Frasier syndrome is a genetic disorder produced by a mutation in intron 9 of the WT1 gene, responsible for renal and genital dysfunctions.
Clinical findingsIt is characterized by discrepancy between the individual karyotype and the individual phenotype and corticosteroid-resistant nephrotic syndrome due to focal segmental glomerulosclerosis. Patients usually have a female phenotype with a 46 XY karyotype, which increases the risk of gonadoblastoma in 50% of cases. Kidney disease requires kidney transplantation in adulthood. Cardiovascular and bone-derived comorbidities such as hyperlipidaemia and osteopenia/osteoporosis, respectively, are also common.
Main diagnosesMutations of the WT1 gene can lead to different clinical entities, most notably Denysh-Drash syndrome, Frasier syndrome, or isolated focal segmental glomerulosclerosis. We present a clinical case of a woman who debuted in childhood with difficult-to-control nephrotic syndrome, the lack of pubertal development, primary amenorrhoea and the absence of ovaries on imaging tests in adolescence, alerted to an underlying genetic problem that, after cytogenetic studies, allowed a diagnosis of Frasier syndrome.
Therapeutic interventionsIt is recommended to remove the gonads due to increased risk of developing gonadoblastoma. Treatment of associated dyslipidaemia and osteopenia is also necessary.
ConclusionFrasier syndrome is an unusual cause of infertility due to gonadal dysgenesis and is associated with kidney problems.
El síndrome de Frasier es un trastorno genético producido por una mutación en el intrón 9 del gen WT1, responsable de disfunciones a nivel renal y genital.
Principales síntomasSe caracteriza por disgenesia gonadal con discrepancia entre cariotipo-fenotipo y síndrome nefrótico resistente a corticoides debido a glomeruloesclerosis focal y segmentaria. Las pacientes presentan habitualmente fenotipo femenino con cariotipo 46 XY, lo que aumenta el riesgo de gonadoblastoma en un 50% de los casos. La enfermedad renal obliga a trasplante renal en la edad adulta. Son habituales también las comorbilidades derivadas a nivel cardiovascular y óseo como hiperlipidemia y osteopenia/osteoporosis, respectivamente.
Diagnósticos principalesLas mutaciones del gen WT1 pueden conducir en distintas entidades clínicas entre las que destaca el síndrome de Denys-Drash, el síndrome de Frasier o la glomeruloesclerosis focal y segmentaria aislada. Se presenta un caso clínico de una mujer que debutó en la infancia con síndrome nefrótico de difícil control y que, durante la adolescencia, ante la falta de desarrollo puberal, la amenorrea primaria y la ausencia de ovarios en las pruebas de imagen alertaron de un problema genético subyacente que, tras estudios citogenéticos, permitió el diagnóstico de síndrome de Frasier.
Intervenciones terapéuticasSe recomienda la exéresis de las gónadas debido al riesgo incrementado de gonadoblastoma. El tratamiento de la dislipemia y la osteopenia asociadas también es necesario.
ConclusiónEl síndrome de Frasier es una causa inusual de infertilidad debido a una disgenesia gonadal y se asocia con problemas a nivel renal.
Frasier syndrome is a rare condition included within the genetic disorders of discrepancy between the individual karyotype and the individual phenotype, with a prevalence of less than 1/1,000,000 and 150 described cases worldwide to date.1 The two defining clinical characteristics are gonadal dysgenesis associated with renal glomerulopathy.1 Patients usually have a female phenotype with poor pubertal development and a male genotype with a 46XY karyotype, as well as nephrotic syndrome with childhood onset, generally due to focal and segmental glomerulosclerosis.2 Frasier syndrome is inherited in an autosomal dominant pattern and, in most cases, is due to de novo mutations in the WT13 gene. There is an increased risk of gonadoblastoma, so prophylactic removal of the gonad is recommended.3
The following is the clinical case of a patient who debuted with a difficult-to-control nephrotic syndrome during childhood and who, later in adolescence, was referred to gynecology consultation for primary amenorrhea. The diagnostic and therapeutic management of pathology at renal and genital level are addressed, as well as comorbidities associated with early ovarian failure at the genitourinary, cognitive, bone and cardiovascular level. It is an interesting case due to its infrequency and that we must take into account in women with primary amenorrhea and kidney problems.
Case reportPatient informationA 10-year-old woman, with no relevant family history, presenting with frank proteinuria and associated hypoalbuminemia. From Nephrology, treatment with low-dose corticosteroids is established, which achieves partial remission of the condition. Blood cell count, kidney function, and serum C3 and C4 complement levels were normal. Subsequently, she presented a relapse of the nephrotic syndrome, for with a renal biopsy was performed, which was reported as mild and diffuse proliferative mesangial glomerulonephritis, with diffuse IgM deposits 38% glomerulosclerosis, moderate atherosclerosis, and interstitial fibrosis with mild/moderate tubular atrophy. At that time, immunosuppressive treatment with Mycophenolate was started, as well as double blockade with Ramipril 10mg/day and losartan 50mg/12h, managing to control the symptoms.
Clinical findingsAt 17 years of age, she was referred to gynecological endocrinology consultations due to primary amenorrhea and poor pubertal development. She reported pubarchy at 14 years old and thelarchy al 16 years old. Gynecological examination revealed normal female external genitalia, with well-shaped vagina and cervix but breasts stadium Tanner 2. She presented hormonal determinations at menopausal range (FSH 192IU/L, LH 88IU/L, estradiol 19pg/ml, AMH 0.06pM) and hypercholesterolemia at the expense of LDL cholesterol and hypertriglyceridemia. Menarche was induced with oral contraceptives (levonorgestrel 0.5mg and estradiol 2mg/day).
Diagnostic evaluationThe vaginal ultrasound revealed a retroverted uterus of 57mm×27mm×34mm (Fig. 1). Internal contour showed a 7mm indentation compatible with septum. Endometrial thickness was 5mm. Right ovary was nor visualized, nor left one. No free liquid was observed at Douglas space. A pelvic magnetic resonance was requested and absence of the ovaries was confirmed (Fig. 2). At that time, due to the suspicion of an underlying syndromic alteration, a genetic study was requested and reported a 46XY karyotype as well as a heterozygous mutation of variant c 1432+4C>T in intron 9 of the WT1 gene (IVS9+4C>T; NM:024426.4), which was diagnostic of Frasier syndrome.
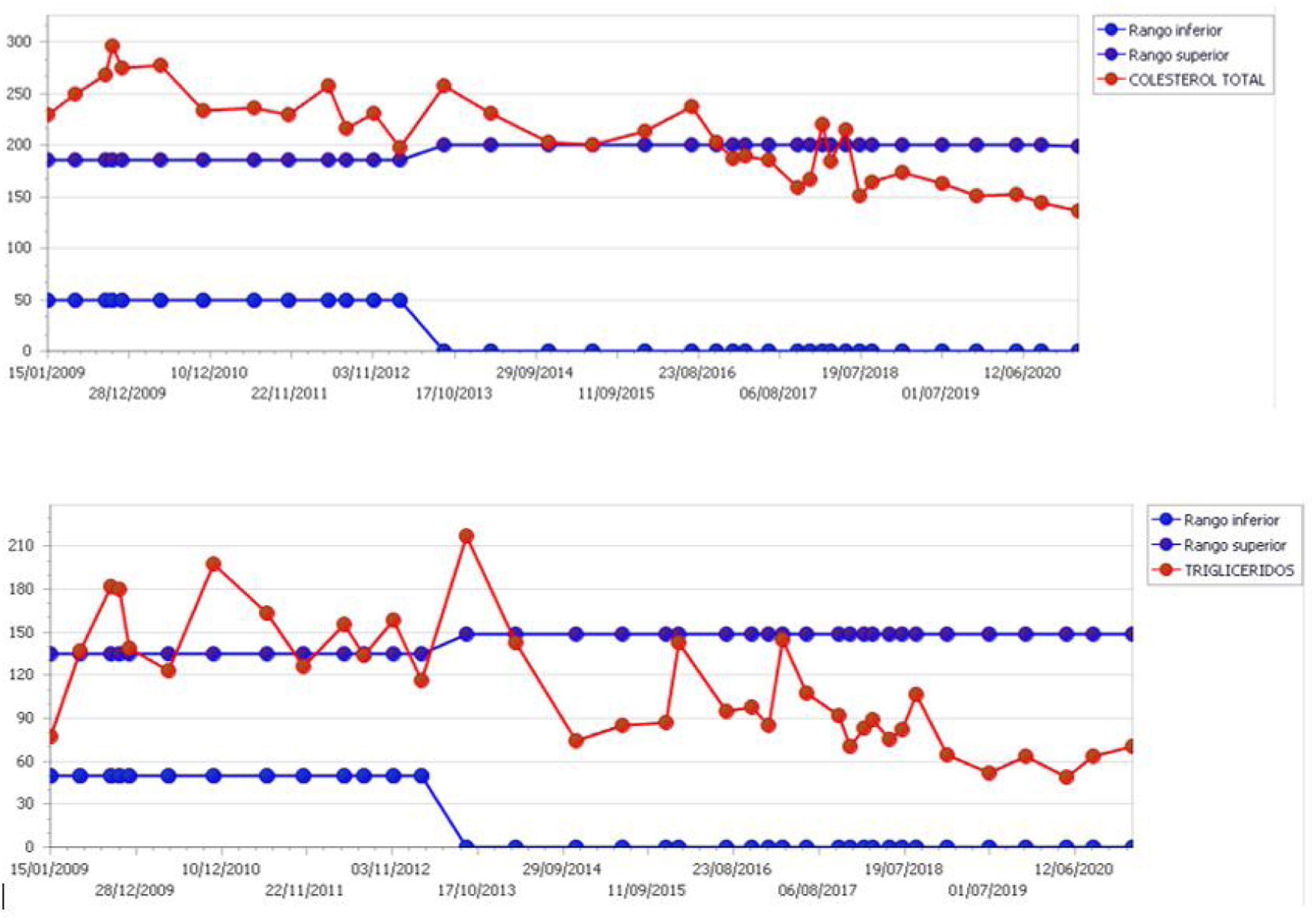
From Genetics, it was reported that the complete reversed sex syndrome is characterized by a 46XY karyotype, normal female external genitalia, underdeveloped gonads and the presence of normal Müllerian structures. Laparoscopic double gonadectomy was performed (Fig. 3) because individuals with a Y chromosome in their karyotype have an increased risk of developing a germ cell tumor, specifically, gonadoblastoma. Pathological report showed absence of ovarian tissue or malignancy. At this time, from Endocrine Reproductive Unit, bone and cardiovascular risks were addressed. First, the bone densitometry of the hip and spine showed osteopenia. Ibandronic acid 150mg monthly and calcium carbonate+cholecalciferol 500mg/400IU daily were prescribed. On the other hand, hypercholesterolemia persisted at level of 322mg/dl of total cholesterol with LDL 205mg/dl and hypertriglyceridemia 216mg/dl. Treatment was started with simvastatin+ezetimibe 40/10mg/night, as well as acetylsalicylic acid 100mg/day. Lipid profile has been controlled to date (Fig. 4).
Monitoring and resultsCurrently, patient is 33 years old and wishes to fulfill her motherhood desire by in vitro fertilization with egg donation. The patient signed the informed consent for us to use the information from her case for this work (Fig. 5).
DiscussionThis study describes the diagnostic process and the therapeutic approach of a patient with Frasier syndrome. The characteristic debut of this type of patient is after renal glomerulopathy resistant to treatment with corticosteroids. Gynecological abnormalities are detected later due to developmental delay during adolescence. Only through a genetic study can the pathogenesis of the problem be ascertained. Mutations of the WT1 gene can lead to different clinical entities associated with corticosteroid-resistant nephrotic syndrome, most notably Denysh-Drash syndrome, Frasier syndrome or isolated focal segmental glomerulosclerosis.4,5 Denysh-Drash syndrome usually occurs with the triad of infantile nephrotic syndrome due to diffuse mesangial sclerosis, ambiguous genitalia, and development of Wilms tumor.6 For its part, Frasier syndrome associates later-onset nephrotic syndrome than Denys-Drash syndrome due to focal and segmental glomerulosclerosis, together with male pseudohermaphroditism and risk of gonadoblastoma.7 Lastly, the nephrotic syndrome due to isolated focal segmental glomerulosclerosis is defined by the lack of association with major malformations or urogenital tumors.8,9 Usually, mutations in Denysh-Drash syndrome are located in exon 8 or 9 of the WT1 gene, while in Frasier syndrome the mutation is located in intron 9 of this gene.10
Kidney disease begins in childhood as asymptomatic proteinuria or nephrotic syndrome that usually underlies focal and segmental glomerulosclerosis and that leads to the progressive development of end-stage chronic renal failure in adulthood despite immunosuppressive treatment that makes kidney transplantation necessary in most cases.5,7
As has already been advanced, the presence of the Y chromosome in the karyotype of these patients is responsible for the increased risk of malignancy of the gonads and developing a gonadoblastoma in up to 50% of cases. Therefore, it is mandatory to carry out the removal of the gonads at the time of diagnosis.11
In turn, patients with Frasier syndrome suffer comorbidities derived as a consequence of a state of ovarian failure and the nephrotic syndrome that leads to states of hypoproteinemia and immunosuppression. Among them, hypercholesterolemia and hypertriglyceridema stand out at an early age, as well as deterioration at the bone level.12,13 For optimal control of the lipid profile, medium-dose statins (Simvastatin 40mg/day) combined with Ezetimibe 10mg/day were required in this case.14 It is interesting, in cases of severe hypercholesterolemia, to include antiplatelet treatment prophylactically (Acetylsalicylic acid 100mg/day). On the other hand, the lack of estrogens generates a low bone density, as in the case of this patient.13 The use of adequate doses of calcium and vitamin D is basic in the treatment and, in certain cases, the use of bisphosphonates is required.
Finally, the only reproductive option presented by this patient is egg donation. The presence of a well-shaped uterus that responds to hormone replacement therapy makes gestation viable, logically, by donating oocytes. There are still no cases described in literature about gestation in patients with Frasier syndrome. However, it will not always be a valid option since, on occasions, the Müllerian structures have not fully reached their development and the patients present rudimentary uteri that, even with hormonal treatment, reach the necessary conditions for a pregnancy.
ConclusionFrasier syndrome is a rare autosomal dominant disease that should be taken into account in adolescent individuals with female phenotype and primary amenorrhea, lack of physical changes during puberty, kidney disease, gonadal dysgenesis, and 46, XY karyotype. Infertility could be managed with egg donation.
Ethical disclosuresProtection of people and animalsThe authors declare that no experiments were carried out on humans or animals for this research.
Data confidentialityThe authors declare that they have followed the protocols of their work center regarding the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Patient consentThe authors have followed hospital protocols for the publication of patient data and have the patient's written consent for publication.
FundingNo funding source.
Conflict interestNo conflict of interest.