The prehabilitation of the abdominal wall through the infiltration of botulinum toxin type A, which induces temporary chemical denervation ("chemical component separation") in the lateral abdominal musculature, is a common practice in units specialized in abdominal wall surgery. However, its use for this indication is currently off-label.
The main objective of this article is to describe a consensus proposal regarding indications, contraindications, dosages employed, potential side effects, administration method, and measurement of possible outcomes. Additionally, a proposal for an informed consent document endorsed by the Abdominal Wall Section of the Spanish Association of Surgeons is attached.
La prehabilitación de la pared abdominal con la infiltración de toxina botulínica tipo A, que produce una denervación química temporal (“separación de componentes química”), en la musculatura lateral del abdomen es una práctica habitual en las unidades con especial dedicación a la cirugía de pared, pero su uso para esta indicación está actualmente fuera de ficha técnica.
El objetivo principal de este artículo es describir una propuesta de consenso sobre las indicaciones, contraindicaciones, dosis empleadas, posibles efectos secundarios, método de administración y medición de los posibles resultados. Además, se adjunta una propuesta de consentimiento informado avalado por la Sección de Pared Abdominal de la Asociación Española de Cirujanos.
Ventral hernias represent a highly heterogeneous group of hernias that include both primary (umbilical, epigastric, Spiegel's hernia or lumbar hernia) and acquired hernias that can be incisional (midline, oblique, stomatal closures, previous insertion of laparoscopic trocars, McBurney incisions, etc.). and parastomal. Inguino-femoral hernias are more prevalent, sometimes giant inguinoscrotal hernias with loss of domain and may require prehabilitation of the abdominal wall. It is estimated that in the United States alone, more than 500,000 patients with these pathologies are operated on each year, so this represents a major socioeconomic problem.1
A 2005 national study carried out in 144 healthcare areas in 13 Autonomous Communities in Spain reported 20,772 ventral or incisional hernia operations (6.66 operations per 10,000 inhabitants per year).2
The objective of hernia repair is to achieve a tension-free fascial closure, reinforced with mesh, that provides direct support to reconstruction and reduces the lateral strain on the repair. In patients with a hernia with a transverse diameter of over 10 cm (W3 of the EHS classification3), with chronically retracted lateral abdominal musculature or with a large volume of herniated visceral contents, closure of the defect may be difficult or even impossible. The placement of bridge meshes in these cases is associated with unacceptably high hernia recurrence rates.4 Surgical techniques of component separation, which are frequently performed during abdominal wall reconstruction, facilitate fascial closure but are associated with greater morbidity (surgical site infection, seromas, hematomas, etc.).
The first experimental study reporting the benefits of botulinum toxin type A (BTA) on rat abdominal wall musculature was published by Cakmak in 2006.5
Preoperative chemical paralysis of the lateral abdominal wall by BTA in humans, first described in 2009 by Ibarra-Hurtado et al., results in increased muscle flexibility and reduces the lateral strain required for midline closure without altering the anatomy.6 Possibly, in some cases, this may even avoid the need to undertake the separation of components, and the placement of a retromuscular mesh following the Rives-Stoppa technique may be sufficient.7,8
In cases with large volumes of herniated contents (ratio of the volume of the sac to the volume of the abdominal cavity or Tanaka index greater than 20%9) insufflation of progressive pneumoperitoneum (PPP) insufflation may be associated with toxin infiltration, a technique described in 1947 by Goñi Moreno.10 This technique enables the abdominal cavity to expand; reduces adhesions; decreases loop oedema; and influences the improvement of respiratory adaptation after the reintroduction of the herniated contents, thus reducing the risk of causing abdominal compartment syndrome.11,12
BTA has also been shown to be useful in delayed closure of the open abdomen and in later closure of the abdominal wall after damage control laparotomy.13
Despite the fact that this is an intervention widely used in units with particular attention to abdominal wall surgery, its use for this indication is currently outside the technical specifications. This obliges us to request this medication for compassionate use, since this is the only legal framework we can use for this medicament outside a clinical trial, under conditions other than those reflected in its technical specifications. In centres where the toxin is needed with a degree of frequency for its use in this indication, the drug can also be obtained by presenting a protocol to the hospital's Pharmacy Committee. Once approved, this avoids the need to request it for each patient as if it were the first time it was to be used. Perhaps publications like this one will help pharmaceutical companies to review the indications for botulinum toxin A and include prehabilitation of the abdominal wall in the technical specifications as one of its indications, so that this can later be updated by the Spanish Agency for Medicines and Medical Devices (AEMPS in its Spanish acronym).14,15
Nor do we have a consensus on the indications, contraindications, doses used, possible side effects, method of administration and measurement of possible outcomes. The main objective of this study is to present a consensus-based document on its use and a proposal for an informed consent document endorsed by the Abdominal Wall Section of the Spanish Association of Surgeons.
Mechanism of actionThe most commonly used subtypes for medical application are BT type A and type B.
Botulinum toxin type A blocks the release of acetylcholine at the level of peripheral cholinergic nerve endings by cleaving SNAP-25, a protein necessary for the proper fixation and release of acetylcholine from vesicles located in the nerve endings. After injection, the toxin initially binds rapidly and with high affinity to specific receptors on the cell surface. The toxin then passes through the plasma membrane via receptor-mediated endocytosis and is released into the cytosol. This last step is linked to a progressive inhibition of acetylcholine release; Clinical signs manifest at 2–3 days, with a maximum effect at 5–6 weeks after injection.
Recovery after intramuscular injection typically occurs at 12 weeks after injection, as the nerve endings branch off and reconnect with the endplates.16
The final effect is a temporary chemical denervation ("chemical component separation") at the neuromuscular junction without producing any physical injury to the nerve structures.17
The benefit of the neurotoxin is based on the decrease in lateral tension on the hernia defect and elongation of the lateral muscles of the abdomen, with a subsequent increase in the volume of the abdominal cavity, enabling tension-free abdominal reconstruction for a period varying between 1 and 3 months.18 In addition, this relaxation can decrease intraabdominal pressure, improving pulmonary ventilation, thus reducing the need for and duration of invasive respiratory support, as described in several publications.13,19,20
Duration of effectWhen a striated muscle is infiltrated, paresis begins after two to five days, peaking at 4 weeks and lasting 2–3 months before gradually disappearing over the next 6–9 months.20,21 That is why hernia repair is recommended after one month, with the advantage that the beneficial effect of BTA will be maintained longer, protecting the excess tension that may occur in the closure of the fascia during the intervention.22
When antibodies to BTA are formed in cases of repeated infiltrations over time, which is not usually the case for use in abdominal wall surgery, the duration of action and the extent of the maximum therapeutic effect are usually after a few infiltrations (partial therapeutic failure) before complete therapeutic failure occurs. The subjective duration of action varies between patients with the same condition and between patients with different conditions. There is a correlation between the amount of BTA applied and the duration and extent of the paresis caused. However, relatively low doses of BTA, in fact, produce significant paresis.21
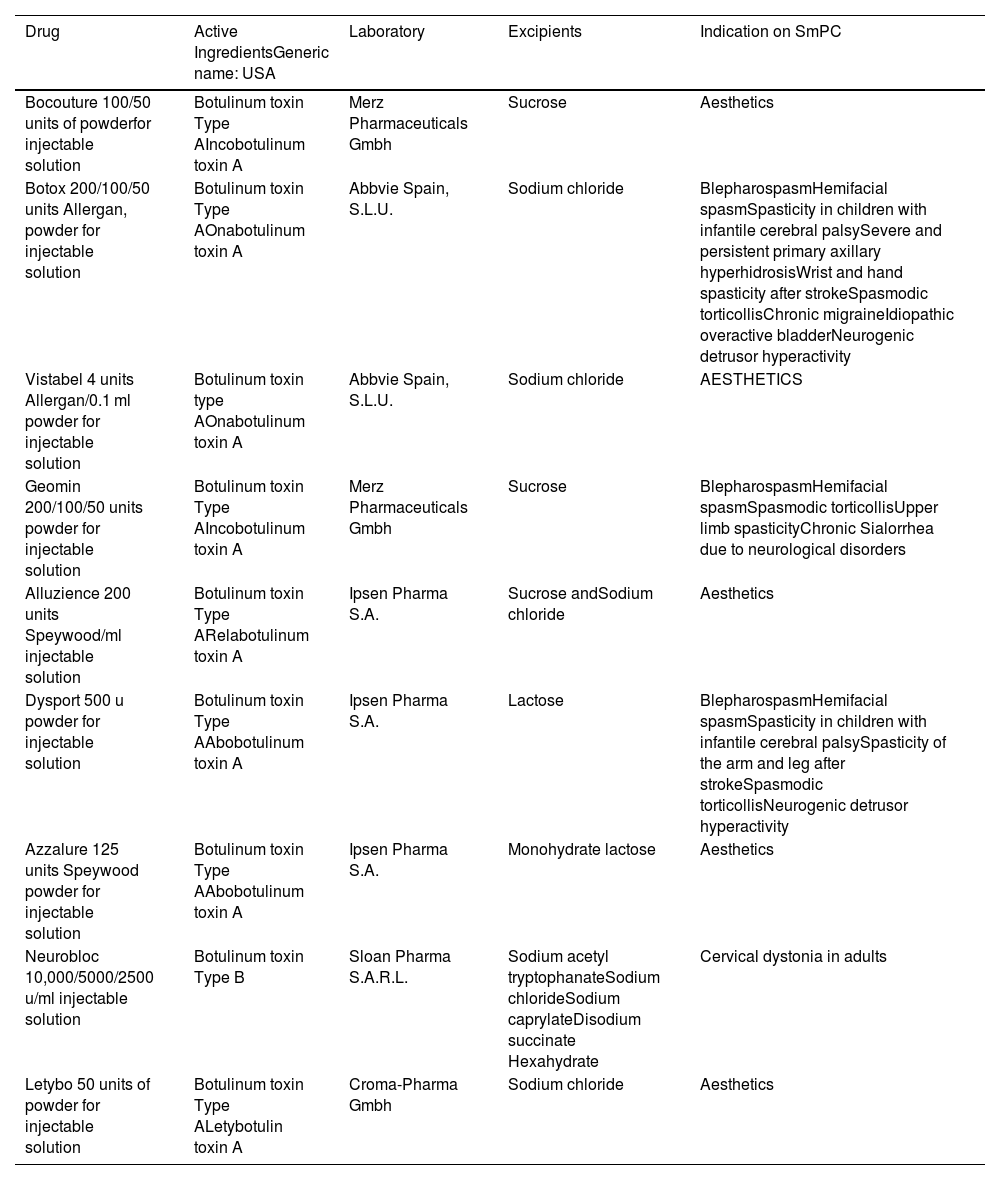
IndicationsUntil Dr Ibarra's publication, its use in hernia pathology was not considered, but BTA had already been shown to be useful for the treatment of many conditions.6 Nowadays it is accepted for use in movement disorders and spasticity (dystonia, bruxism, etc.), hypersecretory disorders (hyperhidrosis, and sialorrhea, etc.), ophthalmic problems (strabismus, nystagmus, etc.), pain (tension headache, migraine, etc.), pelvic floor disorders (vaginismus), gastrointestinal disorders (anal fissure), and cosmetics.21 The indications accepted in the specification is for each of the toxins placed on the market are shown in Table 1.
Table of toxins authorised by the AEMS (Spanish Agency for Medicaments and Medical Devices) and their indications approved on the technical data sheet.
| Drug | Active IngredientsGeneric name: USA | Laboratory | Excipients | Indication on SmPC |
|---|---|---|---|---|
| Bocouture 100/50 units of powderfor injectable solution | Botulinum toxin Type AIncobotulinum toxin A | Merz Pharmaceuticals Gmbh | Sucrose | Aesthetics |
| Botox 200/100/50 units Allergan, powder for injectable solution | Botulinum toxin Type AOnabotulinum toxin A | Abbvie Spain, S.L.U. | Sodium chloride | BlepharospasmHemifacial spasmSpasticity in children with infantile cerebral palsySevere and persistent primary axillary hyperhidrosisWrist and hand spasticity after strokeSpasmodic torticollisChronic migraineIdiopathic overactive bladderNeurogenic detrusor hyperactivity |
| Vistabel 4 units Allergan/0.1 ml powder for injectable solution | Botulinum toxin type AOnabotulinum toxin A | Abbvie Spain, S.L.U. | Sodium chloride | AESTHETICS |
| Geomin 200/100/50 units powder for injectable solution | Botulinum toxin Type AIncobotulinum toxin A | Merz Pharmaceuticals Gmbh | Sucrose | BlepharospasmHemifacial spasmSpasmodic torticollisUpper limb spasticityChronic Sialorrhea due to neurological disorders |
| Alluzience 200 units Speywood/ml injectable solution | Botulinum toxin Type ARelabotulinum toxin A | Ipsen Pharma S.A. | Sucrose andSodium chloride | Aesthetics |
| Dysport 500 u powder for injectable solution | Botulinum toxin Type AAbobotulinum toxin A | Ipsen Pharma S.A. | Lactose | BlepharospasmHemifacial spasmSpasticity in children with infantile cerebral palsySpasticity of the arm and leg after strokeSpasmodic torticollisNeurogenic detrusor hyperactivity |
| Azzalure 125 units Speywood powder for injectable solution | Botulinum toxin Type AAbobotulinum toxin A | Ipsen Pharma S.A. | Monohydrate lactose | Aesthetics |
| Neurobloc 10,000/5000/2500 u/ml injectable solution | Botulinum toxin Type B | Sloan Pharma S.A.R.L. | Sodium acetyl tryptophanateSodium chlorideSodium caprylateDisodium succinate Hexahydrate | Cervical dystonia in adults |
| Letybo 50 units of powder for injectable solution | Botulinum toxin Type ALetybotulin toxin A | Croma-Pharma Gmbh | Sodium chloride | Aesthetics |
In order to establish the correct indications for the toxin, it is essential to perform a preoperative CT scan, if possible with the Valsalva manoeuvre, since this enables us to objectify both the herniated volumes and the measurements of the defect.
Tanaka et al., in 2010, were the first authors to provide an objective method for calculating diameters and volumes, based on tomography findings, by treating a herniated volume as an ellipsoid and calculating the percentage of that herniated volume (hernia sac volume/abdominal cavity volume).9 With tomographic studies in the days prior to surgery, a significant increase in the length of the lateral muscles can be seen and their change in thickness observed after BTA infiltration.8,23
The range of transverse diameters in the different publications where BTA was used is 10–15 cm.24 Most publications indicate infiltration of BTA with transverse diameters greater than 10 cm or W3 in the EHS classification,3 although some groups, such as the Hernia Institute Australia included patients with smaller transverse diameters in their publications.25 In clinical practice, this is usually associated with progressive pneumoperitoneum when the transverse diameter is less than 10 cm but there is a large herniated volume (Tanaka indices greater than 20%). Different groups of researchers have demonstrated the beneficial effect of the combination of progressive pneumoperitoneum insufflation and BTA infiltration in hernias with loss of domain and transverse diameters greater than 10 cm.26,27 The authors' proposal as regards indications for prehabilitation in abdominal wall pathology with botulinum toxin type A (BTA) and/or progressive pneumoperitoneum (PPP) are shown in Figure 1.
Proposed indications for prehabilitation in abdominal wall pathology with botulinum toxin type A (BTA) and/or progressive pneumoperitoneum (PPP).
* Herniated Volume Percentage or Tanaka Index = Hernia Sac Volume (cm3)/Abdominal Cavity Volume (cm3).9.
- 1
Hypersensitivity to the active substance or to any of the excipients.
- 2
Presence of infection at the injection sites.
- 3
Patients with pervasive disorders in muscle activity, such as myasthenia gravis or Eaton-Lambert syndrome.
- 4
Chronic respiratory disorders or a history of bronchoaspiration.
- 5
Other causes of dysphagia.
- 6
Pregnancy, except in cases where its beneficial effects, which are highly unlikely, justify any possible risk to the foetus.
- 7
During breastfeeding. It is not known whether or not BT is excreted in breast milk.
- 8
Patients on anticoagulation because it is an intramuscular injection.
In the cases described in points 3, 4, 5 and 6, BTA should only be administered after a careful assessment of the risk/benefit balance in each individual case, with particular attention to the surveillance of these patients after administration. Extreme caution should be exercised in patients with a history of dysphagia or aspiration.14,15 Contraindication 8 is relative, as long as anticoagulation is reversed (e.g., discontinuation of vitamin K antagonist anticoagulants, switching to bridging therapies with low molecular weight heparins).
Adverse reactions and warnings14,15No major adverse reactions have been reported in abdominal wall muscle infiltration. In some cases, a mild local reaction may occur at the puncture sites that disappears without treatment. Some patients with previous constipation have had slight worsening of this, probably due to the abdominal muscle flaccidity produced by the toxin, that hinders the defecatory effort.
Adverse reactions in locations remote from the site of administration, resulting from the effects of the toxin, have been reported. Patients treated with therapeutic doses may have excessive muscle weakness.
Very rare cases of death in the case of infiltration into other locations have occasionally been reported in the context of dysphagia and lung disease (including, but not limited to, dyspnoea, respiratory failure and respiratory arrest) and/or in patients with significant asthenia following treatment with botulinum toxin A or B.
In some cases of patients with swallowing or breathing problems, although rare, bronchoaspiration has occurred, which is a risk when treating patients with a chronic respiratory disorder or where there is a history of aspiration.
Patients and their caregivers should be advised of the need for immediate medical treatment, should they experience swallowing problems, breathing disorders, or speech disorders.
As with any intramuscular injection, in patients with prolonged bleeding periods, or who have infection or inflammation at the injection site, BTA should only be administered when strictly necessary.
Infiltration into other locations may involve a possible risk of muscle weakness or visual disturbances which, if present, may temporarily impair the ability to drive or use machinery.
This medication contains a small amount of human albumin. This complies with the requirements of the European Union for this type of product. However, the risk of transmission of viral infections cannot be excluded with absolute certainty when using products based on human blood or blood derivatives.
The formation of neutralising antibodies to botulinum toxin has rarely been observed in patients receiving botulinum. Clinically, this could be suspected due to a significant deterioration in the response to treatment and/or the need to systematically administer higher doses, however this fact has not been described in its application for abdominal wall surgery, since the doses are not normally repeated.
FertilityAnimal studies have demonstrated an effect on fertility, as higher than recommended doses produce severe maternal toxicity associated with implantation losses.
Special precautions for useFor its use, the following recommendations mut be followed:
- 1
BTA shall only be used to treat one single patient during a single session.
- 2
When preparing and handling BTA solutions, the use of gloves is recommended.
- 3
If the dry powder or reconstituted solution of the preparation comes into contact with the skin or mucous membranes, these should be thoroughly washed with water.
- 4
Immediately after treatment, any residue that may remain in the vial or syringe should be inactivated by diluted hypochlorite solution (1% available chlorine).
- 5
All material used shall then be disposed of, as per standard hospital practices.
- 6
Any spilled amount of BTA should be rinsed off with an absorbent cloth soaked in dilute hypochlorite solution.
The recommended dosage and frequency of administration should not be exceeded.
In Spain, to date, the toxins authorised by the AEMPS (Spanish Agency for Medicines and Healthcare Products) are for hospital use and their indications approved in the technical specifications are different for each one. The only botulinum toxin type B is Neurobloc® and its indication is exclusively for cervical dystonia (Table 1).
The most commonly used versions in abdominal wall rehabilitation in Spain are Dysport (abobotulinum toxin A) and Botox (onabotulinum toxin A).
On the basis of the recommendations of the Pharmacovigilance Working Party (PhVWP), the AEMPS, in coordination with other European Regulatory Agencies recommends15:
- 1)
Botulinum toxin-based medicines should only be administered by doctors with sufficient experience, including the use of the necessary equipment.
- 2)
Recommended administration techniques and dosing instructions specific to each medicinal product and indication should be followed (including the recommendations to use the minimum effective dose and to adjust the dose to individual needs).
- 3)
Botulinum toxin units are not interchangeable between different medications.
- 4)
Patients or their caregivers should be informed of the risk of spreading the toxin and warned to seek medical attention immediately if breathing, speech, or swallowing disorders occur.
The contents of the BTA vial are diluted to different concentrations with sodium chloride 9 mg/mL (0.9%) solution for injection without preservatives, depending on the individual protocols at each centre. This reconstitution should be done by mixing gently.14
The doses used in each centre are variable.28 Our recommendation is as follows: dilute the vial of Dysport 500 IU in 20 ml of saline solution and do the infiltration of 2 ml in each chosen area (5 punctures on each side/ 50 IU for each puncture/500 IU in total). The equivalence of Dysport 500 IU would be with Botox 300 IU, although the infiltration of Botox 200 IU has not shown any difference in terms of results in abdominal wall pathology.29 In the case of using Botox, a possible recommendation would be to dilute it in 20 ml of saline solution and perform the infiltration of 2 ml in each chosen area (5 punctures on each side/20 IU for each puncture/200 IU in total).
In the case of choosing infiltration only in 3 areas on each side, the IU for each puncture must be recalculated.
Method of administrationThe neurotoxin is applied on an outpatient basis 4–6 weeks before hernia repair surgery. Usually, it is the surgeons themselves who administer it, however in some centres it is the radiologists, anaesthesiologists or neurophysiologists who are in charge of this task. Most of those administering the treatment use ultrasound to visualise the muscles of the lateral abdominal wall and locate infiltration sites.8,19,20,22,26,30–33 (Fig. 2)
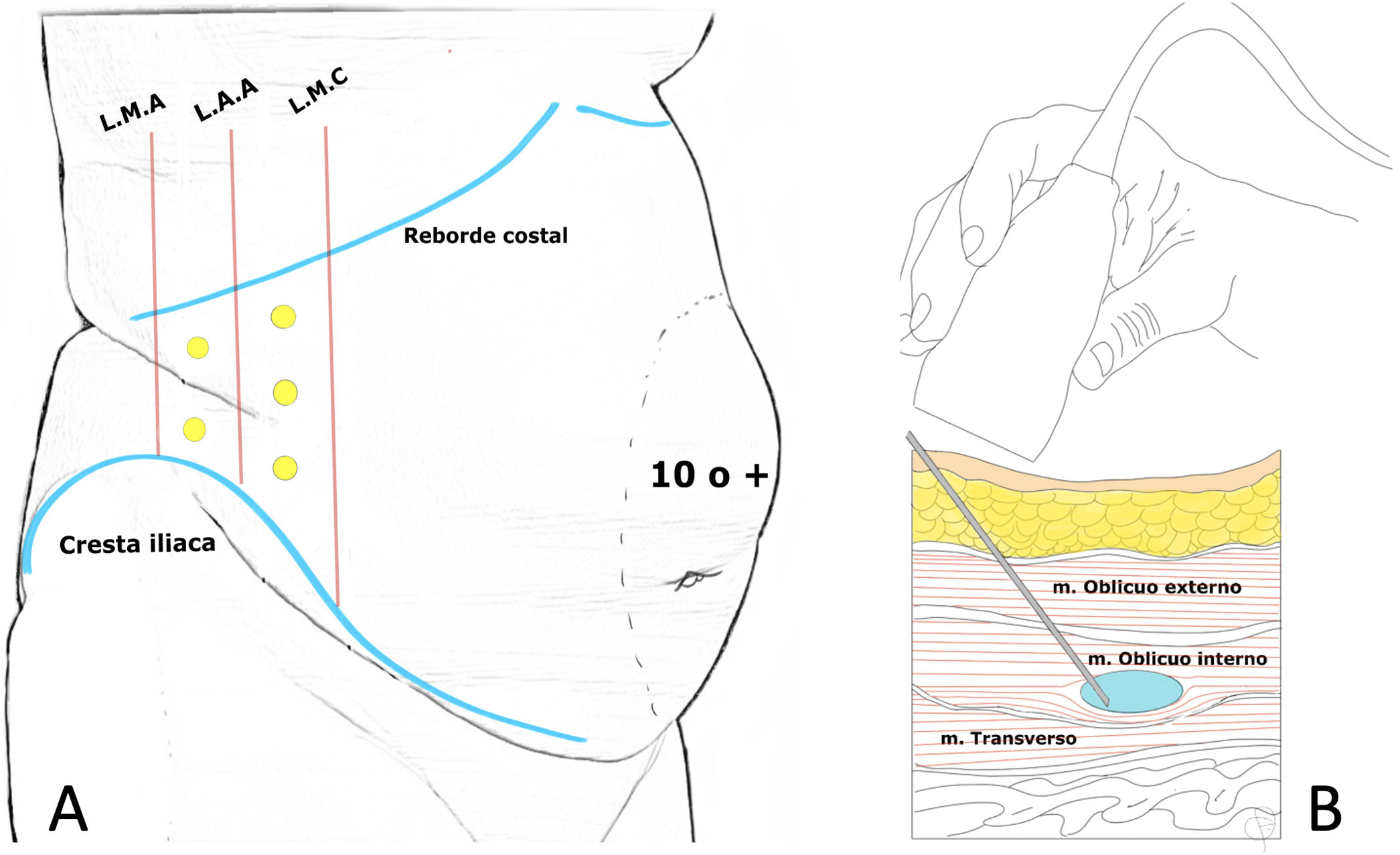
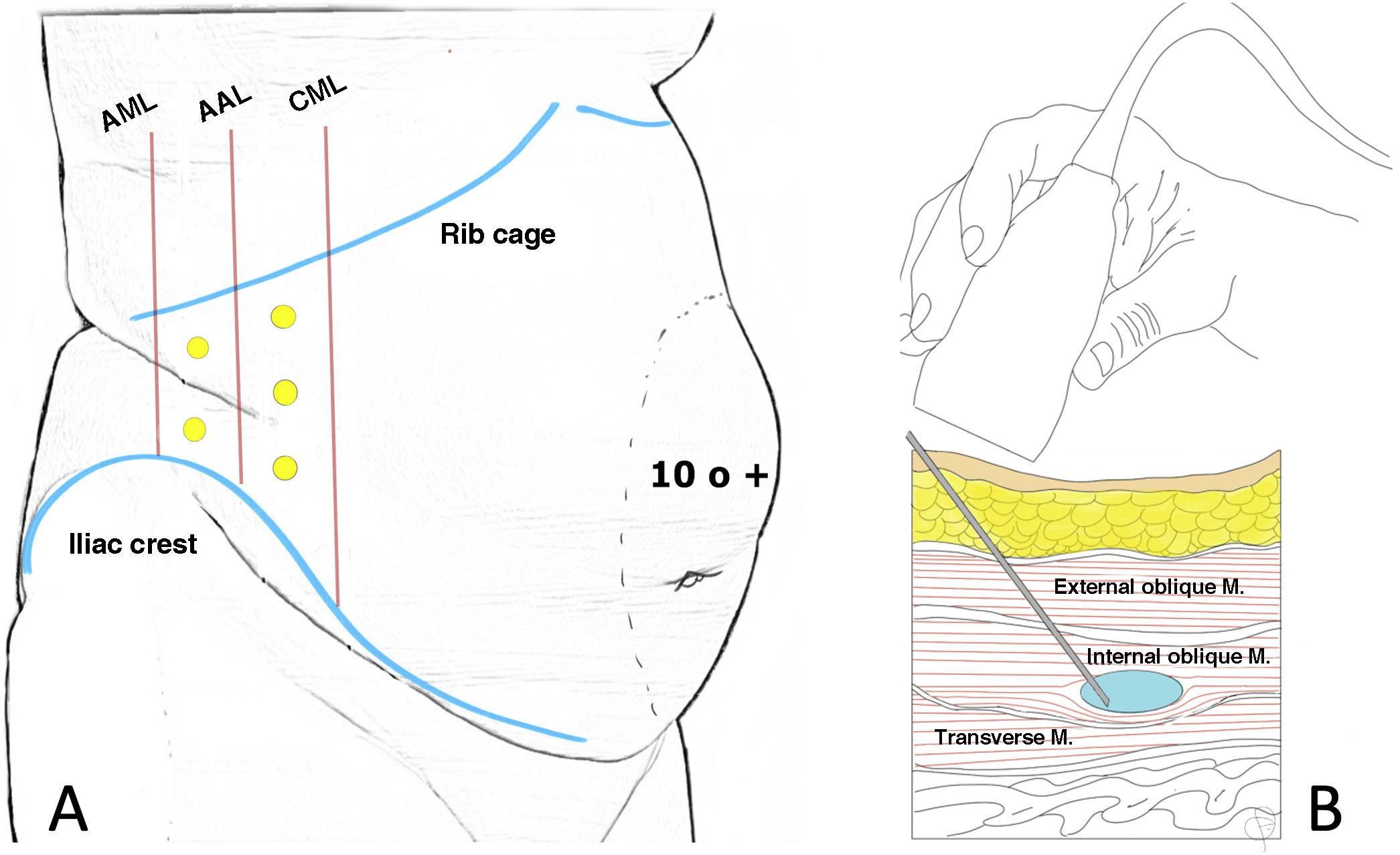
Location of infiltration points as described by Tomás Ibarra6 (A) and ultrasound administration of type A toxin in the lateral abdominal muscles (B).
In some centres electromyography is associated with ultrasound, which adds precision to the procedure as this makes it possible to identify whether the area where it is going to be infiltrated is denervated or fibrotic, enabling the change of location and increasing the desired effect.6,8,26,31–33
In the initial description of the procedure described by Tomás Ibarra, the patient is placed in lateral decubitus and five infiltration points are located on each side (two at the axillary midline, at equidistant points between the costal rim and the iliac crest and three more at the anterior axillary line and midclavicular between the costal margin and the anterosuperior iliac spine).6 (Fig. 2)
Other authors13,22,30 use 3 infiltration points on each side (located on the anterior axillary line, equidistant between the lower edge of the rib and the anterosuperior iliac spine) following Smoot's description.34
No major complications in the procedure have been reported in the latest systematic reviews.35
All three muscle layers can be infiltrated and many authors describe this.36 Ibarra-Hurtado's studies first injected BTA into the external oblique muscle only (2009)6 and then (2014)37 between the external and internal oblique muscles. In the Chan38 and Elstner 39 studies, the injection was performed in only two layers, excluding the transverse abdominal muscle. Elstner compared the standard three-layer injection with the two-layer injection and concluded a significant effect on elongation with both techniques, although there was no difference between the two.39 It is probably sufficient to identify the internal oblique muscle and do the infiltration into this muscle, since when a single bolus is injected into the centre of the muscle, the effects of the injected TB can extend beyond the limits of the target muscle, producing the desired effect also in the external oblique and transverse muscle. However, when the total dose is distributed in small doses throughout the muscle, biological activity can be contained within the target muscle40 (Fig. 2).
There is increasing evidence supporting neuroaxonal transport of the toxin to distal areas,40 therefore, it would not be unlikely that future studies would indicate that perineural infiltration in posterior roots would be more effective.
Measurement of resultsA systematic review and meta-analysis with 23 studies and 995 patients was published in 2021 showing that BTA provides a significant lateral abdominal wall elongation of 3.2 cm per side (95% CI 2.0–4.3; I2 = 0%; p < 0.001); 6.3 cm of total elongation, and a significant but heterogeneous decrease in the cross sectional width of the hernia (95% CI: 0.2–6.8; I2 = 94%; p = 0.04).35 In addition, this meta-analysis shows that prior treatment with BTA in patients with ventral hernia significantly increases the rate of fascia closure [RR 1.08 (95% CI 1.02–1.16; I2 = 0%; p = 0.02)].35
An even more recent systematic review and meta-analysis from this same year, after the quantitative analysis of 7 studies involving a total of 261 patients, revealed a marked advance of 4.11 cm on each side, with a 95% confidence interval (3.76–4.46) and a heterogeneity of I2 = 27% which is considered low, thus confirming the value of the toxin for this benefit.29
CostThe costs of BTA depend on the dose used and on each country41,42 but although costs are rarely reported, they are estimated to be between 400 and 600 euros when used on the muscles of the abdominal wall.8,43
The lab sale price of 2 vials of 500 IU from Dysport is Є284 and that of a vial of Botox 200 Units Allergan, Powder for Injectable Solution is Є143.16,44
Informed consentAppendix 1 shows the informed consent document, with a copy for the patient and the hospital, proposed by the board members of the Abdominal Wall Section and approved by the Quality Section and Scientific Committee of the Spanish Association of Surgeons.
Conflict of interestThe authors declare that they have no conflict of interest.
Appendix AMembers of the Section Board of Abdominal Wall of the Spanish Association of Surgeons are:José Antonio Pereira Rodríguez; Julio Gómez Menchero; Salvador Pous Serrano; Luis Tallón Aguilar; Carles Olona Casas; Alberto López Farias; Antonio Ríos Zambudio; Belén Porrero Guerrero; Monserrat Juvany Gómez; Jacobo Trébol López; Manuel López Cano; Pilar Hernández Granados.
The names of the members of the Sección de Pared Abdominal de la Asociación Española de Cirujanos are listed in Appendix A.