Fructose, alone or in combination with glucose, has been used as a source of added sugars to manufacture sugary drinks and processed foods. High consumption of simple sugars, mainly fructose, has been demonstrated to be one of the causes of developing metabolic diseases. Maternal nutrition is a key factor in the health of the progeny when adult. However, ingestion of fructose-containing foods is still permitted during gestation. Hydrogen sulphide (H2S) is a gasotransmitter produced in the transsulfuration pathway with proved beneficial effects to combat metabolic diseases.
MethodsCarbohydrates were supplied to pregnant rats in drinking water (10% wt/vol) throughout gestation, and the pregnant rats, their foetuses, and adult male descendants were studied. Later, adult male progeny from control, fructose- and glucose-fed mothers were subjected to liquid fructose, and were compared to the control group. Liver H2S production was determined.
ResultsThis study shows that, in pregnancy, either a fructose-rich diet per se or situations that produce an impaired insulin sensitivity such as an excessive intake of glucose, decrease hepatic and placental production of H2S. Furthermore, this effect was also observed in the liver of male offspring (both in foetal and adult stages). Interestingly, when these adult descendants were subjected to a high fructose intake, decreases in H2S synthesis in liver and adipose tissue due to this fructose intake were maternal consumption dependent.
ConclusionsGiven H2S is a protective agent against diseases such as diabetes, obesity, cardiovascular diseases, and metabolic syndrome, the fact that carbohydrate consumption reduces H2S synthesis both in pregnancy and in their progeny could have clear and relevant clinical implications.
La fructosa, sola o en combinación con glucosa, se usa como fuente de azúcares añadidos para elaborar bebidas azucaradas y comidas procesadas. El elevado consumo de azúcares simples, sobre todo fructosa, se ha mostrado como una de las causas del desarrollo de enfermedades metabólicas. La nutrición materna es un factor clave en la salud de la descendencia adulta. Sin embargo, el consumo de alimentos que contienen fructosa está todavía permitido durante la gestación. El sulfuro de hidrógeno (H2S) es un gasotransmisor producido en la ruta de la transulfuración con probados beneficios para luchar contra las enfermedades metabólicas.
MétodosLos carbohidratos se suministraron a las ratas gestantes en el agua de bebida (10% p/v) a lo largo de la gestación, y se estudiaron las ratas preñadas, sus fetos y los descendientes macho adultos. Posteriormente, a la progenie macho adulta procedente de madres control, alimentadas con fructosa o bien con glucosa, se le administró fructosa líquida y se comparó con un grupo control. Se determinó la producción hepática de H2S.
ResultadosEste estudio muestra cómo en la gestación, una dieta rica en fructosa per se o situaciones en las que se produce una empeorada sensibilidad a la insulina tal como un consumo excesivo de glucosa, disminuyen la producción hepática y placentaria de H2S. Más aún, este efecto también fue observado en el hígado de la descendencia macho (tanto en el estado fetal como en la edad adulta). Es destacable que, cuando esta descendencia adulta era sometida a una ingesta elevada de fructosa, las disminuciones en la síntesis de H2S en el hígado y el tejido adiposo debidas a dicho consumo eran dependientes del consumo materno.
ConclusiónDado que el H2S es un agente protector contra enfermedades tales como la diabetes, la obesidad, las enfermedades cardiovasculares y el síndrome metabólico, el hecho de que el consumo de carbohidratos reduzca la síntesis de H2S tanto en la gestación como en su descendencia tendría claras implicaciones clínicas.
Changes in food patterns and unhealthy diets are the main causes of the rising cases of metabolic syndrome (MetS), obesity and non-alcoholic fatty liver disease (NAFLD) all around the world, the excessive intake of sugar-sweetened beverages (SSB) being one the main sources.1 Fructose is used as a source of added sugar in the form of High Fructose Corn Syrup (HFCS) or sucrose (a disaccharide composed of two monosaccharides: glucose and fructose) and a close relationship between its consumption and higher risk of suffering cardiometabolic risk factors has been established.2 Moreover, according to Developmental Origins of Health and Disease (DOHaD) theory, the mother's diet during pregnancy plays a key role in the correct development of the offspring when adult, mainly due to epigenetic changes.3 Indeed, many studies (including some ours) have established an association between fructose intake during pregnancy and detrimental effects in the foetuses and adult descendants.4,5 We have previously seen features of MetS in male, but not female, offspring from fructose fed mothers, such as an impaired insulin signalling, hypoadiponectinemia and steatosis.6 Thus, although a connection between a high consumption of fructose containing beverages and the global epidemic of obesity and metabolic syndrome could exist,5,7 ingestion of these beverages and fruit juices is still permitted during gestation.
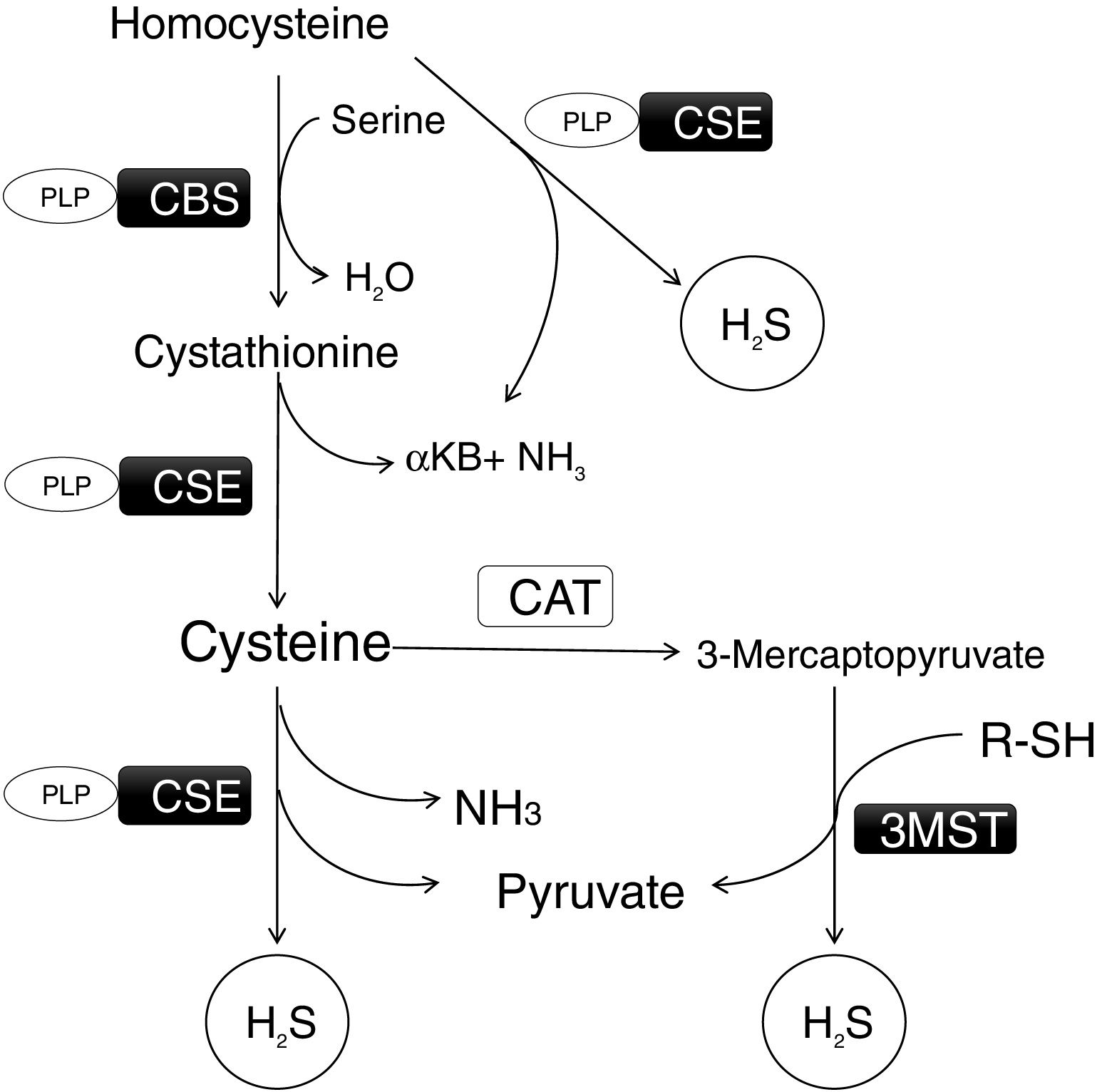
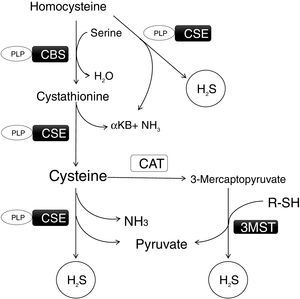
Metabolism is regulated by different chemical species, but only three of them are gasotransmitters: nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S). They are produced enzymatically, are able to cross the cell membrane by simple diffusion and to modulate cellular function.8 Among them, H2S has increased its relevance in the last years due to its emerging role in cardiovascular diseases, due to its anti-inflammatory, antioxidant and vasodilatory effects.9 In fact, solid evidences suggest that endogenous H2S and exogenous supplementation of H2S can ameliorate many of atherogenic processes, inhibiting endothelial cell damage, reducing oxidative stress, blocking inflammation, regulating lipid metabolism, suppressing vascular smooth muscle cells proliferation, attenuating foam cell formation, and reducing platelet aggregation.10 This gasotransmitter is synthesized in the transsulfuration pathway (Fig. 1) in many organs such as liver, kidney, heart and adipose tissue. There are three main enzymes involved in its formation: cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), which work in concert to transform Homocysteine (HCy) into H2S, and 3-mercaptopyruvate sulfurtransferase (3-MST) which uses mercaptopyruvate to produce H2S8. Diminutions in H2S levels and lower expression of transsulfuration enzymes have been linked to an impaired insulin sensitivity in adipose tissue.11 In the liver, H2S donor administration has been demonstrated to decrease liver steatosis by a reduction of lipid production and an activation of the antioxidant systems activity in mice treated with a High-Fat Diet.12 However, some studies have shown increases in CSE expression and H2S production in insulin resistance animal models.13 Interestingly, H2S has been proved to have beneficial effects in cardiovascular diseases (CVD). It works as a cardioprotective factor by reducing oxidative stress, inflammation, fibrosis and apoptosis.14,15 Taking into account all these effects of H2S, some authors have proposed it as a novel agent for therapy for hypertension, atherosclerosis, myocardial hypertrophy and heart failure by administering sulfur donors or increasing endogenous H2S production.16
Transsulfuration pathway and the biosynthetic route of H2S. This gasotrasmitter (H2S) is synthesized in the transsulfuration pathway in many organs such as liver, kidney, heart and adipose tissue. There are three main enzymes involved in its formation: cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), which work in concert to transform Homocysteine into H2S, and 3-mercaptopyruvate sulfurtransferase (3-MST) which uses mercaptopyruvate to produce H2S. α-KB: α-ketobutyrate; PLP: Pyridoxal 5′-phosphate.
Not many studies have evaluated the transsulfuration pathway after fructose intake and, even fewer, if the DOHaD theory could be involved. Therefore, we have investigated here whether maternal liquid fructose (10% wt/vol in drinking water) intake affects this pathway in pregnant rats and their foetuses, and if it differs from glucose consumption (10% wt/vol in drinking water) or water without additives (control). In addition, in order to study a possible foetal programming, the H2S production in several tissues was measured in adult male offspring of mothers having consumed different carbohydrates. Subsequently, we wanted to know if males whose mothers had consumed water without additive, fructose or glucose during gestation maintained the same phenotype after themselves receiving fructose (10% wt/vol in drinking water) for three weeks, when adults.
Material and methodsAnimals and experimental designAn animal model of maternal liquid fructose intake was developed as previously described.5,6 Female Sprague-Dawley rats weighing 200–240g were fed ad libitum, a standard rat chow diet (B&K Universal, Barcelona, Spain), and housed under controlled light and temperature conditions (12-h light–dark cycle; 22±1°C). The experimental protocol was approved by the Animal Research Committee of the University San Pablo-CEU, Madrid, Spain (ref. number 10/206458.9/13).
Pregnant animals were randomly separated into a control group, a fructose-supplemented group (Fructose), and a glucose-supplemented group (Glucose) (five to six rats per group). Fructose and glucose were supplied as a 10% (wt/vol) solution in drinking water throughout gestation. Control animals received no supplementary sugar. The total amount of ingested energy did not differ between fructose-fed, glucose-supplemented and control rats, as described in5 and the amount of total calories obtained from simple sugars was around 25% for the fructose-group and 35% for the glucose-group.17 On gestational day 21, food was removed at 8 a.m. and pregnant rats were sacrificed two hours later. Plasma was obtained by blood centrifugation in Na2-EDTA tubes, aliquoted and stored at −80°C until analysis. Liver and adipose tissue were obtained, placed in liquid nitrogen and kept at −80°C until analysis. The conceptus was dissected, and placentas and foetuses were collected. Placentas from the same litter were pooled and frozen. Foetuses (without being separated by gender) were decapitated. Blood from all pups of the same mother was collected and plasma was obtained in tubes containing Na2-EDTA. The livers of the foetuses were obtained, and those coming from the same mother were pooled and placed in liquid nitrogen to be frozen until processed for further analysis.5,17
Other set of pregnant rats was allowed to deliver and on the day of birth, each suckling litter was reduced to nine pups per mother. After delivery, both mothers and their pups were maintained with water and food ad libitum. At 21 days of age, pups were separated by gender and kept fed on a standard rat chow diet (B&K Universal, Barcelona, Spain) and water without additives. Animals within each experimental group were born to different dams to minimize the “litter effects”. At 240 days of age, one half of the male progeny was randomly separated. When the progeny was 261-days-old, it was sacrificed and blood and tissues were collected. Remarkably, these animals had received no subsequent additive in the drinking water for their entire lives.18
The other half of the male progeny was subjected to the next protocol: First, they were weighed and an aliquot of plasma was obtained from the tail in order to confirm that the values between the experimental groups both for body weight and for several analytes (glucose, triglycerides, non-esterified fatty acids, etc.) were similar.19 Later, independently from the experimental group of mothers to which they had been born, they were maintained on solid pellets and supplied with drinking water containing 10% (wt/vol) fructose. Thus, three experimental groups were formed: C/F, F/F, G/F, the first letter indicating whether the mothers had been supplied during pregnancy with tap water (C, control), or water containing a carbohydrate (F: fructose; G: glucose); and the second letter indicating the period with fructose (F), when they were adults. When the progeny was 261-days-old, they were decapitated at 10 a.m. and blood collected using tubes containing Na2-EDTA. Prior to sacrifice, food was removed at 8 a.m. The period with fructose supplementation was selected to last 21 days (from 240 to 261 days of age) as previously described.19 Liver and lumbar and epididymal adipose tissues were immediately removed, placed in liquid nitrogen and kept at −80°C until analysis. Samples were then centrifuged, and plasma was stored at −80°C until processed for later determinations. In parallel, a fourth experimental group was used, C/C: male progeny from control mothers supplied with water without any additives when adult.19,20 There was no difference in the total amount of ingested energy between fructose-fed rats and control (C/C) group19 and, for the three groups of fructose-fed males (C/F; F/F, G/F), around 25% of the total amount of energy was acquired from fructose19 and this is similar to the daily energy intake from simple sugars observed in heavy consumers of SSB in human populations (20–25%).17
Plasma determinationsPlasma H2S levels were measured with the methylene blue method as previously described with modifications.21 Briefly, 200μL of plasma were deproteinized with 150μL 20% TCA. After centrifugation, 250μL of supernatant were treated with 150μL 1% zinc acetate, 100μL 20mM N1,N1-dimethylbenzene-1.4-diamine sulfate (Fluorochem, UK) in 7.2M HCl and 133μL 30mM FeCl3 (Panreac AppliChem, IL, USA) in 1.2M HCl. Samples were then vortexed and incubated at room temperature for 20min and absorbance measured at 630nm. A standard curve from 1.56 to 200μM of sodium hydrosulfide (NaHS, Fluorochem, UK) was performed following the same procedure as with the samples.
Determination of H2S production in liver, adipose tissue and placentaH2S production in liver, lumbar and epididymal adipose tissue and placenta was evaluated following the Lead Sulfide method as previously described with modifications.22 Briefly, tissues were homogenized in Phosphate-buffered saline (PBS) and their protein levels measured with the BCA Assay Kit (Thermo Fisher, MA, USA). One hundred to 300μg of protein were incubated at 37°C in the presence of 10mM Cys (Sigma–Aldrich, MO, USA) or 30mM HCy (Fluorochem, UK), and 20μM Pyridoxal 5′-phosphate (PLP, Sigma–Aldrich, MO, USA) (for livers) or 2mM PLP (for adipose tissue and placenta) on 96 well plates covered with a lead acetate (II) membrane. Incubations were performed until dots of lead sulfide were detected but not saturated, and this occurred after 2h for liver, 6h for adipose tissue and 3h for placenta. In order to prepare these membranes, Whatman n° 2 paper was soaked into 20mM lead acetate (Sigma–Aldrich, MO, USA) and vacuum dried. Dots were densitometered (BioRad Densitomiter G-800, CA, USA) for quantification. A standard curve from 0 to 1000mM NaHS was performed for each membrane.
RNA extraction and gene expression determination by qPCRTotal RNA was isolated from liver or placenta using Ribopure (Invitrogen, ThermoFisher Scientific, USA). Total RNA was subjected to DNase I treatment using Turbo DNA-free (Invitrogen, ThermoFisher Scientific, USA), and RNA integrity was confirmed by agarose gel electrophoresis. Afterwards, cDNA was synthesized by oligo(dT)-primed reverse transcription with Superscript II (Invitrogen, ThermoFisher Scientific, USA). qPCRs were performed using a Light Cycler 1.5 (Roche, Germany). The reaction solution was carried out in a volume of 20μl, containing 10pmol of both forward and reverse primers, 10× SYBR Premix Ex Taq (Takara Bio Inc., Japan) and the appropriate nanograms of the cDNA stock. Rps29 was used as a reference gene for qPCR. The primer sequences were obtained either from the Atlas RT-PCR Primer Sequences (Clontech, CA, USA) or designed using Primer3 software (University of Massachusetts Medical School, MA, USA).23 Samples were analyzed in duplicate on each assay. Amplification of non-specific targets was discarded using the melting curve analysis method for each amplicon. qPCR efficiency and linearity were assessed by optimization of the standard curves for each target. The transcription was quantified with Light Cycler Software 4.05 (Roche, Germany) using the efficiency correction method.24
Statistical analysisResults were expressed as means±S.E. Treatment effects were analyzed by one-way analysis of variance (ANOVA). When treatment effects were significantly different (P<0.05), means were tested by Tukey's multiple range test, using the computer programme SPSS (version 23). When the variance was not homogeneous, a post hoc Tamhane test was performed.
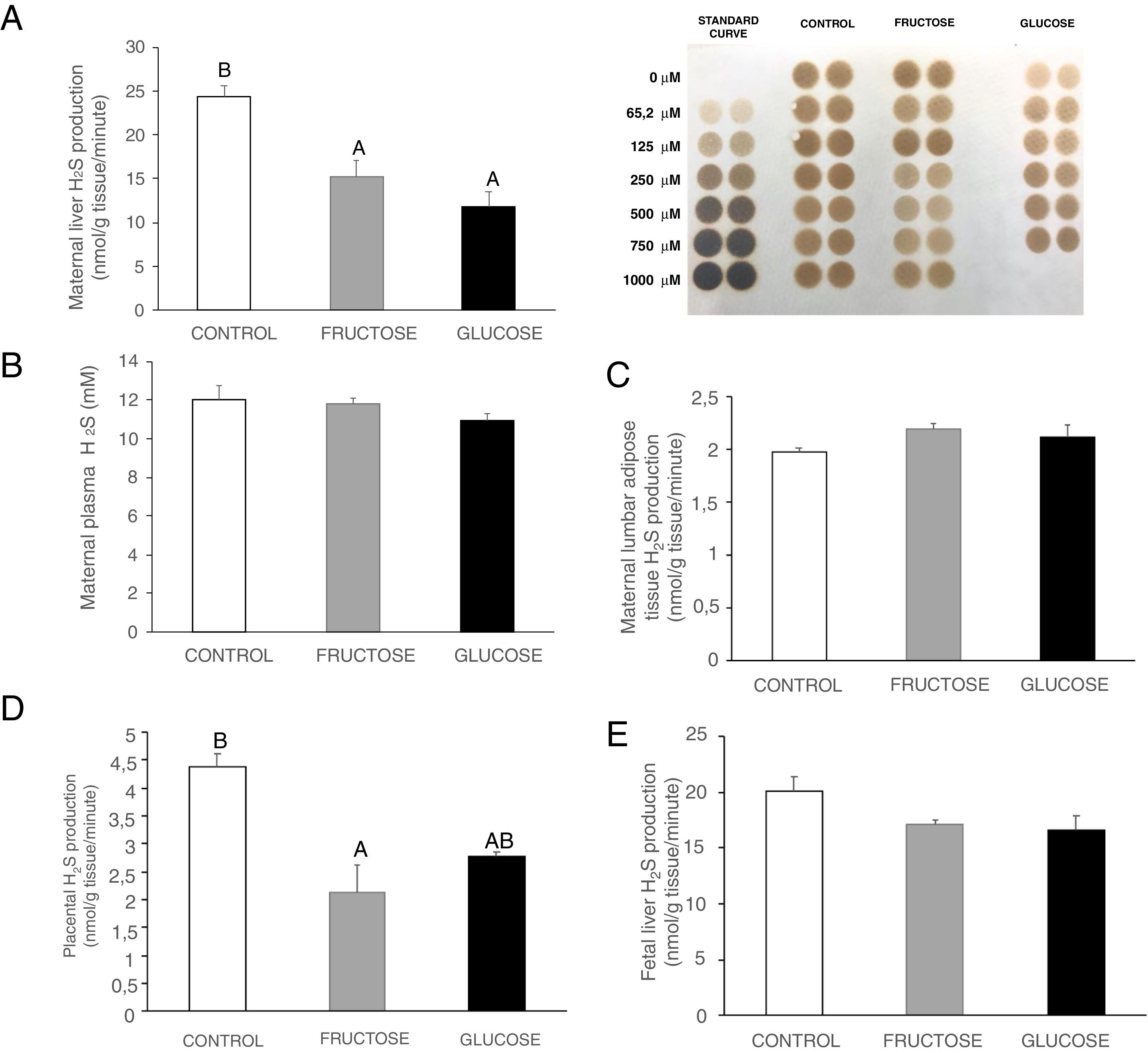
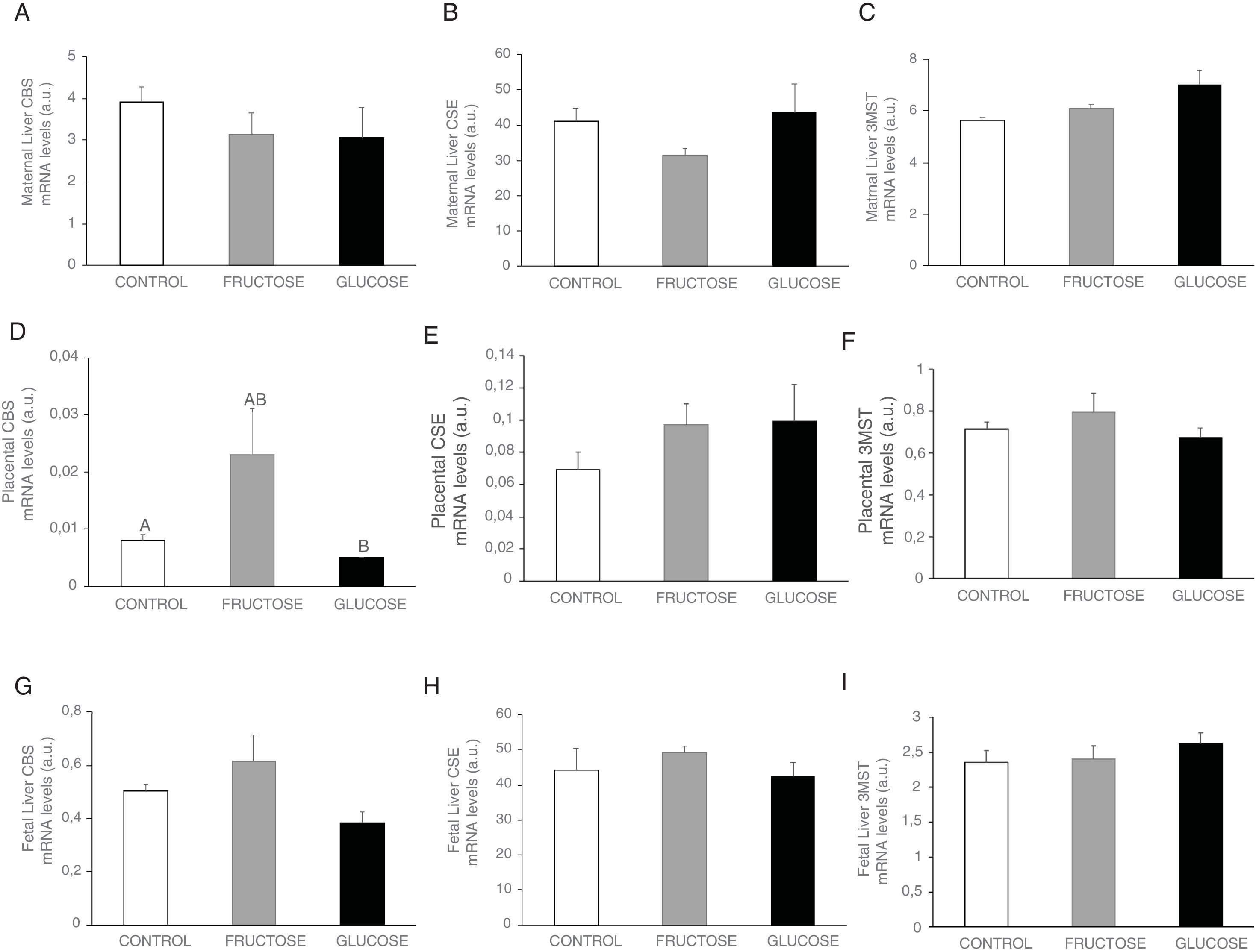
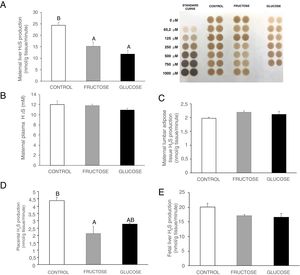
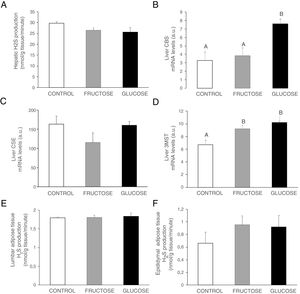
ResultsCarbohydrate intake during pregnancy decreases H2S production in liver and placenta, but not in lumbar adipose tissueHydrogen sulfide is a gasotransmitter produced in the transsulfuration pathway with proved beneficial effects in many metabolic diseases. Surprisingly, H2S production in the liver presented a significant decrease in the two groups of rats that consumed any liquid carbohydrate during gestation in comparison to the control dams (Fig. 2A). This organ is the main producer of H2S in the organism.25 However, plasma H2S levels hardly changed in pregnant rats after fructose or glucose consumption throughout gestation (Fig. 2B). Moreover, the expression of the three main enzymes involved in the transsulfuration pathway did not display significant differences (Fig. 3A–C) in this tissue, although CSE gene expression did show a tendency to decrease in fructose-fed pregnant rats (Fig. 3B). The results obtained in plasma could be related to other tissues that are known to produce this gasotransmitter, although at a lower level, such as adipose tissue.11 In fact, H2S production in lumbar adipose tissue (Fig. 2C) did show a trend to be increased in the two carbohydrate-consuming groups, becoming almost significant when fructose-fed pregnants were compared to control rats (P=0.075).
Carbohydrate (fructose or glucose) consumption during pregnancy affects H2S production in the liver and placenta of pregnant rats, but not adipose tissue. (A) Hepatic H2S production and membrane showing lead acetate precipitates formed in the assay. Darker precipitates indicate higher H2S production in the tissue. (B) Plasma H2S levels of pregnant rats. (C) Lumbar adipose, (D) Placental and (E) Foetal hepatic H2S production of control (empty bar), fructose-fed (grey bar) or glucose-fed (black bar) pregnant rats. Data are means±S.E. from 5 to 6 litters. Values not sharing a common letter are significantly different (P<0.05).
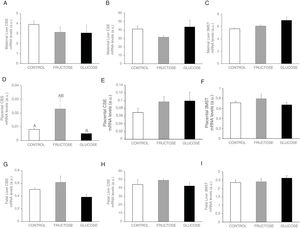
Gene expression (mRNA) of the transsulfuration pathway in the liver (A-C) and placenta (D–F) of control (empty bar), fructose- (grey bar) or glucose-supplemented (black bar) pregnant rats and their foetuses (G–I). Data are means±S.E. from 5 to 6 litters. Different letters indicate significant differences between the groups (P<0.05). Relative target gene mRNA levels were measured by Real Time PCR, as explained in Materials and Methods, and normalized to Rps29 levels and expressed in arbitrary units (a.u.). CBS: cystathionine beta-synthase; CSE: cystathionine gamma-lyase; 3-MST: 3-mercaptopyruvate sulfurtransferase.
In order to evaluate the importance of the placenta in the production of H2S during gestation, both synthesis of H2S and transsulfuration enzymes gene expression were measured. In accordance with the findings observed in liver, placental H2S production was also diminished in carbohydrate-fed dams (Fig. 2D), becoming significant in fructose-fed pregnant rats and showing a trend to decrease, but without reaching statistical significance, in glucose-fed mothers, in comparison to the control rats. As shown in Fig. 3D, CBS gene expression presented an increase in placenta of fructose-fed pregnant rats and it was significant when compared to glucose-fed mothers. On the other hand, CSE and 3MST gene expression did not show significant changes between the three experimental groups (Fig. 3E and F). Nevertheless, gene expression of the three enzymes of the transsulfuration pathway in placenta displayed lower levels than in liver.
As fructose is able to cross the placenta and it can also be produced there,26 all these changes observed after carbohydrate consumption during gestation could also be affecting the foetuses. Thus, H2S production and transsulfuration enzymes gene expression were evaluated. In accordance with the findings found in maternal liver and placenta, H2S production in the liver of foetuses from carbohydrate-fed mothers tended to be decreased when compared to those from control dams (Fig. 2E), although without becoming significantly different. However, neither CBS nor CSE or 3MST gene expression presented changes in liver of foetuses from fructose-, glucose-fed or control mothers (Fig. 3G–I).
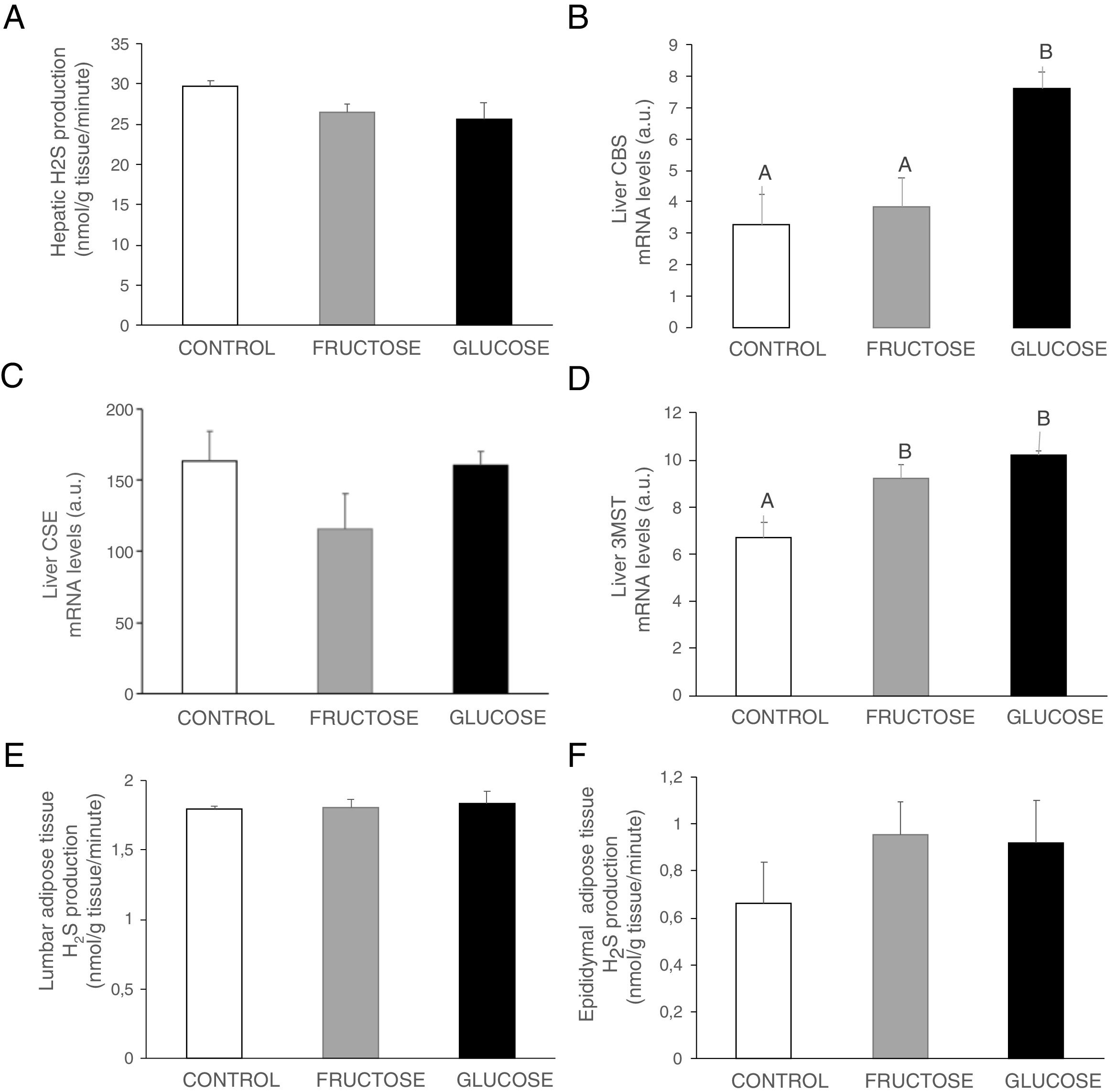
Carbohydrate ingestion during gestation produces changes in the transsulfuration pathway in adult male offspringBearing in mind the results observed in the liver of foetuses from fructose- or glucose-fed mothers, we wanted to evaluate if these changes were maintained in male offspring from carbohydrate-fed dams when adult. As shown in Fig. 4A, H2S production in the liver presented a non-significant decrease in males from fructose- or glucose-fed mothers, in consonance with the effects found in foetal liver (Fig. 2E). Plasma H2S levels were reduced in males from glucose-supplemented mothers in comparison to the other two groups (14.53±2.11; 14.61±0.21; 12.53±0.55μM for males from control, fructose- and glucose-fed dams, respectively; P<0.05, glucose vs. fructose). When gene expression was measured in the liver of male descendants, CBS did show a significant increase in male offspring from glucose-fed mothers compared to control and fructose groups (Fig. 4B), and 3MST displayed higher and significant gene expression in male progeny from carbohydrate-fed mothers versus control offspring (Fig. 4D). This result could be in contrast with the findings observed for H2S production in the liver but CSE, the main enzyme involved in transsulfuration pathway in the liver,27 did show a slight reduction in males from fructose-fed mothers, although this did not become significant (Fig. 4C). On the other hand, although lumbar (Fig. 4E) adipose tissue did not present significant changes in H2S production between the three groups, epidydimal fat pads displayed a non-significant trend to be increased in progeny from carbohydrate-fed rats in comparison to the control group (Fig. 4F).
Carbohydrate intake during pregnancy influences H2S production in the liver and adipose tissue of male offspring. (A) Hepatic H2S production of 261-day-old male progeny from control (empty bar), fructose-fed (grey bar) and glucose-fed (black bar) pregnant rats. Liver gene expression (mRNA) of the transsulfuration pathway enzymes: (B) CBS, (C) CSE and (D) 3-MST of 261-day-old male progeny from fructose- or glucose-supplemented and control mothers. (E) Lumbar and (F) epididymal adipose tissues H2S production. Data are means±S.E. from 5 to 6 litters. Different letters indicate significant differences between the groups (P<0.05). Relative target gene mRNA levels were measured by Real Time PCR, as explained in Materials and Methods, and normalized to Rps29 levels and expressed in arbitrary units (a.u.). CBS: cystathionine beta-synthase; CSE: cystathionine gamma-lyase; 3-MST: 3-mercaptopyruvate sulfurtransferase.
After evaluating the transsulfuration pathway in adult male descendants from carbohydrate-fed mothers, we wanted to discover if that phenotype was conserved by exposing male offspring from control, fructose- or glucose-fed mothers to a fructose consumption and to check if maternal diet influences the response to this fructose exposure.
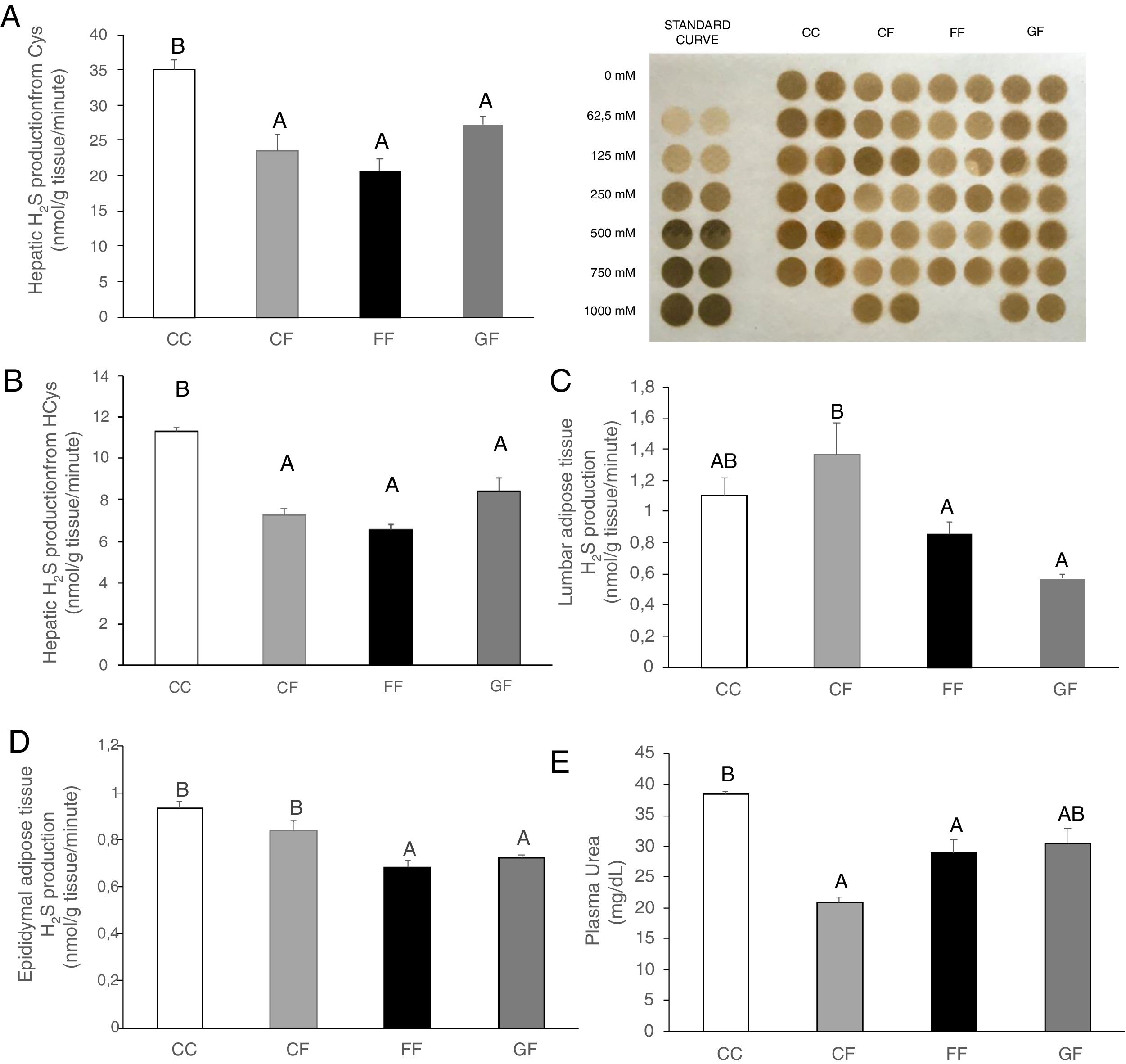
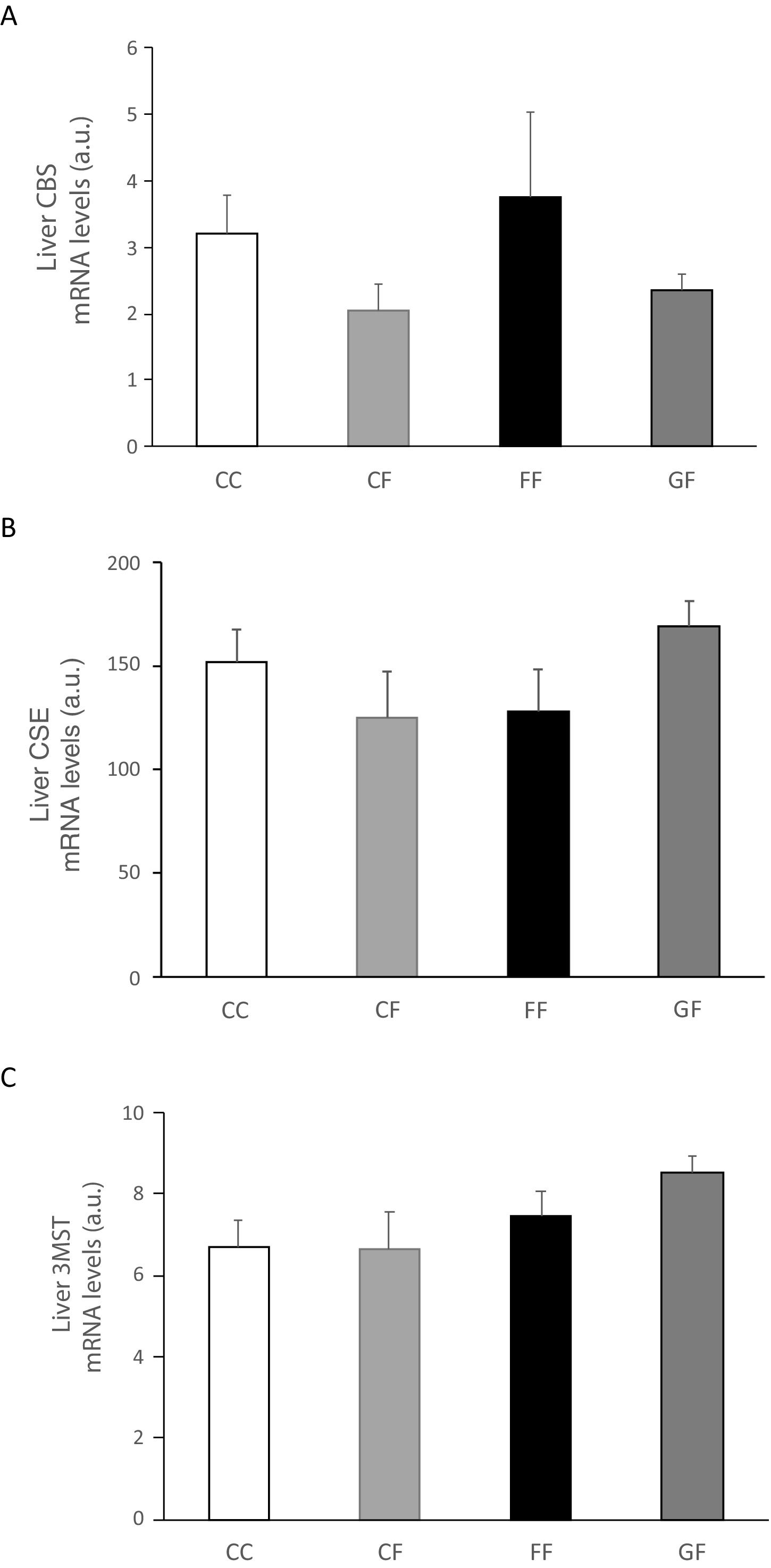
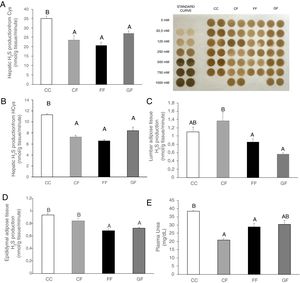
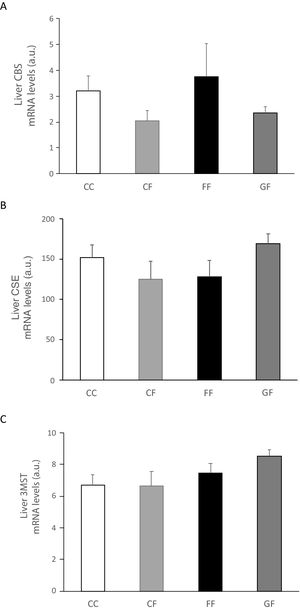
Plasma H2S levels were not affected by fructose ingestion, although they tended to be higher, but not significantly, independently of the maternal consumption (13.34±0.63; 17.72±3.08; 22.22±2.81; 25.06±5.42μM for C/C, C/F, F/F and G/F, respectively). Surprisingly, the hepatic production of this gasotransmitter did seem to present the opposite result to that of plasma data, as shown in Fig. 5A. H2S production was diminished in the liver of male offspring from control, fructose- and glucose-fed mothers after fructose intake (Fig. 5A), this reduction being more pronounced in F/F group. In fact, the effect became almost significant when F/F was compared to G/F group (P=0.065). Moreover, these findings were corroborated when H2S production was measured using HCy as a substrate. It is well known that CSE and CBS use Cys as substrate, whereas HCy is substrate only for CSE.27 Interestingly, the same results obtained for H2S synthesis from Cys (Fig. 5A) were observed when HCy was used as a substrate of the reaction (Fig. 5B). In order to evaluate if these changes were caused by modifications in the trassulfuration pathway enzymes, CBS, CSE and 3-MST gene expression were measured. None of these enzymes presented significant changes in its gene expression (Fig. 6A–C), although CSE, the main enzyme involved in H2S production in the liver,27 tended to be decreased in liver from males that consumed fructose when adult and which mothers had ingested water (C/F) or liquid fructose (F/F) during gestation (Fig. 6B). Interestingly, H2S production in adipose tissue responded to the fructose supplementation in a maternal-intake dependent manner. Thus, both lumbar (Fig. 5C) and epididymal adipose tissues (Fig. 5D) displayed a fructose-induced significant reduction in H2S production in male descendants from fructose (F/F) and glucose (G/F) fed mothers, but not in C/F, when compared to C/C group.
Maternal carbohydrate intake differentially affects fructose-induced diminution of H2S production in the progeny. (A) Hepatic H2S production from Cys and membrane showing lead acetate precipitates formed in the assay. Darker precipitates indicate higher H2S production in the liver. (B) Hepatic H2S production from HCy. (C) Lumbar and (D) Epididymal adipose tissues H2S production of fructose-fed male adult progeny from control (CF, light grey bar), fructose- (FF, black bar), and glucose-supplemented (GF, dark grey bar) mothers. E) Plasma urea. C/C: Control 261-day-old male offspring from control pregnant rats (empty bar, C/C). Data are means±S.E. from 5 to 6 litters. Values not sharing a common letter are significantly different (P<0.05). Cys: cysteine. HCy: homocysteine.
Liver gene expression (mRNA) of the transsulfuration pathway enzymes: (A) CBS, (B) CSE and (C) 3-MST of fructose-fed male adult progeny from control (CF, light grey bar), fructose- (FF, black bar), and glucose-supplemented (GF, dark grey bar) mothers. C/C: Control 261-day-old male offspring from control pregnant rats (empty bar, C/C). Data are means±S.E. from 5 to 6 litters. Different letters indicate significant differences between the groups (P<0.05). Relative target gene mRNA levels were measured by Real Time PCR, as explained in Materials and Methods, and normalized to Rps29 levels and expressed in arbitrary units (a.u.). CBS: cystathionine beta-synthase; CSE: cystathionine gamma-lyase; 3-MST: 3-mercaptopyruvate sulfurtransferase.
In the last decades, obesity, MetS, CVD and diabetes have escalated to epidemic proportions in many countries worldwide.28 The DOHaD theory has linked maternal nutrition to the risk of suffering metabolic diseases in descendants when adult. According to this, early life (pre- and post-natal) nutrition would produce molecular adaptations in pups that will predispose them to a higher risk of metabolic disturbances in later life.3,29 Consumption of SSB and processed foods has increased in recent years and many studies have established a relationship between their consumption and the development of metabolic diseases.18,30,31
H2S is a gasotransmitter whose reduction in plasma has been related to obesity and type 2 diabetes and it seems to protect against the pathogenesis of atherosclerosis.32 Therefore, our goal in the present study is to investigate whether maternal fructose leads to changes in the transsulfuration pathway, which produces H2S, in their pups and if these modifications, if any, are preserved later, when adult. In the present study, neither fructose- nor glucose-fed mothers presented changes in their H2S plasma levels, however a significant reduction in the production of this gasotrasmitter was observed both in liver and in placenta. This discrepancy between H2S plasma levels and its production in liver has been previously reported by Peh et al., but using a high fat diet,33 and they proposed that it could be caused by H2S produced in non-hepatic tissues,34 such as adipose tissue, kidney or gut. In fact, lumbar adipose tissue presented in our carbohydrate-fed mothers a non-significant increase in the H2S production. In contrast to Peh et al.,33 we did not observe significant changes in the gene expression of the transsulfuration pathway enzymes in neither liver nor placenta. Nevertheless, modifications in H2S synthesis have been related not only with the gene expression of transsulfuration pathway but also with the activity of these enzymes.35 Moreover, H2S has been inversely related to oxidative stress35 and, in a previous report, we have showed an imbalanced redox status in these carbohydrate-fed pregnant rats.17
We and others have demonstrated that maternal fructose produces profound molecular and metabolic changes in the foetuses,5,17,30 but there are no studies that evaluate its relationship with imbalances in the transsulfuration pathway in the offspring. Our findings indicate a slight reduction in H2S production in the liver of foetuses from fructose- and glucose-fed mothers and, interestingly, this diminution induced by maternal carbohydrate intake was maintained in male descendants when adult. These results would indicate an example of foetal programming in the offspring. In fact, we have recently demonstrated that maternal fructose is able to modify the methylation state of gene promoters of key proteins in cholesterol metabolism in the adult progeny.36
Bearing in mind that epigenetic modifications can cause different responses to environmental factors,37 we have demonstrated that the fructose-induced diminution in H2S production observed here in liver and adipose tissue of male rats is a maternal nutrition-dependent effect. Beneficial effects of H2S in cardiovascular events have been widely studied,38,39 so a reduction in the gasotransmitter levels could be one of the links between fructose consumption and symptoms of CVD and MetS. In fact, we have previously reported that fructose intake provokes characteristic features of MetS, such as oxidative stress and lipid abnormalities, in the adult offspring and that these imbalances are influenced by maternal intake of carbohydrates.19,20 However, H2S plasma levels were not reduced by fructose consumption independently of that had been taken by their mothers. Therefore, more specific studies are needed to connect the diminution in H2S production, H2S plasma levels and characteristics of MetS observed in our rats. Nevertheless, as mentioned above, other tissues that can also produce H2S could be involved.33 In addition, reductions in H2S production have been related to insulin resistance in adipose tissue11 and, interestingly, our animals which were fed liquid fructose showed such a decrease. We were not able to detect systemic insulin resistance in these male rats,19 but it could possibly exist locally, in adipose tissue.
The administration of food containing 75% fructose in rats conduced to a reduction in CSE levels in thoracic aortas producing an impaired endothelium-dependent vasorelaxation40 has been described and, further, a diet with 65% fructose did lead to oxidative stress and insulin resistance in rats with decreased levels of H2S in plasma.41 In the present study, we reported similar alterations in the transsulfuration pathway both in liver and adipose tissue but using a much lower fructose amount (10% w/v). The reduction in H2S production observed here in the liver could be due to a diminished CSE, the main transsulfuration enzyme,27 gene expression in C/F and F/F groups, but not in G/F. Nevertheless, since H2S is generated from HCy,42 this reduction of H2S production could possibly be related to lower plasma levels of HCy which we have found in the three groups that received fructose (data not shown). Furthermore, modifications described here in H2S synthesis could also be due to changes in activity of the enzymes in the transsulfuration pathway, where fructose would be affecting it directly or indirectly through diverse factors such as calcium, oxidative stress, hormones or glucose.35 Interestingly, the effects observed here for H2S in liver and adipose tissue are probably more relevant than if we had found them in plasma.33 Whereas plasma H2S levels are the sum of production from many tissues, H2S synthesis is key in the functioning of the tissue. This would be the situation for, among others, the aorta and relaxation of endothelium, the adipose tissue and insulin sensitivity, the kidney and blood pressure, and the placenta and preeclampsia.11,40,43,44 In fact, although we did not see changes in plasma H2S levels, the fructose-induced modifications in tissue production of H2S found here would parallel the low levels of plasma urea (Fig. 5E). Since it is known that ammonia, produced along with the H2S in the transsulfuration pathway (Fig. 1), participates in the synthesis of urea, these results observed for urea are confirmatory of the reduced transsulfuration pathway after a fructose intake.
In summary, this study shows in pregnancy that a fructose-rich diet per se or situations producing an impaired insulin sensitivity such an excessive intake of glucose5 generate a clear diminution in the hepatic and placental production of H2S. And this effect is observed not only in pregnant mothers, but also in male offspring (both in foetal and in adult stages) and, mainly, when their offspring are themselves subjected to a high fructose intake. Interestingly, these results correlate with others published where proatherogenic diets also caused H2S depletion.33,34,41,45 Given this gasotransmitter has been proposed as a possible treatment for CVD and as a protective factor in developing MetS alterations,38 the importance of these findings lies in the escalating worldwide intake of SSBs and its, increasingly clearer, relationship with non-communicable diseases.2,46 Altogether, the clinical significance of the transsulfuration pathway studies and its connection with fructose intake should be taken into consideration.
Authors’ contributionC.B. conceived and designed the study. S.R., E.F., R.A., P.O. and M.I.P. contributed reagents/materials/analysis tools for gene expression studies and parameter analysis. L.R. handled the animals. M.I.P. and J.J.A.-M. analyzed the data. C.B. and E.F. wrote the paper. None of the authors have any conflicts of interest to report.
The authors thank Jose M. Garrido and his team for their help in handling the rats, and Brian Crilly for his editorial help. This work was supported by funds from the SEA-FEA grant “Manuel de Oya” for research in nutrition (2016), the Ministerio de Ciencia, Innovación y Universidades (MICINN) (SAF2017-89537-R) and European Union FEDER funds. S.R. was supported with a FUSP-CEU fellowship. E.F. is supported with a FPU fellowship from MICINN.