The main objective of our study is to analyze the results in our hospital after launching a treatment protocol without antibiotic therapy for patients diagnosed with acute uncomplicated diverticulitis.
MethodsOur observational, prospective, single-center study was developed after launching a treatment protocol without antibiotic therapy for patients diagnosed with acute uncomplicated diverticulitis (AUD) in January 2021. The follow-up period was from January 1, 2021 to September 30, 2023. Variables evaluated by the study have included demographic and analytical variables, as well as those related to diagnosis and whether the patients needed to start antibiotic treatment, inpatient treatment, or surgical procedures.
ResultsIn total, 199 patients were diagnosed with AUD, 75 of whom were treated without antibiotic therapy as outpatients. Seven of these patients needed to start antibiotic treatment because of adverse evolution; none of these patients required surgical procedures. The need for inpatient treatment, urgent care, or surgical procedures is similar to the group of patients treated with antibiotics.
The main risk factor of failure of outpatient treatment without antibiotic therapy identified by the study was the presence of bacteriuria at diagnosis.
ConclusionsOur results confirm previous reports, observing that treatment without antibiotic therapy in selected patients with AUD is safe.
El objetivo de nuestro estudio es analizar los resultados obtenidos en nuestro centro tras la puesta en marcha de un protocolo de tratamiento sin antibioterapia en pacientes diagnosticados de diverticulitis aguda no complicada.
MétodosSe trata de un estudio observacional prospectivo unicéntrico tras la implantación de un protocolo de tratamiento sin antibioterapia en pacientes con diverticulitis aguda no complicada (DANC) en enero de 2021. El periodo de seguimiento fue desde la implantación del protocolo el 1 de enero de 2021 hasta el 30 de septiembre de 2023. Se recogieron variables demográficas, analíticas y diagnósticas de los pacientes valorados, así como si precisaron el inicio de antibioterapia, ingreso o intervención quirúrgica.
Resultados199 pacientes fueron diagnosticados de DANC, de los cuales, 75 fueron tratados de forma ambulatoria sin antibioterapia. En 7 de los casos fue preciso iniciar el tratamiento antibiótico por evolución desfavorable, sin que en ninguno de los casos fuera necesario realizar una intervención quirúrgica o drenaje percutáneo urgente. Los resultados, en términos de necesidad de ingreso, reconsulta en urgencias o necesidad de intervención, fueron similares a aquéllos en el grupo de pacientes tratados con antibioterapia.
El principal factor de riesgo de fracaso del tratamiento sin antibioterapia identificado ha sido la presencia de bacteriuria al diagnóstico.
ConclusionesNuestros resultados demuestran lo publicado hasta la fecha, que el tratamiento sin antibioterapia en pacientes muy seleccionados diagnosticados de DANC es seguro.
Acute uncomplicated diverticulitis (AUD) is a very common pathology that affects 5%–25% of patients who present diverticulosis, a condition that is increasingly common in the general population and is progressively increasing with age.1–3
In recent years, the pathophysiological mechanism causing AUD has been researched, and theories include ischemic, microtrauma or inflammatory mechanisms.4,5
Due to these advances in the study of the pathophysiology of AUD, it has been suggested that the treatment of this pathology may not need to be based on antibiotic therapy, since it is due more to pathophysiological mechanisms of inflammation and not so much to micro-perforation and subsequent infection.2,4,5
Based on this new pathophysiological concept of AUD, several randomized clinical trials (RCT) published in recent years have shown that treatment without antibiotic therapy is safe in selected patients.6–9 In the initial trials that sought to study the effectiveness of treatment with anti-inflammatories alone, the experimental groups always used a regimen with hospitalization, which could be considered an important limitation for the implementation of this therapeutic strategy. However, the latest RCT (a national multicenter DINAMO trial by Mora L et al.) was conducted with an experimental group that received treatment without antibiotics on an outpatient basis, which is a fundamental argument for the implementation of this strategy.
In addition to the publication of these RCT, several meta-analyses12–15 have also been carried out on the subject, and all have reached the same conclusion about the safety and effectiveness of the strategy without antibiotics in AUD. All of this emerging research has had a direct impact on the latest international guidelines.10,11 In several, the current recommendation is to manage these patients on an outpatient basis and with anti-inflammatories when a series of strict selection criteria are met.
Given the existing evidence and the modified recommendations proposed by the latest international guidelines, our group implemented a treatment protocol without antibiotic therapy to improve care for our patients. In order to implement this innovation safely, we did it in the context of a strict prospective registry of the results, which allowed us to analyze the evolution of these patients and verify that it was a safe and effective strategy in our clinical context as well.
The objective of our study is to analyze the results in terms of efficacy and safety after the establishment of the treatment protocol without antibiotic therapy in selected patients.
MethodsWe conducted a prospective, longitudinal, descriptive, observational study that included patients diagnosed with uncomplicated acute diverticulitis at a tertiary hospital after the implementation of an outpatient treatment protocol without antibiotic therapy. All patients diagnosed with AUD from January 1, 2021 to September 30, 2023 have been registered prospectively.
Treatment protocolPatients who met the inclusion criteria were proposed participation in the study, which entailed treatment with a regimen of ibuprofen (600 mg/8 h), paracetamol (1 g/8 h) and omeprazole (40 mg/24 h), associated with specific dietary and monitoring guidelines (Addendum I).
Patients who agreed to be included in the study signed an informed consent form; in addition, the follow-up was explained, and recommendations were given. Patients received a phone call after 24 h, during which they were asked about pain control, oral tolerance, presence of fever, and intestinal transit. Some 48 h after the diagnosis, patients had an in-person clinical follow-up visit with lab work-up. At that time, and depending on the results, either the established treatment was continued, or the decision was made to start antibiotic therapy, or hospital admission was deemed necessary depending on the findings.
The inclusion criteria for outpatient treatment without antibiotic therapy are listed in Addendum II.
The main study variable is the failure of outpatient treatment without antibiotic therapy, defined as the need for antibiotic therapy, hospital admission, percutaneous drainage, or urgent surgery.
Statistical analysisThe necessary sample size was calculated based on the fact that the incidence of failure of outpatient treatment without antibiotic therapy in acute uncomplicated diverticulitis (defined as the need for hospital readmission8) is around 3%. To reach a power of 80% to detect differences in the contrast of the null hypothesis H0: π1 = π2 using Fisher’s exact test with a bilateral contrast for 2 independent samples, while taking into account a level of significance of 5%, it was necessary to include 59 patients.
The data obtained from each patient have been entered into a database in pseudonymized form and then analyzed with the statistical data program SPSS® version 24 (Statistical Package for the Social Sciences, IBM, Armonk, NY).
The qualitative variables have been defined by the number of events and percentage. Quantitative variables that followed normal distribution are described as mean and standard deviation (SD); those that did not comply with normal distribution are presented as median and interquartile range.
To determine whether the variables follow normal distribution, the Kolmogorov-Smirnov test was used. For the bivariate analysis of the normal quantitative variables, either the Student’s t-test (in the case of 2 media) or the ANOVA test (for more than 2 media) was applied. To compare quantitative variables that do not comply with normal distribution, the Mann–Whitney U test (comparison of 2 means) or Kruskal–Wallis (more than 2 means) has been used.
To compare qualitative variables, the chi-squared test was used. When an absolute frequency ≥5 was not met in any of the cells of the contingency table, the Fisher’s exact test was used (univariate analysis).
ResultsDuring the time period analyzed, 245 patients were evaluated for 259 episodes of acute diverticulitis, 76.8% (n = 199) of which were AUD.
Among the 199 episodes of AUD, 97 (48.7%) met inclusion criteria to be treated without antibiotic therapy. Ultimately, 75 patients were included in the analysis, as 22 patients had been excluded for various reasons (Fig. 1).
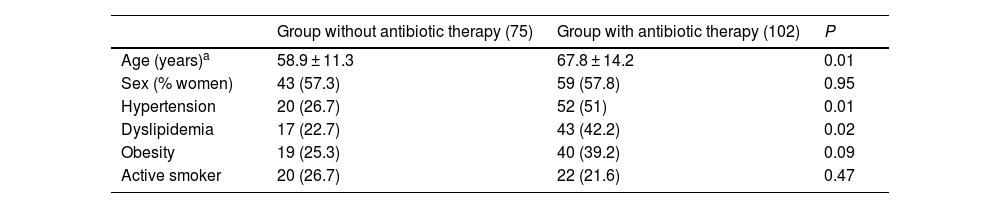
Tables 1 and 2 summarize the general characteristics of the patients diagnosed with AUD and the analytical values at diagnosis in both treatment groups.
Demographic variables and comorbidities of patients from both cohorts included in the study.
| Group without antibiotic therapy (75) | Group with antibiotic therapy (102) | P | |
|---|---|---|---|
| Age (years)a | 58.9 ± 11.3 | 67.8 ± 14.2 | 0.01 |
| Sex (% women) | 43 (57.3) | 59 (57.8) | 0.95 |
| Hypertension | 20 (26.7) | 52 (51) | 0.01 |
| Dyslipidemia | 17 (22.7) | 43 (42.2) | 0.02 |
| Obesity | 19 (25.3) | 40 (39.2) | 0.09 |
| Active smoker | 20 (26.7) | 22 (21.6) | 0.47 |
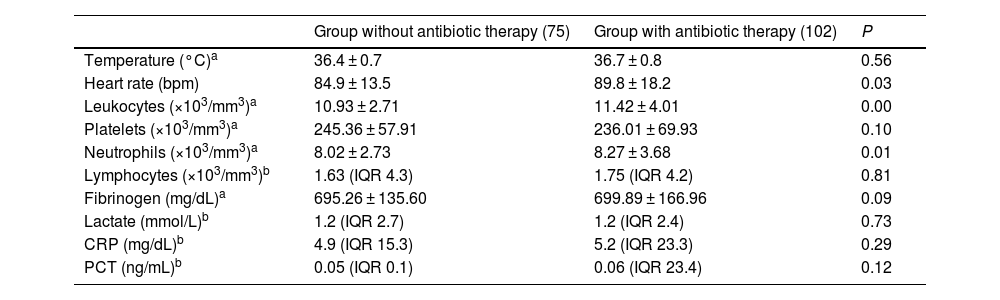
Analytical variables upon diagnosis in patients from both cohorts included in the study. CRP = C-reactive protein; PCT = procalcitonin.
| Group without antibiotic therapy (75) | Group with antibiotic therapy (102) | P | |
|---|---|---|---|
| Temperature (°C)a | 36.4 ± 0.7 | 36.7 ± 0.8 | 0.56 |
| Heart rate (bpm) | 84.9 ± 13.5 | 89.8 ± 18.2 | 0.03 |
| Leukocytes (×103/mm3)a | 10.93 ± 2.71 | 11.42 ± 4.01 | 0.00 |
| Platelets (×103/mm3)a | 245.36 ± 57.91 | 236.01 ± 69.93 | 0.10 |
| Neutrophils (×103/mm3)a | 8.02 ± 2.73 | 8.27 ± 3.68 | 0.01 |
| Lymphocytes (×103/mm3)b | 1.63 (IQR 4.3) | 1.75 (IQR 4.2) | 0.81 |
| Fibrinogen (mg/dL)a | 695.26 ± 135.60 | 699.89 ± 166.96 | 0.09 |
| Lactate (mmol/L)b | 1.2 (IQR 2.7) | 1.2 (IQR 2.4) | 0.73 |
| CRP (mg/dL)b | 4.9 (IQR 15.3) | 5.2 (IQR 23.3) | 0.29 |
| PCT (ng/mL)b | 0.05 (IQR 0.1) | 0.06 (IQR 23.4) | 0.12 |
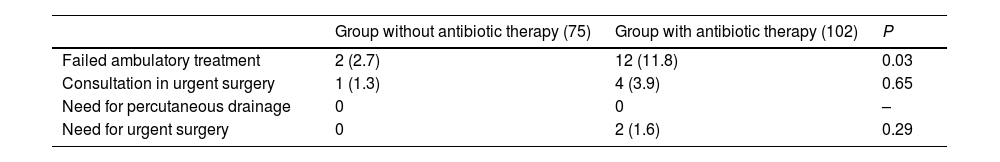
Seven patients (9.3%) included in the protocol required initiation of treatment with antibiotics due to worsening symptoms and lab work-up alterations. In 2 patients who required antibiotic therapy, this was initiated while in hospital. None of the patients included in the study protocol required percutaneous drainage or urgent surgical intervention (Table 3).
Comparison of the main clinical result variables in the sample analyzed according to treatment groups.
| Group without antibiotic therapy (75) | Group with antibiotic therapy (102) | P | |
|---|---|---|---|
| Failed ambulatory treatment | 2 (2.7) | 12 (11.8) | 0.03 |
| Consultation in urgent surgery | 1 (1.3) | 4 (3.9) | 0.65 |
| Need for percutaneous drainage | 0 | 0 | – |
| Need for urgent surgery | 0 | 2 (1.6) | 0.29 |
In the group of patients treated with antibiotic therapy, 12.2% (12) required hospital admission due to failed outpatient treatment, while 2.1% (2) needed urgent surgical intervention (Table 3).
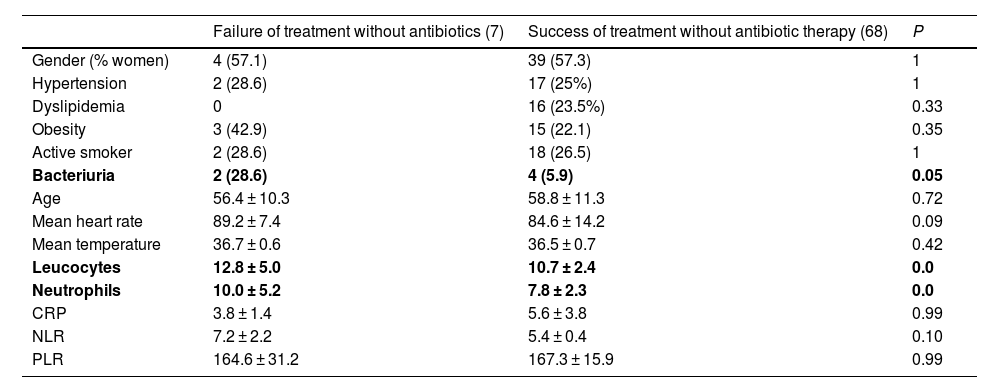
Statistically significant differences were found. Specifically, mean values of leukocytes and neutrophils were higher in the group of patients in whom treatment without antibiotic therapy failed compared to patients in whom it did not; likewise, we observed higher rates of patients with bacteriuria in the same group (Table 4).
Comparison between groups in which treatment without antibiotic therapy failed or succeeded.
| Failure of treatment without antibiotics (7) | Success of treatment without antibiotic therapy (68) | P | |
|---|---|---|---|
| Gender (% women) | 4 (57.1) | 39 (57.3) | 1 |
| Hypertension | 2 (28.6) | 17 (25%) | 1 |
| Dyslipidemia | 0 | 16 (23.5%) | 0.33 |
| Obesity | 3 (42.9) | 15 (22.1) | 0.35 |
| Active smoker | 2 (28.6) | 18 (26.5) | 1 |
| Bacteriuria | 2 (28.6) | 4 (5.9) | 0.05 |
| Age | 56.4 ± 10.3 | 58.8 ± 11.3 | 0.72 |
| Mean heart rate | 89.2 ± 7.4 | 84.6 ± 14.2 | 0.09 |
| Mean temperature | 36.7 ± 0.6 | 36.5 ± 0.7 | 0.42 |
| Leucocytes | 12.8 ± 5.0 | 10.7 ± 2.4 | 0.0 |
| Neutrophils | 10.0 ± 5.2 | 7.8 ± 2.3 | 0.0 |
| CRP | 3.8 ± 1.4 | 5.6 ± 3.8 | 0.99 |
| NLR | 7.2 ± 2.2 | 5.4 ± 0.4 | 0.10 |
| PLR | 164.6 ± 31.2 | 167.3 ± 15.9 | 0.99 |
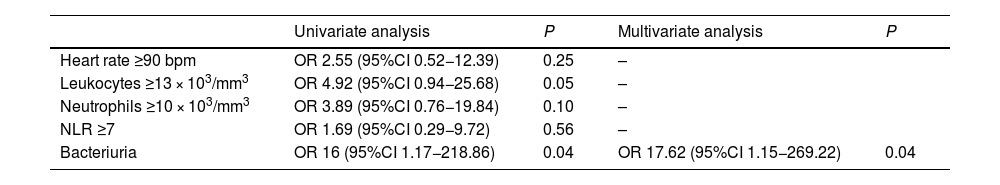
In the univariate and multivariate analyses, the only variable that has been identified as a possible risk factor for treatment without antibiotic therapy is the presence of bacteriuria at diagnosis (Table 5).
Univariate and multivariate analyses of risk factors for failed treatment without antibiotic therapy.
| Univariate analysis | P | Multivariate analysis | P | |
|---|---|---|---|---|
| Heart rate ≥90 bpm | OR 2.55 (95%CI 0.52−12.39) | 0.25 | – | |
| Leukocytes ≥13 × 103/mm3 | OR 4.92 (95%CI 0.94−25.68) | 0.05 | – | |
| Neutrophils ≥10 × 103/mm3 | OR 3.89 (95%CI 0.76−19.84) | 0.10 | – | |
| NLR ≥7 | OR 1.69 (95%CI 0.29−9.72) | 0.56 | – | |
| Bacteriuria | OR 16 (95%CI 1.17−218.86) | 0.04 | OR 17.62 (95%CI 1.15−269.22) | 0.04 |
The telephone interview was completed by 76% (57) of study participants. During the call, adequate oral tolerance was reported by 96.5% of patients, with preserved transit in 64.9%. Only one patient reported having fever at the time of the call; during re-evaluation 48 h after diagnosis, this patient’s treatment regimen was changed, and oral antibiotic therapy was started.
DiscussionOur study shows that the introduction of a treatment protocol without antibiotic therapy in selected patients diagnosed with acute uncomplicated diverticulitis is safe.
We have obtained treatment failure rates without antibiotic therapy of 9.3%, a percentage that is in line with previous publications,6–9 but no surgical intervention or urgent percutaneous drainage were required.
To date, only one RCT established treatment on an outpatient basis, defining treatment failure as the need for hospitalization.9 In this regard, our study had slightly better figures, since only 2.7% were hospitalized, versus the 3.3% reported in the literature.9
Furthermore, in RCT in which treatment is performed in-hospital, urgent surgery or drainage rates are higher than in our study.6,7 Nevertheless, it should be noted that the only RCT whose management was entirely outpatient presented the same rates of need for urgent surgery or drainage.9
In several RCT and previous retrospective studies, the variability of selection criteria for patients treated without antibiotic therapy is notable. Early publications had very strict criteria, while recent ones focus more on clinical exclusion criteria. Perhaps due to this evolution in selection, the results have been increasingly encouraging in terms of decreased rates of surgery and urgent drainage.
When we analyzed possible risk factors for treatment failure without antibiotic therapy, we observed that the presence of bacteriuria in the urinary sediment and high levels of leukocytes and neutrophils were statistically significant. It seems that these differences may not be clinically relevant since the differences in numerical terms are small, but perhaps it would be advisable to consider these data when proposing new exclusion criteria.
In our study, leukocyte counts greater than 12 000, together with other clinical alterations, was an exclusion criterion. However, in light of the results obtained, it would be necessary to reconsider whether it could be considered an isolated criterion alone.
Lastly, only the presence of bacteriuria at diagnosis has been identified by the univariate and multivariate analyses as a risk factor for failure of said treatment. However, these results have not been demonstrated in any other study to date and should perhaps be taken with caution, as in many cases the samples could be contaminated.
Other analytical parameters have been analyzed, such as the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), as previous studies have shown them to be good markers that indicate both the severity of the condition as well as the risk of needing surgical treatment in cases of complicated acute diverticulitis.16,17 In our study, the results do not present statistically significant differences; however, with a larger sample size, these differences could perhaps be observed given the results published in the literature. With regards to these variables, a national multicenter study is being conducted to analyze the role of these indices as predictors of outpatient treatment failure in patients diagnosed with AUD.18
Of course, it seems clear that proper patient selection for treatment without antibiotic therapy is key to achieving good results in terms of the need for urgent surgery or percutaneous drainage, as our series presents lower rates than RCT with in-hospital treatment.
The results obtained are promising in terms of the need for unscheduled emergency consultations, and admission rates are very low. This is probably thanks to the implementation of a scheduled consultation 48 h after diagnosis, which is why we closely monitor patients to determine whether they are progressing adequately or not and whether a change in treatment is needed.
On the other hand, it seems that the telephone follow-up within 24 h may lack importance, since only one patient presented symptoms (persistent fever), which led to a modification in treatment only after being re-evaluated in person after 48 h.
It should be noted that none of the patients treated without antibiotic therapy presented worsened radiological results with progression of diverticulitis symptoms to a more complicated degree.
It is important to keep in mind that, while adequate patient selection is of vital importance, collaboration with other hospital departments like the Emergency Department seems to be a key part of obtaining good clinical results.
Similar to the DINAMO9 study, we have not considered it necessary to perform a 90-day follow-up, as we believe that choosing one or another treatment in the acute phase should not influence the appearance of long-term recurrences.
The application of this type of protocols in routine clinical practice is difficult, as demonstrated in a recent study19 based on a national survey, in which only 25% of the hospitals represented have treatment protocols without antibiotic therapy. The reasons given to justify the non-implementation of these protocols are mainly logistical difficulties and lack of evidence. It seems necessary to obtain a better level of scientific evidence in this type of outpatient approaches in other population groups, such as patients with mild immunosuppression, for which different diagnostic analysis tools may be useful.
The main limitation of our study is that it is an observational study with patients selected after the implementation of a protocol, as it is not a RCT. Therefore, we may encounter certain difficulties when comparing treatment groups, since patients treated with antibiotic therapy are those who are not candidates for anti-inflammatory therapy.
In light of these results, and supported by current evidence, we believe that the implementation of similar protocols at the national level for the treatment of AUD in selected patients is safe.
FundingNone.
Conflict of interestsThe authors declare that no funding was received for the preparation of this study, and that there are no conflicts of interests. No experiments in animals or humans were conducted for its implementation. All patients signed the informed consent form for the publication of this manuscript as well as the necessary access to medical records in accordance with clinical ethics and confidentiality protocols. The signing authors have read and approved the manuscript, meeting its authorship requirements.
Authors Alba Correa Bonito and Carlos Cerdán Santacruz have made equal contributions to the development of this manuscript.