity. The sample was divided into four groups three of which describe the socio-economic background of the types of schools in South Africa and one which represents a specific group of teachers of interest to policy makers and those involved in professional development. Group A consisted of 18 teachers from independent schools. These are schools that are considered private schools, while registered with the National Department of Basic Education, they are funded privately, located largely in the affluent areas of the Province and their school exit examination is independent from the national secondary school exit examination. The second group, group B comprised of 20 experienced chemistry teachers who are Zimbabwean expatriates, teaching in diverse public schools in Gauteng. The Zimbabwean expatriate teachers are a unique group in a sense that they are perceived to have been exposed to a higher quality of science education than their South African counterparts. The third group, group C are 11 teachers sampled from ex-model C schools. These are public schools were exclusively accessible to the white communities under apartheid. They are known for having well equipped infrastructure and perceived to have qualified science teachers. These schools have since been opened to all communities and many have experienced a swing in demographics now reflecting a high population of black learners and teachers. The last group, group D, were 15 experienced chemistry teachers from disadvantaged communities located in the black settlements known as townships. These schools are often under-resourced and characterized by feeding scheme programmes, and learners exempted from paying school fees because of non-affordability.
The range of qualifications and experience of the teachers is summarized for the 4 groups in Table 1.
Range of Qualifications and experience of teachers in the study.
| Group | School type | Teacher qualification breakdown | Experience teaching electrochemistry | No. of teachers |
| A | Private | 16 science degrees with additional teaching diplomas, 2 science graduates no teaching qualification. | 5-25 yr | 18 |
| B | Zimbabwean | 2 science degrees with no teaching qualification, 3 teaching degrees in science, 5 licentiates (Cuba), 2 diplomas. | 5-18 yr | 20 |
| C | Ex-Model C | 7 science degrees with teaching qualification, 3 teaching degrees in science, 1 diploma in teaching | 5-25 yr | 11 |
| D | Township | 14 had an initial 3 year teaching diploma qualification, one had initial 4 year teaching degree equivalent. 13 of the three year diplomas upgraded to degree equivalent through part time study. | 5-19 yr | 15 |
All the groups had similar ranges of teaching experience and most were qualified to teach the subject in South African terms. Those who would have been considered unqualified would have a science degree with no teaching qualification such as the two private school teachers mentioned in table 1. However the most visible difference between the groups is that almost all the township teachers had a three year teaching diploma obtained from teacher colleges which were all closed in the late 1990s. These apartheid era teacher colleges were characterised by rote based teaching and little exposure to practical work. It is also of interest that none of the teachers in the other groups were qualified in this way. The township teachers had all upgraded to degree status through part time study for a qualification called an Advanced Certifícate in Education which offered varying levels of content knowledge instruction depending on the institution. This combination of qualifications would provide these teachers with the equivalent content knowledge of freshman chemistry in the US context.
Tools used in the studyTeachers in these categories were given two research instruments. The first instrument was a diagnostic content instrument that determines the quality of the content knowledge (ck) in the topic of electrochemistry. The second instrument was a tspck tool in electrochemistry measuring teachers understanding about teaching the topic.
The ck instrument consisted of 21 mainly multiple choice questions covering core concepts of electrochemistry including spontaneous and non-spontaneous reactions, redox reactions, galvanic and electrolytic cells and the difference between them, the understanding of processes and reactions taking place in these cells, electrical neutrality and half-cell reactions and electrode potential. Items used in the instrument were taken from reported tools in the literature (e.g. Ogude and Bradley, 1996) therefore their credibility was regarded as established. The duration of the ck instrument was found to be about an hour.
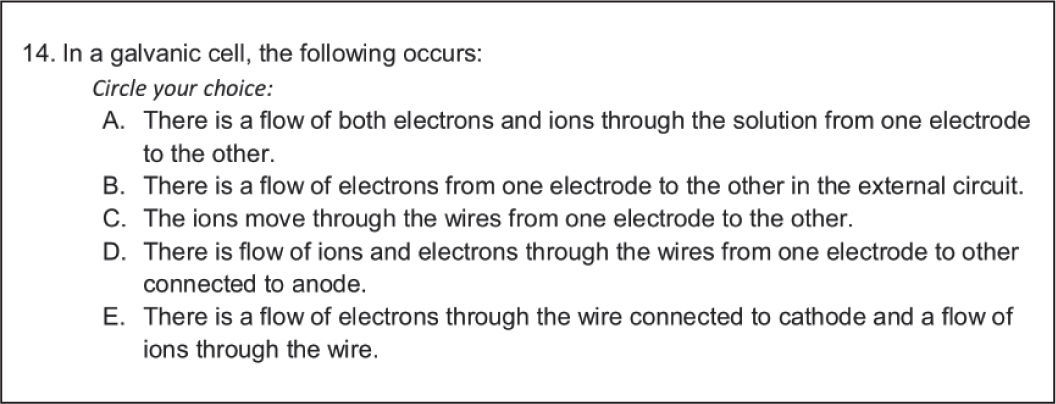
A sample item from the ck instrument on maintaining cell neutrality is shown in Figure 2.
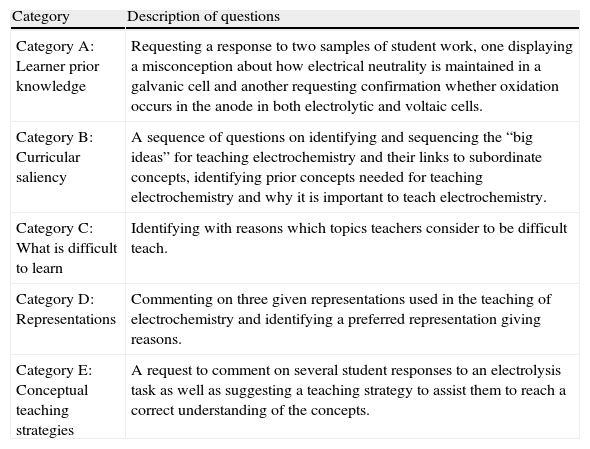
The tspck instrument had five test items corresponding to the tspck categories outlined above, viz., These five knowledge components are explained in detail in Geddis (1993) as: (i) Students’ Prior Knowledge, (ii) Curricular Saliency, (iii) What is difficult to teach, (iv) Representations and (v) Conceptual teaching strategies. Each item consisted of one or more questions as shown in Table 2.
Description of questions designed for the various components of tspck
| Category | Description of questions |
| Category A: Learner prior knowledge | Requesting a response to two samples of student work, one displaying a misconception about how electrical neutrality is maintained in a galvanic cell and another requesting confirmation whether oxidation occurs in the anode in both electrolytic and voltaic cells. |
| Category B: Curricular saliency | A sequence of questions on identifying and sequencing the “big ideas” for teaching electrochemistry and their links to subordinate concepts, identifying prior concepts needed for teaching electrochemistry and why it is important to teach electrochemistry. |
| Category C: What is difficult to learn | Identifying with reasons which topics teachers consider to be difficult teach. |
| Category D: Representations | Commenting on three given representations used in the teaching of electrochemistry and identifying a preferred representation giving reasons. |
| Category E: Conceptual teaching strategies | A request to comment on several student responses to an electrolysis task as well as suggesting a teaching strategy to assist them to reach a correct understanding of the concepts. |
An example, of a test item in category A is shown in Figure 3.
The test items focus on the teaching of the concepts rather than the correctness of the concepts and contain no wrong content knowledge. They are located in a specific teaching context so as to focus on teacher tasks. The questions are semi-open allowing the respondent to choose and/ or expand on their response in their own words.
Data collection and analysisThe instruments were administered to individual educators using a combination of prior arrangements with groups and individuals. The completed questionnaires were coded and kept within the respective teacher groups for scoring. A memorandum of correct answers was used to score the ck instrument.
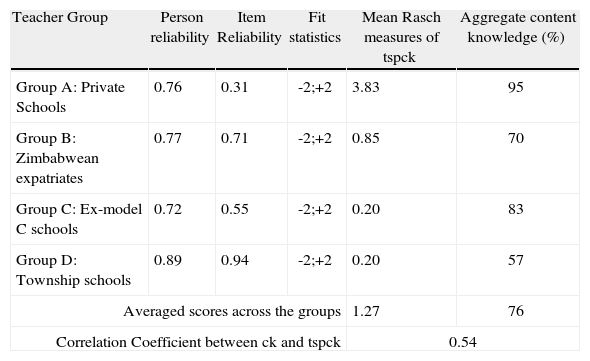
The tspck instrument was scored using a rubric corresponding to the five components with each being rated on a four point scale reflecting the quality of tspck as: ‘limited’ assigned a score of 1; ‘Basic’ a score of 2; ‘Developing’ a score of 3 and ‘Exemplary’ a score of 4. The scoring of each test in each of the teacher groups was validated by three raters producing agreement in 85% of the scores. The analysis of the generated scores was done using the Rasch statistical model (Winstep, version 3.72.3). The reliability indices as indicated by person reliability and item reliability for each group are found to be high and acceptable as shown in Table 4.
Reliability and Validity of generated scores.
| Teacher Group | Person reliability | Item Reliability | Fit statistics | Mean Rasch measures of tspck | Aggregate content knowledge (%) |
| Group A: Private Schools | 0.76 | 0.31 | -2;+2 | 3.83 | 95 |
| Group B: Zimbabwean expatriates | 0.77 | 0.71 | -2;+2 | 0.85 | 70 |
| Group C: Ex-model C schools | 0.72 | 0.55 | -2;+2 | 0.20 | 83 |
| Group D: Township schools | 0.89 | 0.94 | -2;+2 | 0.20 | 57 |
| Averaged scores across the groups | 1.27 | 76 | |||
| Correlation Coefficient between ck and tspck | 0.54 | ||||
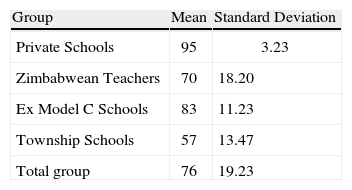
The ck instrument yields a score that reflects achievement and is obtained when the participant has chosen a correct response. The scores were expressed as a percentage. Table 3summarises the scores of the group as a whole and those of the different groups.
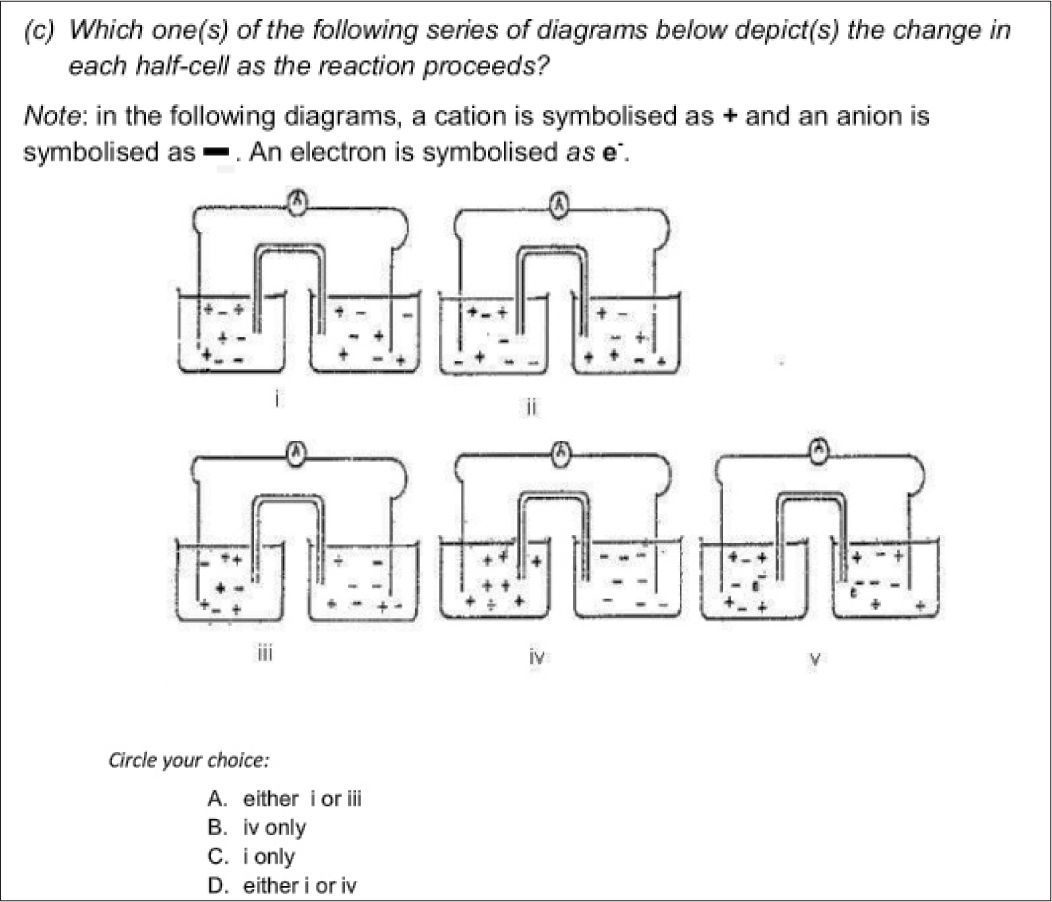
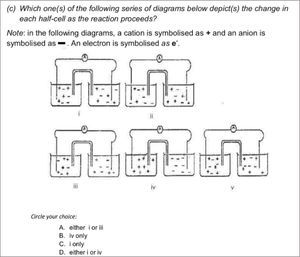
Two issues are immediately apparent from this table. Firstly the scores are very diverse and secondly the Private school and model C scores show less variation than the Zimbabwean teachers’ scores. Given that the content tool is testing no more than what the students are expected to learn, the scores of the township teachers are a cause for concern. The township teachers’ scores need to be considered in the light of their very different teaching qualifications as seen in Table 1. There was however one question that presented challenges to teachers in all the groups including the private school teachers. This question related to understanding cell neutrality and was taken from the work of Ogude and Bradley (1994). The question is shown in Figure 4.
The correct answer for this question is C as it is the only representation that reflects equal numbers of charges in both cells and does not show any electrons in solution, a common misconception. Most teachers struggled with the notion that neutrality will be maintained in both half cells throughout the electrode process. Some of the private school teachers complained about the quality of the diagram, but the symbols were clearly explained. The performance of teachers on this task shows that the idea of balance of charges in the two half cells of an electrochemical cell still causes problems for almost all teachers in the sample.
Topic specific pck (tspck)As explained above the tspck tool was subjected to Rasch analysis as the Rasch analysis converts the ordinal data (values 1, 2, 3 and 4) to continuous values on a linear scale with a mean set at zero. The Rasch measures obtained in this way can then be used to calculate reliability and validity measures and the person scores thus obtained can be compared across the sample. Table 4 shows the Rasch measures for the sample together with person and item reliability, fit and mean statistics. The score on the content tool is reproduced for convenience.
The persons and item scores reflect measures well inside the conventionally acceptable range of -2 and +2 for all teacher groups. The scores presented in Table 4 indicate that teachers from the private schools scored highest, well above the group mean, in both the ck tool and in the tspck tool. This means that they possess good understanding of the content concepts in electrochemistry and the knowledge for teaching them in comparison to their counterparts around Gauteng Province. The results also show expatriates from Zimbabwe to have performed better in their tspck scores than teachers from the township and ex model C schools though it should be borne in mind that they would be teaching in both these school types. Teachers from both ex-Model C and township schools seem to have the same level of knowledge for teaching tspck despite the difference in their ck scores.
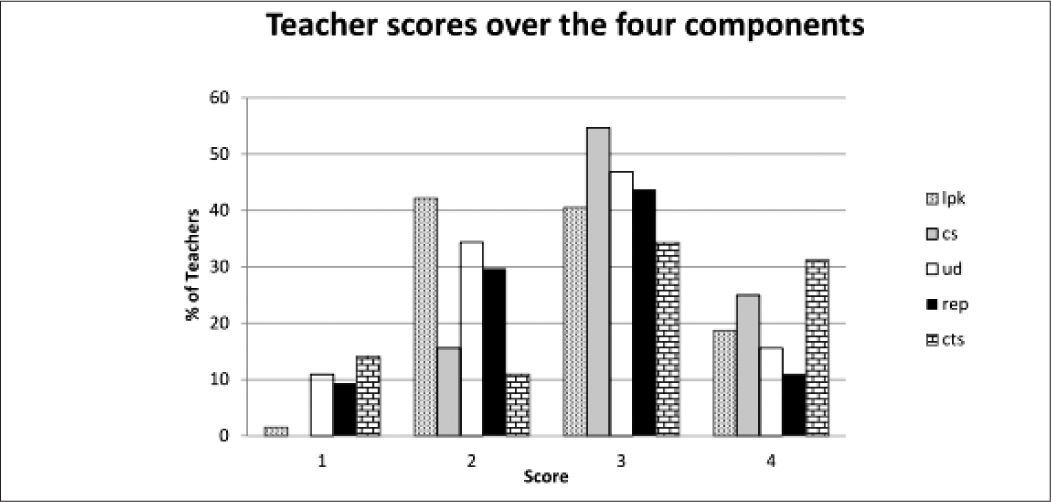
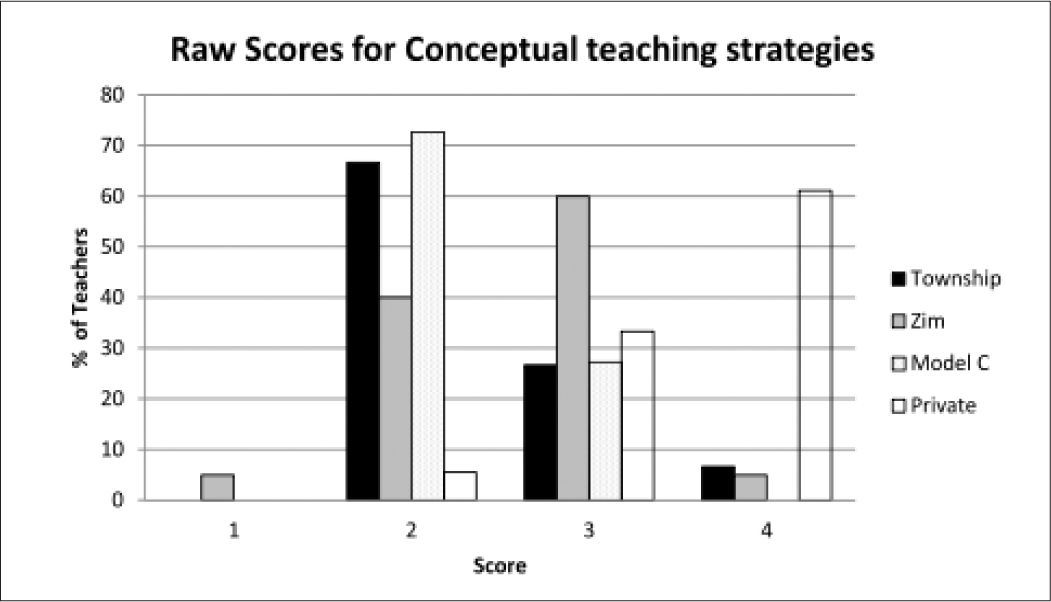
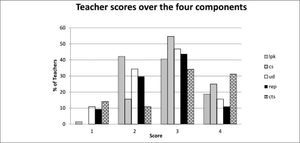
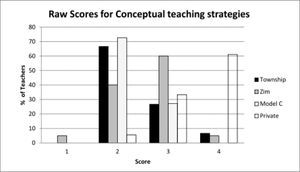
It is also useful to examine the distribution of the raw scores which give a qualitative picture of the status of the teachers’ topic specific pck. Figure 5 shows the distribution of the whole group of teachers across the five components showing the percentage of teachers placed at limited (1), basic (2), developing (3) and exemplary (4) levels.
As can be seen, the scores centre around the developing level (3) with learner prior knowledge (lpk) yielding the lowest scores with most teachers on the basic level (2). Curricular saliency (cs) which includes the ability to recognise big ideas shows the least spread and is concentrated at the developing level. Surprisingly the more complex component of conceptual teaching strategies (cts) showed a high proportion of teachers at developing and exemplary level. Here the presence of the private school teachers was an important factor as shown by the breakdown in Figure 6.
The ability to use conceptual teaching strategies requires integration of all the other components and hence should provide the most challenge for teachers as it requires use of the other four components as shown by the extract below from the rubric for scoring it in Table 5.
Rubric for scoring Conceptual Teaching Strategies.
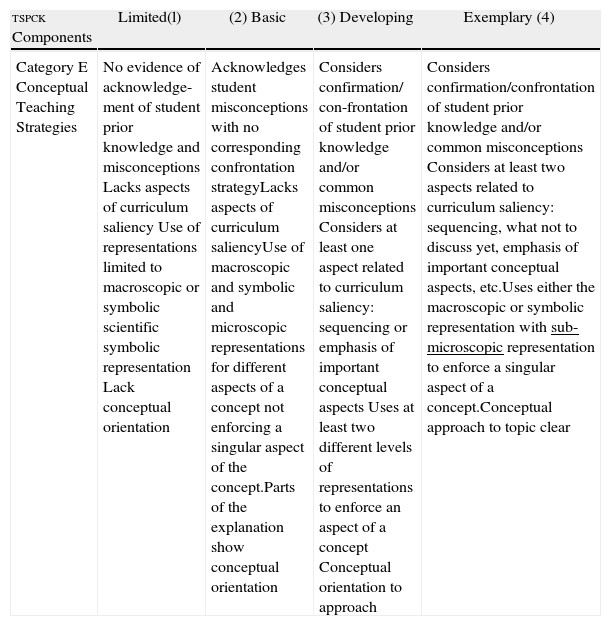
| tspck Components | Limited(l) | (2) Basic | (3) Developing | Exemplary (4) |
| Category E Conceptual Teaching Strategies | No evidence of acknowledge-ment of student prior knowledge and misconceptions Lacks aspects of curriculum saliency Use of representations limited to macroscopic or symbolic scientific symbolic representation Lack conceptual orientation | Acknowledges student misconceptions with no corresponding confrontation strategyLacks aspects of curriculum saliencyUse of macroscopic and symbolic and microscopic representations for different aspects of a concept not enforcing a singular aspect of the concept.Parts of the explanation show conceptual orientation | Considers confirmation/ con-frontation of student prior knowledge and/or common misconceptions Considers at least one aspect related to curriculum saliency: sequencing or emphasis of important conceptual aspects Uses at least two different levels of representations to enforce an aspect of a concept Conceptual orientation to approach | Considers confirmation/confrontation of student prior knowledge and/or common misconceptions Considers at least two aspects related to curriculum saliency: sequencing, what not to discuss yet, emphasis of important conceptual aspects, etc.Uses either the macroscopic or symbolic representation with sub-microscopic representation to enforce a singular aspect of a concept.Conceptual approach to topic clear |
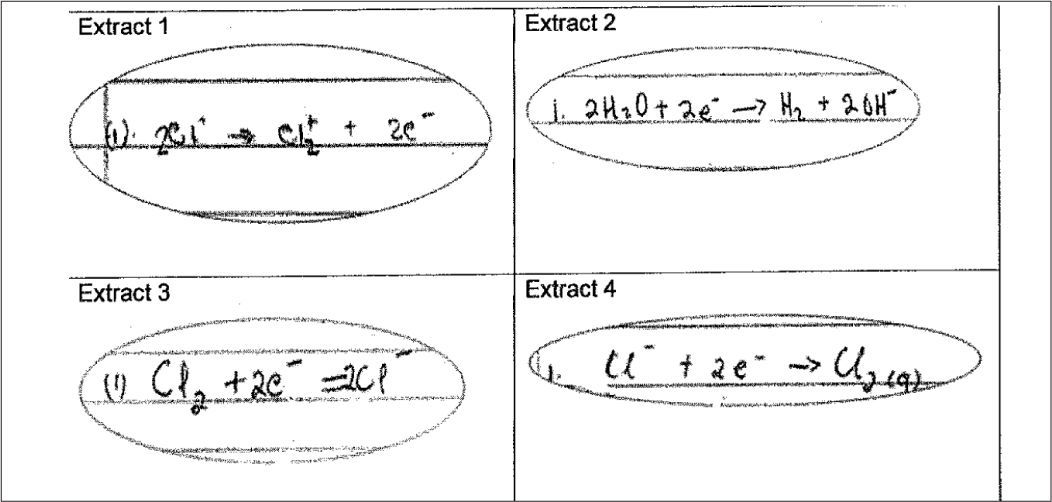
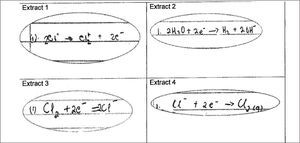
The question in the tspck tool targeting conceptual teaching strategies required teachers to analyse student responses and provide a strategy to respond to them. Students asked to provide an equation describing the half reaction at the anode of an electrolytic cell in the chloralkali process provided the following responses (see Figure 7).
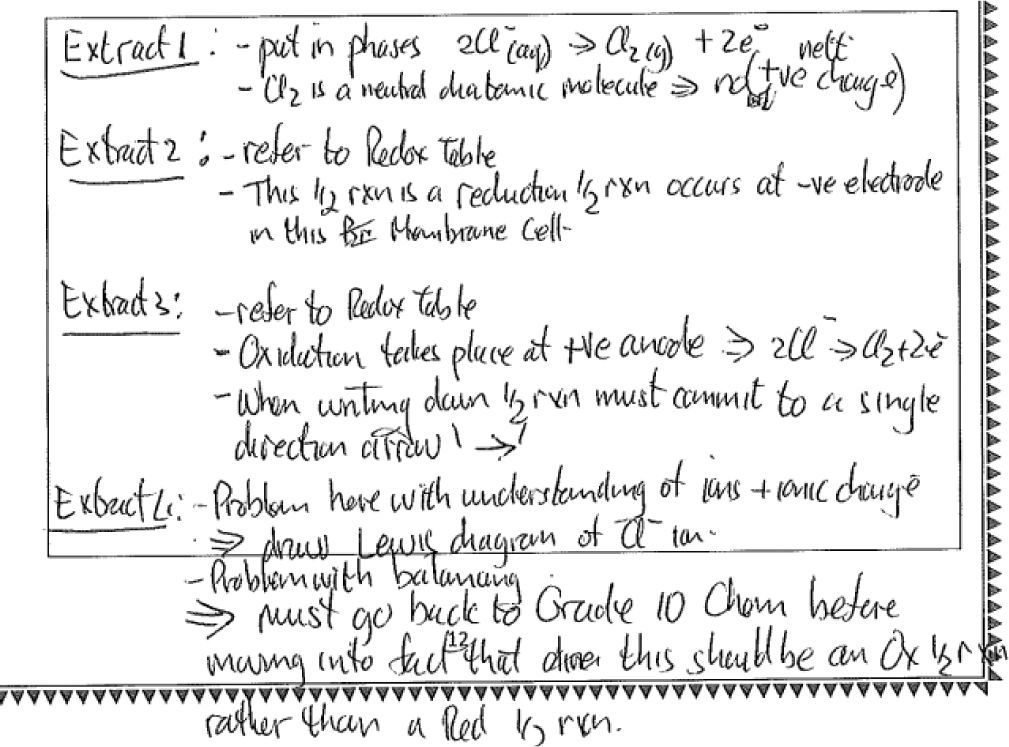
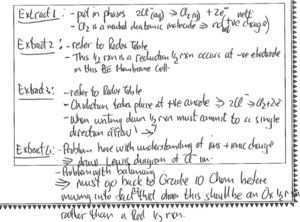
Figure 8 shows a response to the above task that was scored at the exemplary level (4).
The response to extract 4 in Figure 8 shows the teacher’s awareness of learner prior knowledge, common difficulties and thus what constitutes appropriate or inappropriate errors from learners at this level and thus knowledge of prior concepts in the curriculum. The teacher has diagnosed the student’s difficulty in understanding charges on ions and proposes working on a Lewis diagram with the student to try and assist with this problem. There is a further suggestion to return to grade 10 work on balancing equations and distinguishing between oxidation and reduction half reactions. The proposed course of action is a considered result of many factors which include learner prior knowledge, curricular saliency and symbolic representations.
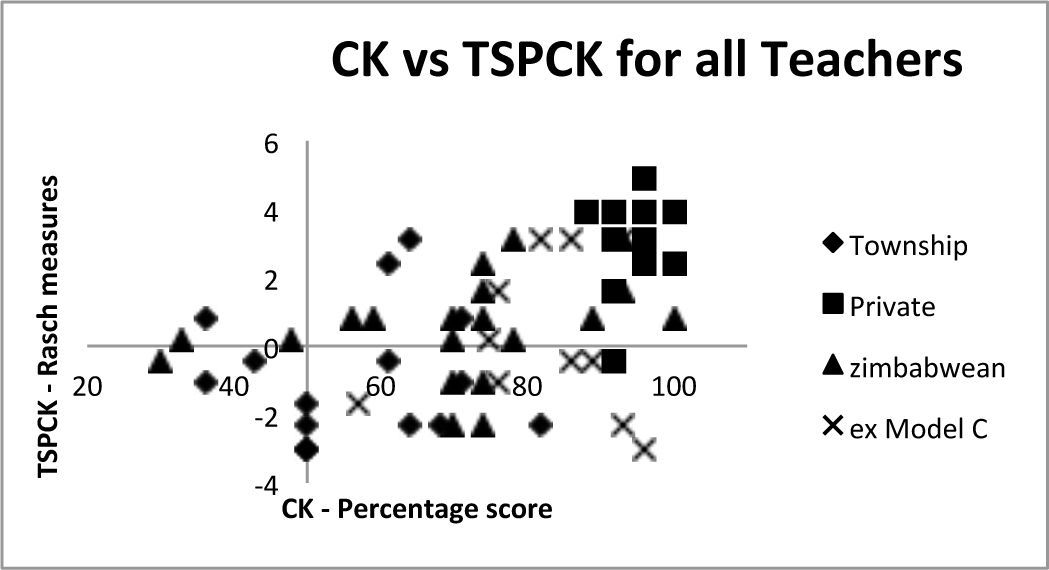
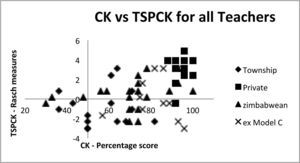
Relationship between ck and pckAs table 4 shows there is a moderate correlation of 0.54 between the Rasch measures for the tspck and the ck scores. Given that ck is a necessary precursor for tspck, we do not expect any teachers with low ck scores and high tspck scores. However observations of high ck scores associated with low tspck scores are possible in teachers who lack the capacity to transform content knowledge into teachable form. Equally it is possible to find low scores in both constructs. Figure 9 below shows a scatter gram where an acceptable level of ck has been arbitrarily set at 50% and the Rasch measures for tspck are centred around 0. The regions above these values have been designated as high ck and tspck respectively and correspondingly below these values are considered as low ck and pck.
As can be seen most of the teachers fall into the top right hand quadrant as should be expected in teachers who have taught the topic for 5 years or more. However, about a third of the teachers (19) fall into the bottom right hand quadrant, suggesting that they have reasonable ck but poor tspck. 6 teachers in the whole sample scored below 50% in the ck test and one of them had a tspck score slightly above the 0 Rasch value. Two were on the zero line.
It is worth noting that only three of the township teachers fell into the top right hand quadrant. Their ck mean was significantly lower than all the other groups but Figure 9 suggests that even those with moderate to high ck scored low on the tspck suggesting that their knowledge about teaching the content in a conceptual way is poor. On the other end of the spectrum the very high scores of the private school teachers are reinforced with good tspck with one exception.
Discussion and conclusionsIn the discussion above we have defined tspck as the knowledge for teaching a topic by transforming its core concepts into versions that are teachable. According to Shulman transformation of content concepts is important for teaching, as seen in his statement: “[teacher’s]… comprehended ideas must be transformed in some manner if they are to be taught” (Shulman, 1987, p. 16). Following the observed poor performance of students in chemistry in the National Examination, specifically in questions on electrochemistry, we explored measurement of the ability of the teachers in the Gauteng province to transform concepts in these topics in planning for teaching. The results indicates that teachers across the different types of public schools scored below the mean of Rasch measures for tspck of 1.27 as compared to their counterparts in the private schools. While their achievement scores in the ck tool was found to be acceptable and above a mean of 76%, with the exception of the group of teachers from previously disadvantaged township schools, the ability to transform concepts in the topic of electrochemistry was generally lacking. These findings are in line with those reported in the literature that while ck is necessary for the development of pck, it is however, not an automatic guarantee for the existence of the knowledge for teaching a topic (Kind, 2009). The value of this study lies in establishing a baseline of tspck in teachers to inform future teacher development programmes, as the country continue in the effort to improve the quality of science education in schools.
The instruments and their results may also be of value to researchers in other countries who wish to establish baseline knowledge of teachers in electrochemistry. The use of tools like the one in this study has the advantage of requiring less testing time than would normally be required to obtain such information from teachers. However teachers found the tspck instrument onerous to complete and ways need to be found to gain their commitment in doing so.