This is the fourth update of the therapeutic planning tables. These tables are a simple desktop tool, to help in determining the most appropriate oral cholesterol-lowering therapy for patient. This can either be with monotherapy or combination therapy (statins plus ezetimibe), taking into account the patient LDL cholesterol (LDL-C) and the therapeutic objective to be achieved. These therapeutic indications are based on 2 fundamental principles: the causality of LDL-C, and that the effect of cardiovascular protection depends on the decrease in LDL-C. It is based on a colour code that indicates the drugs that have the necessary power to meet the therapeutic objectives of the patient. We provide some recommendations on the strategy to follow to implement the most effective treatment. It is assessed up to what levels a decrease in LDL-C can be expected by adding a PCSK9 inhibitor.
Se presenta la cuarta actualización de las tablas de planificación terapéutica. Esta tabla es una sencilla herramienta de sobremesa que permite determinar la terapia hipocolesterolemiante oral más apropiada para su paciente, ya sea con monoterapia o terapia combinada (estatinas más ezetimiba), teniendo en cuenta su colesterol LDL (cLDL) de partida y el objetivo terapéutico a alcanzar. Estas indicaciones terapéuticas se basan en dos principios fundamentales: la causalidad del cLDL y que el efecto de protección cardiovascular depende del descenso del cLDL. Se han diseñado como un código de colores que señala los fármacos que tienen la potencia necesaria para llevar a su paciente a objetivos terapéuticos. La figura 1 se asocia a unas recomendaciones sobre la estrategia a seguir para implementar el tratamiento más eficaz. La figura 2 permite valorar hasta que niveles podemos esperar un descenso de cLDL al añadir un inhibidor de PCSK9.
Fifteen years ago a simple and practical table was published to facilitate the prescription of the most appropriate cholesterol-lowering therapy for each patient based on the efficacy of the different drugs that were available.1 The emergence of new molecules and clinical criteria for dyslipidaemia control led to the publication of successive updates in 2010 and 2015.2,3 This tool has been widely used in primary care and more complete and computerised versions have validated their use as a means to increase the number of patients who achieve their therapeutic targets.4 Recently, a study conducted by a separate group validated its usefulness by demonstrating that its use very significantly increases the number of patients who achieve their therapeutic targets.5
Since the publication of the last edition of the table, new scientific evidence has emerged, new lipid-lowering drugs have been added such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and new therapeutic guidelines have been published by several scientific societies. In light of the above, we consider it necessary to update this table, as well as to provide an overview of the impact that treatment with PCSK9 inhibitors may have on low-density lipoprotein cholesterol (LDL-C) levels.
Current dyslipidaemia control for cardiovascular disease prevention. The problem continuesDespite considerable evidence, recommendations from experts and clinical guidelines advising that certain LDL-C levels be reached to optimise the vascular risk of the population in accordance with their overall risk, the number of patients not achieving therapeutic targets continues to be alarmingly high.
Data from the EUROASPIRE V registry are worrying with regards to the level of LDL-C control. Only 29% of patients who have been diagnosed with coronary heart disease have LDL-C levels below 70 mg/dL and 31% have values above 100 mg/dL. The fact that, even with these figures, a quarter of patients are not aware that they must control their cholesterol, is striking.
In terms of the therapy administered, it is noteworthy that, although a high percentage (84%) followed lipid-lowering treatment, half used inappropriately low doses.6
Not achieving therapeutic targets undoubtedly leads to a preventable excess of cardiovascular morbidity and mortality. The DYSIS study showed that those patients who do not achieve therapeutic targets maintain LDL-C concentrations almost 1 mmol/L off the recommended targets. This implies that we stop reducing the risk of these patients by up to 20%.7
This percentage is very relevant if we bear in mind that in Spain approximately 120,000 cases of acute coronary syndrome occur every year, one third of which die before reaching hospital, i.e. in whom prevention is their only chance.8
Statins as a key part of cardiovascular disease preventionThe results of the Scandinavian Simvastatin Survival Study changed the focus of the prevention of cardiovascular disease (CVD).9 Several randomised controlled trials (RCT) ratified the data of the Scandinavian Simvastatin Survival Study and also demonstrated that the greater the potency and dose of the statin, the greater the benefit will be. Based on these data, various scientific societies established some LDL-C therapeutic targets to be achieved according to cardiovascular risk, which for very high-risk patients must be below 70 mg/dL.10
Due to the fact that other lipid-modulating drugs, such as fibrates, niacin or cholesteryl ester transfer protein (CETP) inhibitors, did not provide additional benefits in cardiovascular disease prevention when they were tested along with statins and that the studies with statins were not strictly designed to achieve an LDL-C target, the American College of Cardiology and the American Heart Association published new guidelines in 2013 emphasising the prescribing of statins to risk groups instead of recommending the attainment of a particular LDL-C target.11 This point of view is different from the perspective of European societies, which recommend achieving an LDL-C target.10
Since 2013, studies have emerged which have supported the idea that LDL-C should be the therapeutic target, based on the recognition of two principles: first, that LDL-C is an aetiological factor of atherosclerosis; and second, that the protective cardiovascular effects of lipid-lowering drugs are measured by LDL-C reduction.
The scientific support for these two statements comes from different types of evidence: new RCTs of non-statin lipid-lowering drugs, new meta-analyses and genetic data based on Mendelian randomisation studies.
New randomised controlled trialsSince 2013, five studies with lipid-lowering drugs have proven their efficacy on cardiovascular risk.
In the IMPROVE-IT study, 18,144 patients with acute coronary syndrome were assigned to receive ezetimibe plus simvastatin versus simvastatin alone. The mean LDL-C reached was 69.5 mg/dL in the simvastatin arm and 53.7 mg/dL in the combination arm. After seven years of follow-up, a significant 2% reduction in absolute risk (HR 0.936; 95% CI: 0.89–0.99; p = 0.016) was observed.12 The clinical effect was exactly that expected according to the meta-analyses of the Cholesterol Treatment Trialists’ (CTT) Collaboration, which establish a relative risk reduction of 22% for a 1 mmol/L (38.67 mg/dL) reduction in LDL-C.13
Three RCTs with the new PCSK9 inhibitors have demonstrated the protective effect of LDL-C reduction beyond the effect of statins. In the FOURIER study, patients with established CVD were randomly assigned to receive evolocumab, a fully human anti-PCSK9 monoclonal antibody, or placebo in patients with optimised statin therapy. The active arm had a significant reduction in number of episodes of 15% compared to the placebo in only 2.2 years of follow-up.14 Alirocumab, another fully human anti-PCSK9 antibody, obtained similar data in the ODYSSEY OUTCOMES study in patients with acute coronary syndrome. In the active arm, a significant reduction of 15% was observed in the primary target after 2.8 years.15 The SPIRE-1 and SPIRE-2 studies had a similar design, using bococizumab, which obtained confirmatory results despite the fact that the study was stopped due to a progressive lack of effectiveness of the antibody, in this case a “humanised” monoclonal antibody.16 A reduction in the expected number of episodes based on the reduction in LDL-C and the time of exposure was seen in the three studies, in accordance with the CTT Collaboration meta-analyses. Anacetrapib is a CETP inhibitor which has also demonstrated a significant effect in the reduction of cardiovascular events. The effect size was again that expected according to the reduction of LDL-C when it was evaluated by means of β-quantification.17
The general conclusion of these studies is that non-statin LDL-C-lowering agents induce a reduction in relative risk of CVD in line with that of statins per reduced cholesterol unit and time of exposure, which supports the assumption that reduction of LDL-C is vital, regardless of the drug used.
Confirmatory meta-analysesThe above concept was reaffirmed by an extensive meta-analysis which showed that the main determinant of relative risk reduction is the extent of LDL-C reduction, regardless of the drug or lipid-lowering method used. The impact of surgery, diet or medicines such as resins, ezetimibe, PCSK9 inhibitors or statins was similar.18
Mendelian randomisation studiesThe studies known as Mendelian randomisation studies provide new evidence. These studies are similar to RCTs, but in this case randomisation is determined by nature. Human beings are genetically diverse, which is why we are different. Variations in the genes involved in lipid metabolism cause lipid plasma concentrations to increase or decrease: genetic variants in the HMG-CoA reductase, LDLR, PCSK9, NPC1L1 and ABCG5/G8 genes, which affect cholesterol synthesis, the LDL receptor, PCSK9 expression and cholesterol absorption mimic the effect of statins, PCSK9 inhibitors, ezetimibe or resins. These give rise to lower levels of LDL-C and are identically associated with fewer cardiovascular events per unit of reduced LDL-C and irrespective of the metabolic pathway affected. Mendelian randomisation studies also show that small differences in LDL-C that persist throughout life due to the fact that they are genetically determined, are associated with a greater risk reduction than that induced in a shorter period of time by drug therapy, pointing to the importance of cholesterol load over time and, therefore, to the importance of early control of this metabolic disorder.19
Once again, this evidence confirms that the benefit of lipid-lowering drugs is measured by their effect on LDL-C, meaning that the concept of “high-intensity statin therapy” should be replaced by “high-intensity cholesterol-lowering therapy”.20
This new concept suggests that lipid-lowering therapy should focus on the LDL-C target to be achieved, regardless of the way in which it is achieved, whether with maximum doses of statins, intermediate doses combined with ezetimibe or maximum doses of statins with ezetimibe when the therapeutic target is very far off. The relative risk reduction will correspond to the resulting LDL-C reduction.
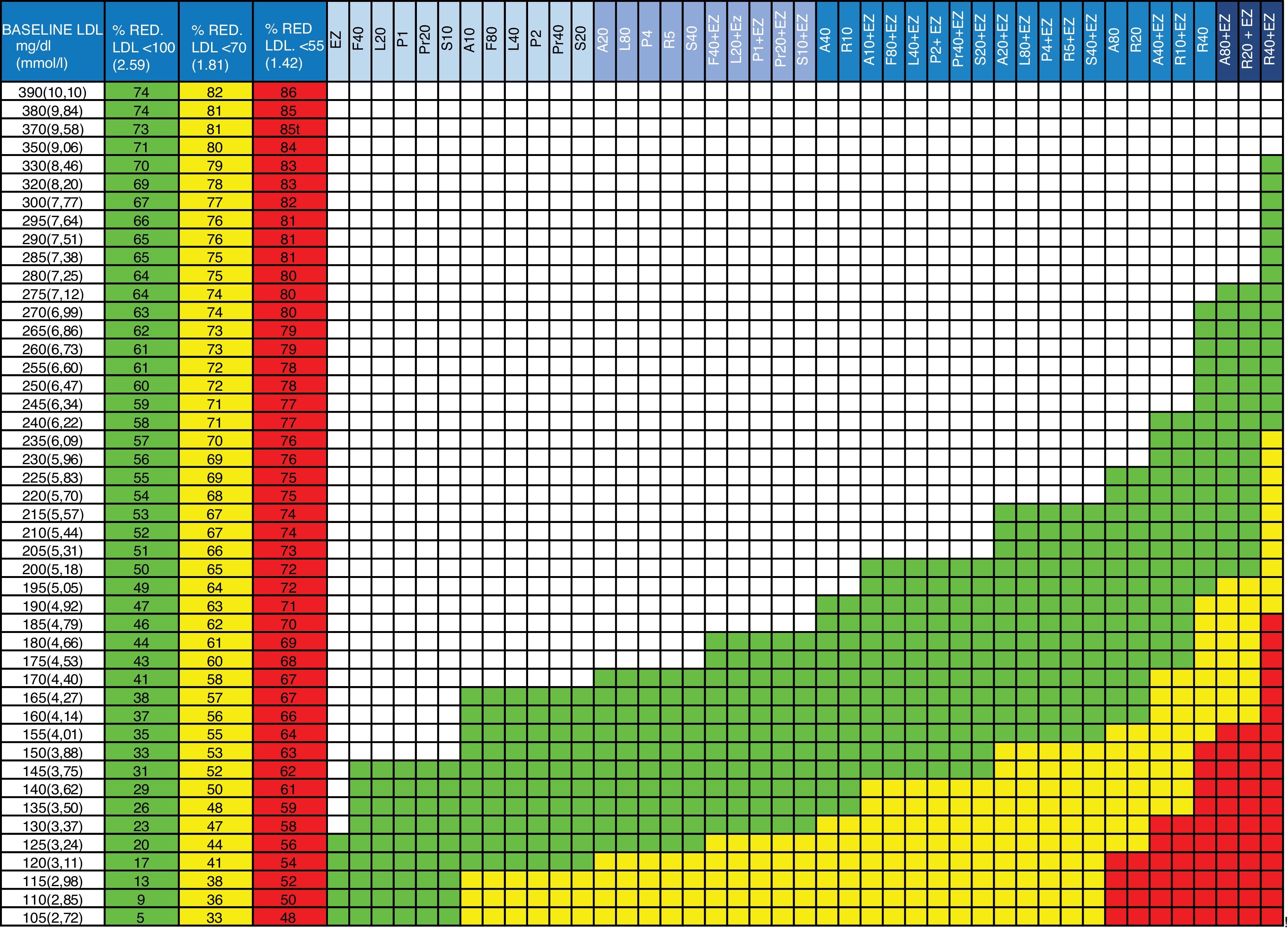
Updates to the tableIn the new version of the table (Fig. 1), the therapeutic target columns have been updated considering only the targets to be achieved in patients at high and very high risk (<100 and<70 mg/dL, respectively) according to the latest European guidelines, and a column has been added with the target <55 mg/dL, given the existing evidence of extreme risk groups and following the recommendations of the American Association of Clinical Endocrinologists and the American College of Endocrinology21 and the data from recent RCTs. Statins and combination therapy (statin + ezetimibe) have been ordered progressively from lowest to highest therapeutic efficacy so that the physician decides, in each action range, the strategy to use with his/her patient: monotherapy or combination. The effect of ezetimibe in monotherapy has also been added, which is useful in those cases when patients do not tolerate statins. Calculations performed for this new value are not the result of the direct sum of the effects of the drugs, but instead the impact of the reduction of one of them on the efficacy of the next one has been calculated to assess the effect on the baseline concentration of LDL-C. This concept and the formula applied are explained in another publication.3,22
Guidelines for the prescription of cholesterol-lowering therapy aimed at achieving the therapeutic targets.
Column 1: baseline LDL-C value of the patient to be treated.
Columns 2, 3 and 4: percentage of LDL-C reduction required to achieve the therapeutic targets. Column 2 (green): in patients with high overall cardiovascular risk (LDL-C <100 mg/dL). Column 3 (yellow): in patients on secondary prevention or at very high overall cardiovascular risk (LDL-C <70 mg/dL). Column 4 (red): in patients with extremely high risk (LDL-C <55 mg/dL).
Columns 5 to 47: drugs or drug combinations (statins + ezetimibe) that facilitate the necessary LDL-C reduction to achieve the therapeutic targets. In green: for high overall cardiovascular risk (LDL-C <100 mg/dL). In yellow: secondary prevention or very high overall cardiovascular risk (LDL-C <70 mg/dL). In red: for extremely high cardiovascular risk (LDL-C <55 mg/dL).
A: atorvastatin; EZ: ezetimibe; F: fluvastatin; L: lovastatin; LDL: LDL cholesterol; P: pitavastatin; Pr: pravastatin; R: rosuvastatin; S: simvastatin; %RED.: % reduction.
The number beside the letter indicates the dose, for example, A20 is atorvastatin 20 mg. The dose of ezetimibe is always 10 mg/day. gr1
Although it has not been included in the figure, it is important to mention that ion-exchange resins (cholestyramine, colesevelam) can produce a similar effect to ezetimibe. Bempedoic acid is not mentioned given that it was not marketed when this manuscript was written. However, it may become a clinical reality in the coming years and with a similar cholesterol-lowering effect to ezetimibe.
Advice for prescribing the appropriate doses and therapies to each individual patient has also been added to the instructions on use. The use of the figure is identical to that of previous editions (Table 1 and Fig. 1).
Instructions for using the table and therapeutic implementation.
| 1. Locate the baseline LDL-C value in column 1. If the patient is being treated with statins, calculate the approximate baseline LDL-C by multiplying the value by 1.5 |
| 2. Check the reduction required to achieve the target of LDL-C <100, <70 or <55 mg/dL in the adjacent columns 2, 3 or 4 |
| 3. Follow the row to the right |
| 4. The green cells indicate the therapies suitable for achieving the target <100 mg/dL (see header row in blue) |
| 5. The yellow cells indicate the therapies suitable for achieving the target <70 mg/dL (see header row in blue) |
| 6. The red cells indicate the therapies suitable for achieving the target <55 mg/dL (see header row in blue) |
| 7. Apply the appropriate therapy to achieve the targets. If treatment with statins at maximum doses is opted for, we recommend starting with the mean dose in patients over the age of 75 or who may be more susceptible to statin toxicity. |
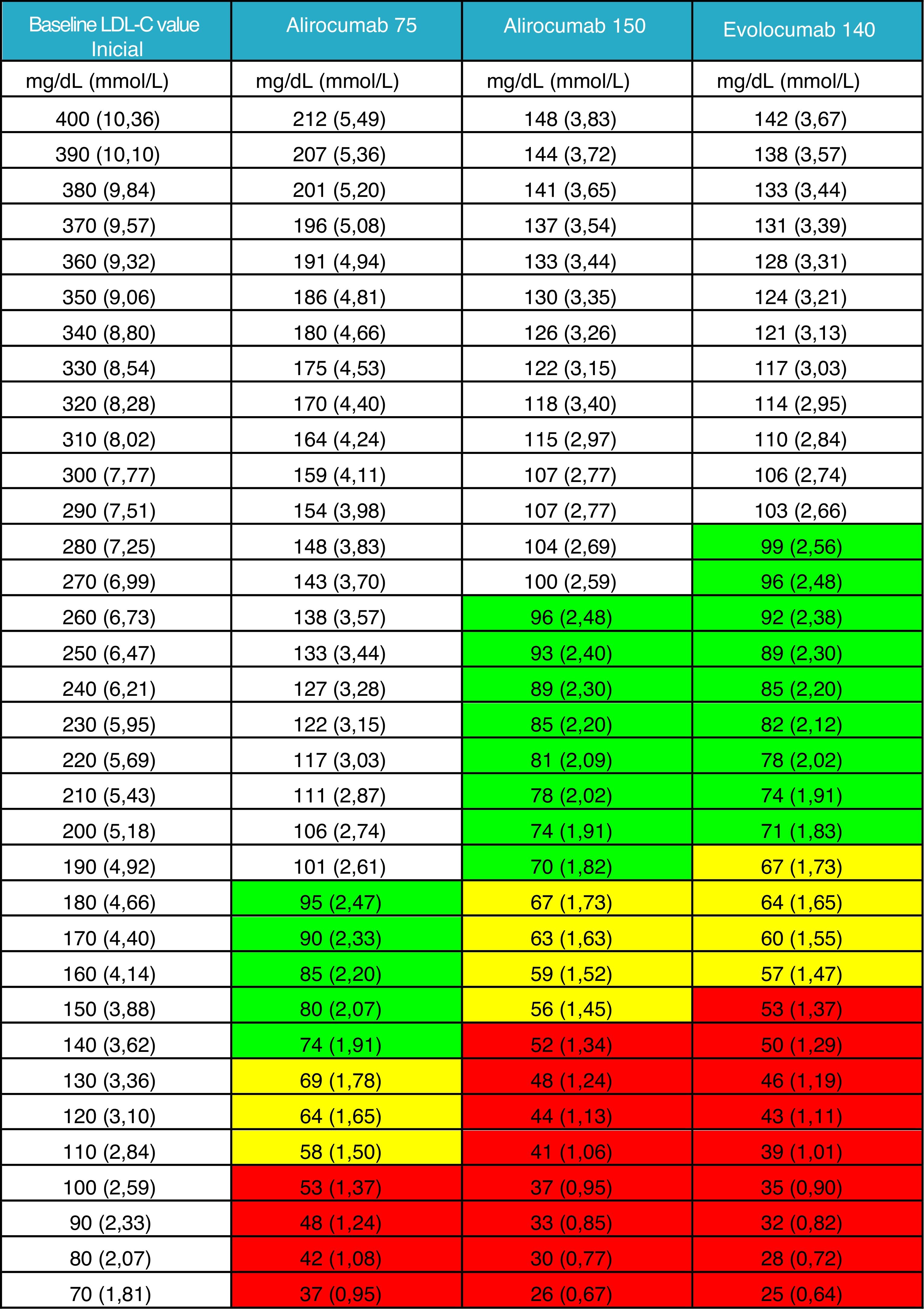
It was not considered appropriate to add the effect of a triple association with the inclusion of PCSK9 inhibitors to the general figure given that this therapy must be prescribed after routine oral therapy. Instead, a new table is provided in which the clinician can obtain guidance on the LDL-C levels that his/her patient can achieve based on measured baseline levels, generally after optimised oral lipid-lowering therapy. We have deemed this information to be currently more relevant and of greater clinical utility Fig. 2).
LDL-C value achieved after the administration of PCSK9 inhibitor.
Mean theoretical value which would be achieved when treating with any of the three therapeutic options of PCSK9 inhibitors available, taking into account the LDL-C value of the patient at the time of the indication, with prior therapy or without prior therapy in the case of intolerant patients.
The efficacy of PCSK9 inhibitors has been defined as the intermediate value of those reported in the summary of product characteristics for alirocumab 75 mg and evolocumab 140 mg. The value of alirocumab 150 mg has been based on the data from the ODYSSEY LONG TERM study.23
Lluís Masana has received conference and/or consultancy fees from Amgen, Sanofi, MSD and Mylan. Núria Plana has received conference fees from MSD, Amgen, Sanofi and Alexion.
Please cite this article as: Masana L, Plana N. Actualización de las tablas de planificación terapéutica hipocolesterolemiante orientadas a la obtención de los objetivos terapéuticos. Clin Investig Arterioscler. 2019;31:271–277.