Familial chylomicronemia (FCS) is a very rare, underdiagnosed disorder that can cause abdominal pain and recurrent pancreatitis from childhood -potentially life-threatening- and chronic complications such as diabetes mellitus and exocrine pancreatic insufficiency. FCS affects the quality of life and mental health of those who suffer from it, aspects that must be taken into account in its treatment, based on a strict low-fat diet, which is difficult to adhere to and persist. People with FCS lack the lipolytic capacity to hydrolyze triglycerides (TG) and have a minimal or null response to conventional lipid-lowering treatments. ApoCIII antagonists, specifically volanesorsen, olezarsen and ARO-APOC3, are the most promising drugs to reduce TG concentrations in patients with FCS. Anti-ANGPTL3 therapies appear to be less effective. More clinical trials and new pharmacological treatments are needed to improve the quality of life and prognosis of people with FCS.
La Quilomicronemia Familiar (QF) es un trastorno muy infrecuente, infradiagnosticado y que puede provocar dolor abdominal y pancreatitis recurrente desde la infancia -potencialmente amenazan la vida- y complicaciones crónicas como diabetes mellitus e insuficiencia pancreática exocrina. La QF afecta a la calidad de vida y salud mental de quienes la padecen, aspectos que hay que tener en cuenta en su tratamiento, basado en una dieta estricta baja en grasas, de difícil adherencia y persistencia. Las personas con QF carecen de capacidad lipolítica para hidrolizar los triglicéridos (TG) y tienen una respuesta mínima o nula a los tratamientos hipolipemiantes convencionales. Los antagonistas de la ApoCIII, específicamente volanesorsen, olezarsen y ARO-APOC3, son los fármacos más prometedores para reducir las concentraciones de TG en pacientes con QF. Las terapias anti-ANGPTL3 parecen ser menos efectivas. Son necesarios más ensayos clínicos y nuevos tratamientos farmacológicos, para mejorar la calidad de vida y el pronóstico de las personas con QF.
In addition to severe HTG, FCS is clinically characterised by recurrent episodes of pancreatitis since childhood, occasional xanthomas, lipaemia retinalis and a significant impact on the quality of life and mental health of those affected.1,2 One of the most important features of this disease is the delay in diagnosis, as it is a rare disease and therefore unknown to most healthcare professionals. In fact, according to initial data from the APPROACH study,3 the mean age at diagnosis was 24 years.
PrevalenceIt is a very rare disorder and its prevalence is unknown, partly because it is difficult to diagnose, as genetic testing is not always available. In Europe, according to different studies, it affects between one and 19 people per million.4–6 In Spain, there are at least two registries7,8 of 13 and 26 patients with compatible genetics.
PhysiopathologyAs mentioned above, the basic disorder is the almost total absence of activity of the LPL enzyme, a key enzyme in the hydrolysis and catabolism of TGs, due to the presence of pathogenic biallelic loss-of-function variants in the 5 genes previously described (LPL, GPIHBP1, APOA5, APOC2, and LMF1).9
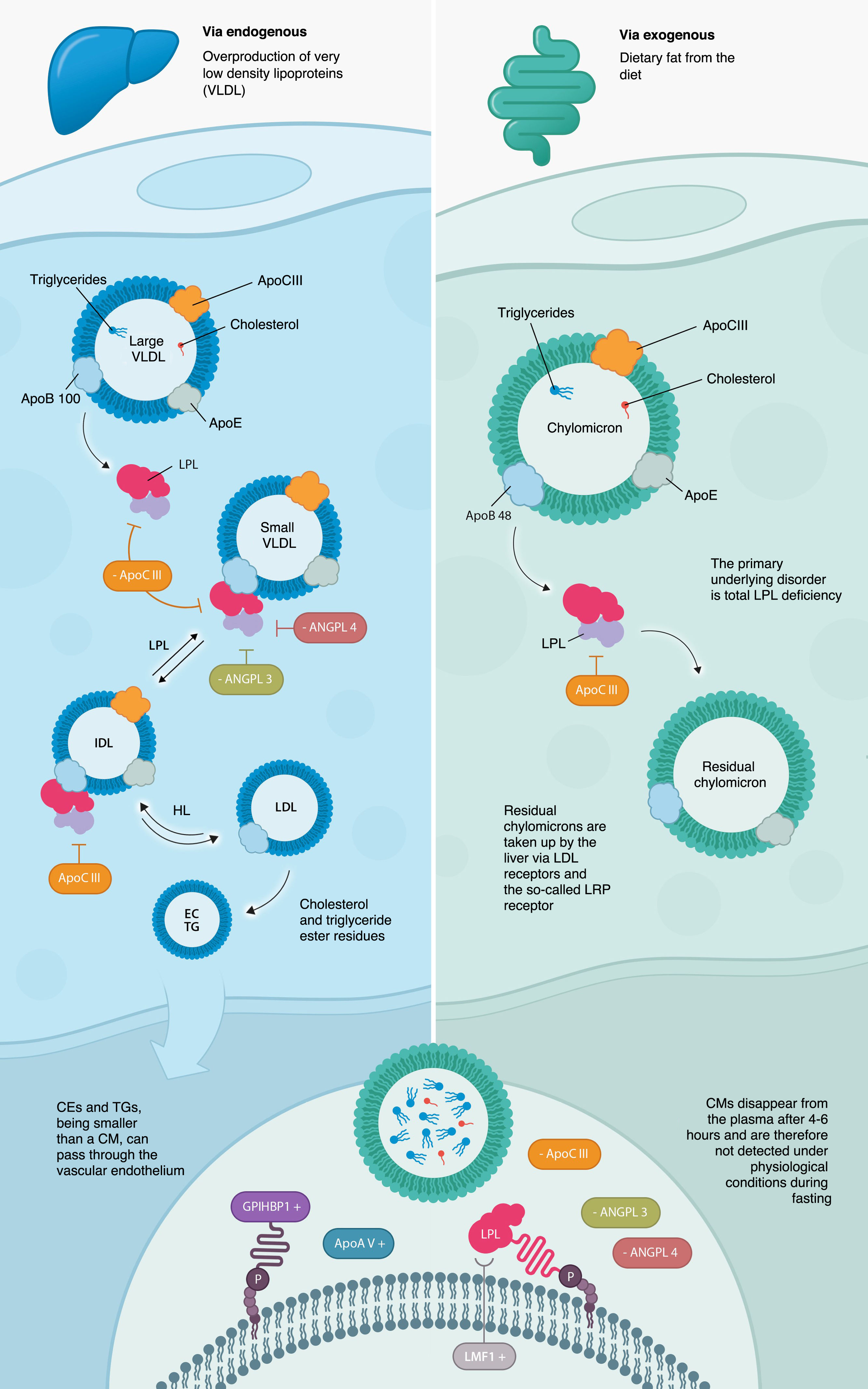
Under normal conditions, chylomicrons (CMs) are synthesised in the intestine after ingestion of fat and carry large amounts of TG (exogenous pathway), which are hydrolysed by LPL, which requires other cofactor proteins such as ApoAV, ApoCII, LMF1, and GPIHBP1 for its normal function. The remaining CMs are taken up by the liver via LDL receptors and the so-called LRP receptor (LDL receptor-related proteins), which use apolipoprotein E (ApoE) as a ligand.10 CMs disappear from the plasma after 4−6 hours, and therefore cannot be detected under physiological conditions on an empty stomach (Fig. 1). What characterises this disorder is the persistence of CMs in the plasma, even after fasting for more than 14 h, due to the impossibility of metabolising them, as there is virtually no LPL activity, and they cannot be metabolised.
Clinical manifestationsThe fat content of the diet is essential to avoid the main manifestations, i.e., episodes of pancreatitis and abdominal pain (Fig. 2). Recurrent acute pancreatitis11 is a serious and life-threatening complication that can lead to long-term complications such as exocrine pancreatic insufficiency, partial or total necrosis of the pancreas, and secondary diabetes. There is also an association between TG levels and the development of pancreatitis. Although acute pancreatitis usually occurs at very high TG levels, above 2000 mg/dl, much lower levels can also trigger pancreatitis.12,13 A 100 mg/dl increase in TG concentration increases the risk of acute pancreatitis by about 4%. The exact pathophysiological mechanisms by which chylomicronaemia causes pancreatitis are not known. It has been postulated that blood hyperviscosity due to excess circulating CMs would lead to local ischaemia and pancreatic acidosis. Other mechanisms include excess oxidised radicals from free fatty acids poorly hydrolysed by pancreatic lipase or mitochondrial inflammation of pancreatic cells.1 There is no correlation between the absolute TG peak value and the severity of pancreatitis.14
Patients with FCS often have recurrent episodes of abdominal pain with or without pancreatitis, often leading to voluntary fasting. Other manifestations include the appearance of eruptive xanthomas, nausea, vomiting, lipaemia retinalis, hepatosplenomegaly, growth retardation, confusion with 'mental fog', and changes in mood and mental health. The prevalence of different symptoms varies according to published results from different cohorts.8,15,16
A very important aspect is the impact on the quality of life of these patients, who often experience fear and anxiety about new episodes of pancreatitis.16–18 Social relationships and their emotional world are also affected.19 Patients experience frustration at the lack of understanding from family and friends about the nature of their disease and the implications for their treatment. Up to 23% of patients report eating disorders.
DiagnosisThe diagnosis is genetic. In a patient with severe HTG (>880 mg/dl) and the presence of CMs in ultracentrifugation, in the absence of secondary causes (alcohol consumption, poorly controlled diabetes, obesity, insulin resistance, drugs, chronic kidney disease, paraproteinemia, etc.), a genetic study should be considered. As this is a very rare disease and the clinical picture may go unnoticed in relation to other aetiologies, Moulin et al.20 have developed a diagnostic algorithm for suspected FCS. In general, a normal/low apolipoprotein B (ApoB) level, together with a normal or low body mass index, recurrent symptoms of abdominal pain since childhood, multiple episodes of severe HTG, recurrent pancreatitis and lack of response to conventional lipid-lowering therapy, make the diagnosis of FCS more plausible and a diagnosis of MCS less likely.
If genetic diagnosis is not available or is negative despite high clinical suspicion, LPL activity11 could be measured after heparin injection. An LPL activity of less than 20% would confirm the diagnosis, although unfortunately this technique is only available in research centres and is not routinely performed.
Recently, the presence of LPL or GPIHBP1 autoantibodies21,22 has been described. Therefore, the possibility of autoimmune chylomicronaemia should be considered when genetic testing comes back negative. Unfortunately, the test to detect the presence of autoantibodies is not available in most centres.
TreatmentTreatment should include a comprehensive approach to the patient, with the primary goals of avoiding recurrent episodes of pancreatitis, controlling chronic HTG and thus chylomicronaemia, and treating and managing mental health and improving the patient's quality of life as much as possible. The mainstays of treatment are diet and pharmacological treatment. The diet should be very restrictive in terms of fat23,24 (<20 g per day of any type of fat), requiring the involvement of dieticians and nutritionists in the management of these patients. The main source of energy will be carbohydrates and medium-chain triglycerides (MCTs), which can be used as crude oil. A summary of foods to avoid and foods that can be safely consumed is given in Table 1.
Food group recommendations in familial chylomicronaemia syndrome.
| Foods to include in the diet | Foods to avoid in the diet |
|---|---|
| Vegetables and legumes without added oil | Alcohol |
| Wholegrain cereals | Added sugars |
| Skimmed milk | Sugar-sweetened drinks and juices |
| Lean meat | Industrial cakes and pastries |
| Skimmed products | Oils of any kind |
| Complex carbohydrates | Processed/ultra-processed foods |
| Essential fatty acids such as linoleic and α-linoleic | Fatty foods |
For episodes of acute pancreatitis, the management of these patients is the same as for other aetiologies that can cause pancreatitis. For example, gastrointestinal rest with absolute diet, fluid and electrolyte replacement with intravenous fluid therapy, and analgesia are usually effective in rapidly reducing HTG and controlling pain. Plasma exchange therapies have not been shown to be superior to conventional treatment in the long term, and their use is limited in pregnancy. In diabetic patients with hyperglycaemia, intravenous insulin may be useful.
Pharmacological treatment of FCS: volanesorsenPharmacological treatment is essential to control chylomicronaemia in FCS, as dietary recommendations are necessary but not sufficient. In this context, fibrates can help to reduce hepatic overproduction of VLDL and improve TG levels in the presence of a V phenotype, but they do not correct chylomicronaemia in people with FCS.
Volanesorsen is the only drug approved by the European Medicines Agency (EMA) and available in Spain with proven efficacy in FCS. It is an antisense oligonucleotide hybridised to the messenger RNA of apolipoprotein CIII (ASO-ApoCIII) in the cell nucleus, forcing the cell to destroy it (Fig. 3). The ultimate mechanism of action by which it reduces HTG remains to be elucidated, although it has been postulated that by reducing ApoCIII (Apo-CIII) there would be a much larger pool of LPL available, although in these patients LPL does not regain activity. The pivotal study for volanesorsen was the APPROACH trial,25 a phase 3 clinical trial in which 66 patients with FCS were randomised 1:1 to receive volanesorsen 285 mg or placebo, both administered subcutaneously weekly for 52 weeks. At three months, the reduction in TG concentration (primary endpoint) was 77%, corresponding to a TG reduction of 1712 mg/dl, while Apo-CIII concentration was reduced by 84%. Finally, 77% of the participants treated with volanesorsen achieved mean TG concentrations below 750 mg/dl versus 18% in the placebo group. The most common adverse events were local injection site reactions (redness and itching) and thrombocytopenia below 100,000 platelets/mm3 in 15 of the 33 patients treated with volanesorsen.
In Spain, conditions for funding to use volanesorsen include a confirmed genetic diagnosis of FCS (biallelic variant), recurrent pancreatitis, and TG concentrations above 750 mg/dl. Clinical outcomes in the follow-up of patients treated with volanesorsen should be included in the Ministry of Health's VALTERMED platform, and the efficacy of treatment should be reviewed after 3 months and discontinued if a TG reduction of more than 25% above baseline and levels below 750 mg/dl (8. 5 mmol/l) are not achieved, or if the TG concentration does not fall below 2000 mg/dl (22.6 mmol/l) after 3 months of weekly treatment with 285 mg. Regular assessment is also required, at least every 3 months, with discontinuation of the drug if TG levels return over time to levels similar to those at baseline.
The side effect that most limits its use is thrombocytopenia. It should be remembered that many of these patients have had previous pancreatitis and already have complications such as splenomegaly or thrombosis of the splenic artery, which means that their platelet count is often lower than normal. The label states that treatment should not be initiated in patients with thrombocytopenia (platelets <140,000/mm3).
Other drugs in developmentOlezarsen: Apo-CIII (ASO-ApoCIII) inhibitor antisense oligonucleotide conjugated to triantennary N-acetylgalactosamine (GalNAc3), a carbohydrate ligand for the asialoglycoprotein receptors that are abundant on the surface of hepatocytes, thus facilitating their entry into the hepatocyte nucleus where Apo C3 messenger RNA is generated (Fig. 3) and inhibiting apo-C3 production. A recently published phase 3 clinical trial26 that enrolled 66 patients with FCS, of whom 22 were assigned to the 80 mg olezarsen treatment group, 21 to the 50 mg olezarsen group, and 23 to the placebo group, all administered subcutaneously every 4 weeks. At baseline, the mean (±standard deviation) TG level was 2630 ± 1315 mg/dl and 71% had a history of acute pancreatitis in the previous 10 years. TG levels at 6 months were significantly reduced with the 80 mg dose of olezarsen (–43.5%; 95% confidence interval [CI]: –69.1 to –17,9; p < .001), but not with the 50 mg dose (–22.4%; 95% CI: −47.2–2.5; p = .08). The difference in mean percentage change in apolipoprotein C-III levels from baseline to six months in the 80 mg group compared to placebo was −73.7% (95% CI −94.6 to −52.8) and in the 50 mg group compared to placebo was −65.5% (95% CI −82.6 to −48.3). At 53 weeks, there were 11 episodes of acute pancreatitis in the placebo group and one episode in each olezarsen group (combined olezarsen versus placebo rate ratio: .12, 95% CI: .02–.66). There were no significant rates of thrombocytopenia.
Plozasiran: small interfering RNA (ARO-ApoCIII) designed to reduce Apo-CIII expression in the liver. A very recently published phase 3, double-blind, randomised, double-blind trial27 in at least 3 consecutive tests, refractory to standard lipid-lowering therapy and at least one additional criterion (previous genetic diagnosis of FCS, absent or low post-heparin LPL activity ―less than 20% of normal value― history of acute pancreatitis not caused by alcohol or cholelithiasis, recurrent hospitalisations for severe abdominal pain with no other identified cause, childhood pancreatitis, or family history of HTG-induced pancreatitis). A total of 75 patients were randomised in a 2:1:2:1 ratio to receive 25 mg of plozasiran or volume-matched placebo or 50 mg of plozasiran or volume-matched placebo subcutaneously every 3 months for 12 months. At 10 months, the mean change from baseline in fasting TG concentrations (primary endpoint) was –80% in the 25 mg plozasiran group, –78% in the 50 mg plozasiran group, and –17% in the placebo group (p < .001). Secondary endpoints showed better outcomes in the plozasiran groups than in the placebo group, including the incidence of acute pancreatitis (odds ratio: .17, 95% CI: .03–.94; p = .03). The risk of adverse events was similar in all groups, with serious and severe adverse events occurring less frequently with plozasiran than with placebo. Hyperglycaemia occurred with plozasiran in some patients with pre-diabetes or diabetes at baseline. TG concentrations in patients with persistent chylomicronaemia were significantly lower with plozasiran than with placebo, as was the incidence of acute pancreatitis.
Evinacumab: Angiopoietin-like protein 3 (ANGPTL3) is a circulating protein encoded by the ANGPTL3 gene on chromosome 1p31 that is synthesised in the liver and partially regulates lipid metabolism by inhibiting LPL and endothelial lipase activity. Evinacumab28 is a monoclonal antibody against ANGPTLT3 and is approved for the treatment of homozygous familial hypercholesterolaemia based on the results of the ELIPSE HoFH trial,29 in which patients treated with evinacumab showed a mean reduction in TG concentration of 55%. To evaluate the potential of evinacumab in patients with severe HTG, a randomised, double-blind, phase 2 trial30 (evinacumab versus placebo) was conducted in patients with severe HTG and at least one previous hospitalisation for acute pancreatitis. One cohort of this trial included only patients with FCS; in this cohort, evinacumab was not associated with a reduction in TG levels compared to placebo. Very high doses of evinacumab may be required in patients with no or very low LPL activity. A clinical trial in patients with MCS is ongoing.
Lomitapide: a drug that inhibits the microsomal triglyceride transfer protein (MTP), thereby lowering TG concentrations by preventing the transfer of TG to nascent Apo B-containing lipoproteins, including chylomicrons.31 It was initially approved for use in homozygous familial hypercholesterolaemia after anecdotal use in a patient with FCS with an acceptable clinical outcome, but with marked worsening of the patient's fatty liver.32
FundingThis work was funded by an unconditional grant from Sobi, which was not involved in the design of the work or drafting of this manuscript.
Information about the supplementThis paper is part of the supplement entitled Severe hypertriglyceridaemia which has been funded by the Sociedad Española de Arteriosclerosis, with sponsorship from Sobi.
Please cite this article: Blanco Echevarría A., Ariza Corbo M.J., Muñiz-Grijalvo O., Díaz-Díaz J.L., Quilomicronemia familiar: nuevas perspectivas, Clin Investig Arterioscl. 2025. https://doi.org/10.1016/j.artere.2025.100747