Surgery is the only potentially curative treatment for colorectal cancer liver metastases (CRLM) and its indication and results have varied in the last 30 years.
MethodsAll patients operated on for CRLM in our centre from 1990 to 2021 were prospectively collected, establishing 3 subgroups based on the year of the first surgery: group A 1990–1999, group B 2000–2010, group C 2011−2021. Clinical characteristics and the results of survival, recurrence and prognostic factors were compared.
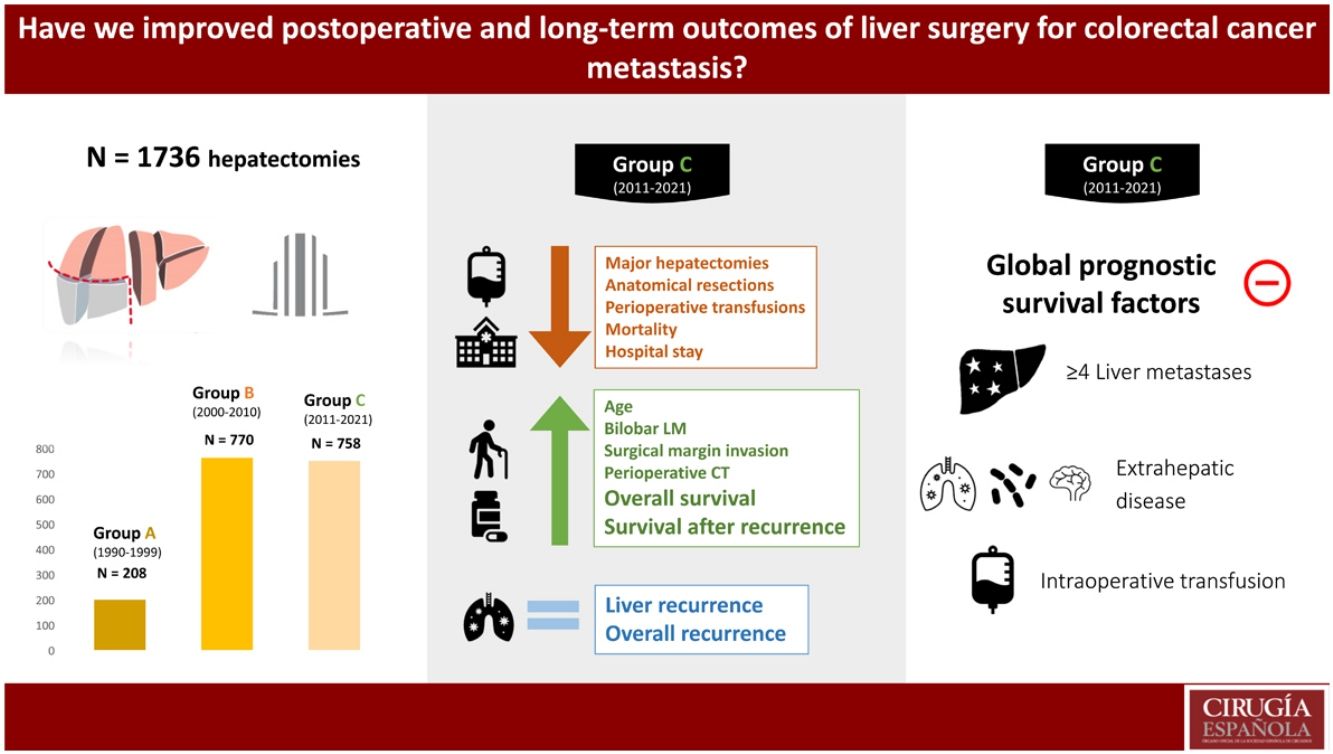
Results1736 hepatectomies were included (Group A n = 208; Group B n = 770; Group C n = 758). Patients in group C had better survival at 5 and 10 years (A 40.5%/28.2%; B 45.9%/32.2%; C 51.6%/33.1%, p = 0.013), although there were no differences between groups in overall recurrence at 5 and 10 years (A 73%/75.7%; B 67.6%/69.2%, and C 63.9%/66%, p = 0.524), nor in liver recurrence (A 46.4%/48.2%; B 45.8%/48.2%; and C 44.4%/48.4%, p = 0.899). An improvement was observed in median survival after recurrence, being 19 months, 23 months, and 31 months (groups A, B and C respectively). Prognostic factors of long-term survival changed over the 3 study periods. The only ones that remained relevant in the last decade were the presence of >4 liver metastasis, extrahepatic disease at the time of hepatectomy, and intraoperative blood transfusion.
ConclusionsSurvival after surgery for CRLM has improved significantly, although this cannot be explained by a reduction in overall and hepatic recurrence, but rather by an improvement in post-recurrence survival. Involvement of the resection margin has lost prognostic value in the last decade.
La cirugía es el único tratamiento potencialmente curativo de las metástasis hepáticas de cáncer colorrectal (LMCCR) y su indicación y resultados han variado en los últimos 30 años.
MétodosTodos los pacientes operados por LMCCR en nuestro centro de 1990 a 2021 fueron recogidos prospectivamente, estableciendo 3 subgrupos en función del año de la hepatectomía: grupo A 1990–1999, grupo B 2000–20010, grupo C 2011−2021. Se compararon características clínicas, supervivencia, recidiva y factores pronósticos.
ResultadosSe incluyeron 1736 hepatectomías (Grupo A n = 208; Grupo B n = 770; Grupo C n = 758).En el grupo C mejoró la supervivencia a 5 y 10 años (A: 40,5%/28,2%; B: 45,9%/32,2%; C: 51,6%/33,1%, p = 0.013), sin encontrar diferencias en recidiva global a 5 y 10 años (A: 73%/75,7%; B: 67,6%/69,2%, C: 63,9%/66%, p = 0,524), ni en recidiva hepática (A: 46,4%/48,2%; B: 45,8%/48,2%; C: 44,4%/48,4%, p = 0,899). Se observó una mejoría en la supervivencia mediana tras recidiva, siendo de 19 meses, 23 meses y 31 meses (grupos A, B y C respectivamente). Los factores pronósticos de supervivencia a largo plazo variaron en las 3 épocas, los únicos que mantuvieron relevancia en la última década fueron la presencia de >4 metástasis, la enfermedad extrahepática al momento de la hepatectomía y la transfusión perioperatoria.
ConclusionesEn los pacientes operados por LMCCR la supervivencia ha mejorado de forma significativa, sin que pueda explicarse por una reducción de la recidiva global y hepática, pero si por una mejoría de la supervivencia después de la recidiva. La afectación del margen de resección ha perdido valor pronóstico en la última década.
Colorectal cancer (CRC) is the second cause of cancer death in Europe. Up to 40% of patients will develop liver metastases (LM), these being the cause of the majority of deaths.1–3 The most common indication for liver surgery is CRLM as it is the only potentially curative treatment that improves survival,2,3 which is described as 40%–60% at 5 years, and 25%–30% at 10 years.4–8
Since the beginning of liver surgery for CRLM, patient characteristics have evolved and gained complexity. We have seen the emergence of perioperative chemotherapy, resections in bilobar and more advanced disease, parenchyma-sparing resections and minimally invasive approaches, surgery combined with ablation of lesions that are difficult to access for resection, as well as important improvements in diagnostic and radiological techniques, interventional techniques and perioperative care.9–11
The prognostic factors that confer worse long-term survival to patients operated on for CRLM have varied over time, but include: CEA value >200 ng/mL, synchronous extrahepatic disease, number of LM, synchronous LM, perioperative transfusions, severe postoperative complications, invasion of the surgical margin or the impossibility of receiving perioperative chemotherapy.4–7,10,12
The objectives of this study were the analysis of the evolution of the characteristics of patients with indication for surgery for CRLM in 3 historical periods in the last 30 years, the comparison of the results of morbidity and mortality, survival and tumour recurrence, and the study of the evolution of the prognostic factors in those three periods.
Material and methodsAll patients who underwent hepatectomy for CRLM between 1990 and 2021 were included, data were collected prospectively, and a retrospective analysis was performed. Patients who were unresectable during surgery, those who only underwent ablation, as well as those whose definitive pathological anatomy was different from CRLM were excluded. The cases were divided into 3 decades based on the year of hepatectomy: Group A (1990–1999), Group B (2000–2010) and Group C (2011−2021).
For diagnosis and preoperative staging, the CRLM management guidelines available in each period were followed. Surgery was indicated if complete treatment of all lesions could be carried out while maintaining a sufficient liver remnant. All cases were discussed in a multidisciplinary committee. Liver metastases were defined as synchronous when their diagnosis was simultaneous with the primary tumour or in the following 6 months.13 The type of liver resection was described following the Brisbane terminology.14
Any degree of Clavien–Dindo15 complication was taken into account in morbidity. Liver failure, biliary fistula, and postoperative haemorrhage were defined according to the ISGLS16–18 classification. Postoperative mortality was defined as death within 90 days after surgery or before hospital discharge. To define the number of resected metastases and their size, the final pathological anatomy report was taken into account. Prolonged hospital stay was one that lasted more than 9 days, based on the definition of “text book outcome” proposed by Görgec.19
Rehepatectomies due to liver recurrence were considered new patients, although for the survival analysis they were excluded as they were the same patient. In the case of 2-stage liver surgery, survival was calculated from the second procedure (if it was not completed, survival was calculated from the first procedure). In those whose liver surgery was performed before liver-first surgery, survival was calculated from the date of liver surgery.
Statistical analysisContinuous variables are presented as frequencies and percentages, and were analysed using the one-way ANOVA test. Categorical variables are presented as mean and standard deviation, and were analyzed using the Chi-square test or Fisher's exact test. Overall survival was calculated from the date of hepatectomy to the last follow-up contact. Overall and liver recurrence data were calculated from the date of hepatectomy to recurrence. Survival after recurrence was calculated from the date of first recurrence to the last follow-up contact. For the analysis of survival and recurrence, the Kaplan-Meier method was used and they were compared using log-rank. The variables with statistical significance (p < .05) were selected for multivariate analysis with the Cox regression model. A P value less than .05 was considered significant. The statistical package SPSSv21.0 (SPSS Inc., Chicago, IL) for Windows was used.
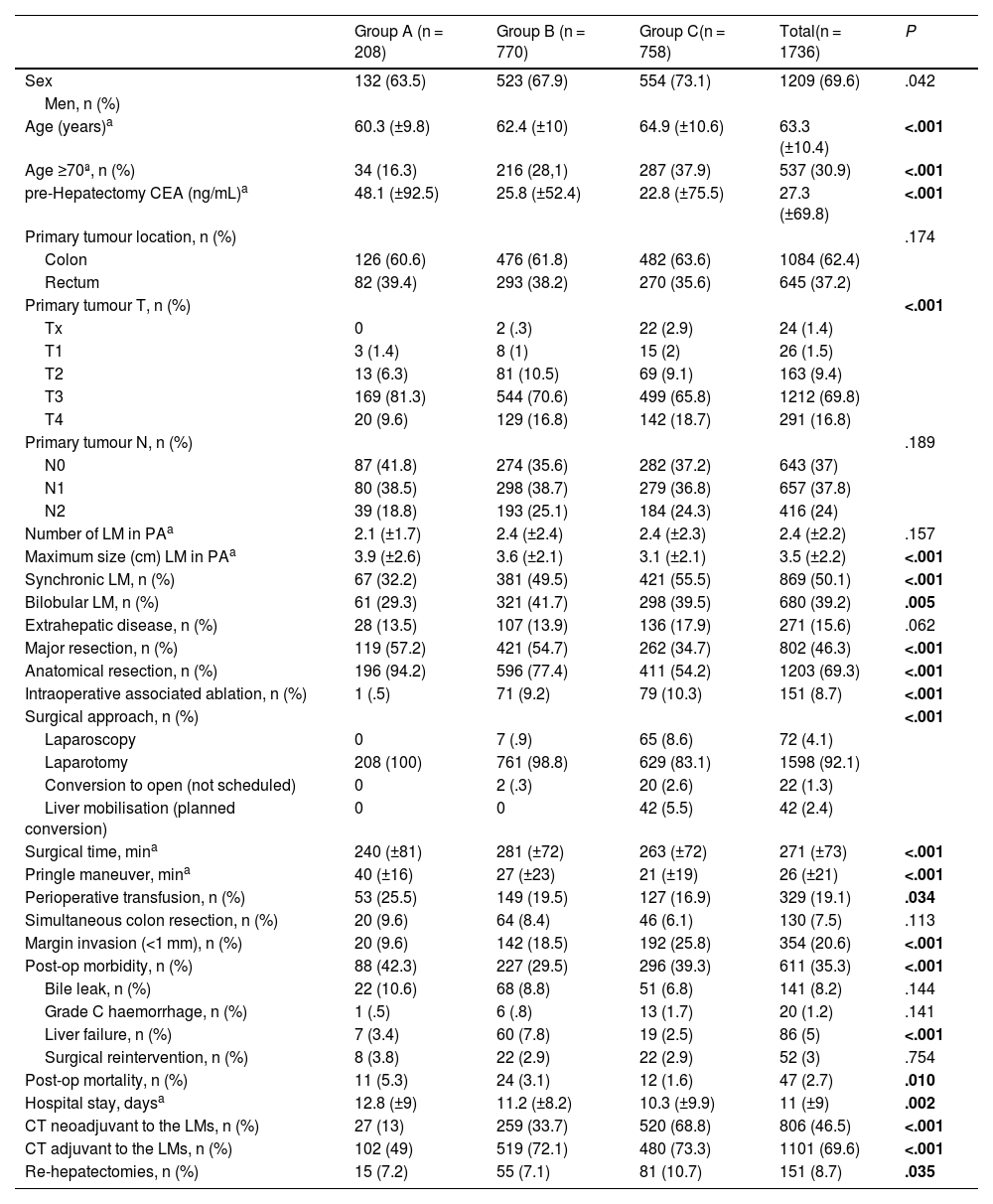
ResultsFrom January 1990 to December 2021, 1,736 liver resections were performed that met the inclusion criteria. According to the decade of hepatectomy, the cases were divided into 3 groups: 208 in group A (1990–1999), 770 patients in group B (2000–2010) and 758 patients in group C (2011−2021). The demographic and clinical characteristics of the 3 groups are contained in Table 1.
Comparison between groups of the demographic and clinical characteristics of the patients operated on for CRLM.
| Group A (n = 208) | Group B (n = 770) | Group C(n = 758) | Total(n = 1736) | P | |
|---|---|---|---|---|---|
| Sex | 132 (63.5) | 523 (67.9) | 554 (73.1) | 1209 (69.6) | .042 |
| Men, n (%) | |||||
| Age (years)a | 60.3 (±9.8) | 62.4 (±10) | 64.9 (±10.6) | 63.3 (±10.4) | <.001 |
| Age ≥70ª, n (%) | 34 (16.3) | 216 (28,1) | 287 (37.9) | 537 (30.9) | <.001 |
| pre-Hepatectomy CEA (ng/mL)a | 48.1 (±92.5) | 25.8 (±52.4) | 22.8 (±75.5) | 27.3 (±69.8) | <.001 |
| Primary tumour location, n (%) | .174 | ||||
| Colon | 126 (60.6) | 476 (61.8) | 482 (63.6) | 1084 (62.4) | |
| Rectum | 82 (39.4) | 293 (38.2) | 270 (35.6) | 645 (37.2) | |
| Primary tumour T, n (%) | <.001 | ||||
| Tx | 0 | 2 (.3) | 22 (2.9) | 24 (1.4) | |
| T1 | 3 (1.4) | 8 (1) | 15 (2) | 26 (1.5) | |
| T2 | 13 (6.3) | 81 (10.5) | 69 (9.1) | 163 (9.4) | |
| T3 | 169 (81.3) | 544 (70.6) | 499 (65.8) | 1212 (69.8) | |
| T4 | 20 (9.6) | 129 (16.8) | 142 (18.7) | 291 (16.8) | |
| Primary tumour N, n (%) | .189 | ||||
| N0 | 87 (41.8) | 274 (35.6) | 282 (37.2) | 643 (37) | |
| N1 | 80 (38.5) | 298 (38.7) | 279 (36.8) | 657 (37.8) | |
| N2 | 39 (18.8) | 193 (25.1) | 184 (24.3) | 416 (24) | |
| Number of LM in PAa | 2.1 (±1.7) | 2.4 (±2.4) | 2.4 (±2.3) | 2.4 (±2.2) | .157 |
| Maximum size (cm) LM in PAa | 3.9 (±2.6) | 3.6 (±2.1) | 3.1 (±2.1) | 3.5 (±2.2) | <.001 |
| Synchronic LM, n (%) | 67 (32.2) | 381 (49.5) | 421 (55.5) | 869 (50.1) | <.001 |
| Bilobular LM, n (%) | 61 (29.3) | 321 (41.7) | 298 (39.5) | 680 (39.2) | .005 |
| Extrahepatic disease, n (%) | 28 (13.5) | 107 (13.9) | 136 (17.9) | 271 (15.6) | .062 |
| Major resection, n (%) | 119 (57.2) | 421 (54.7) | 262 (34.7) | 802 (46.3) | <.001 |
| Anatomical resection, n (%) | 196 (94.2) | 596 (77.4) | 411 (54.2) | 1203 (69.3) | <.001 |
| Intraoperative associated ablation, n (%) | 1 (.5) | 71 (9.2) | 79 (10.3) | 151 (8.7) | <.001 |
| Surgical approach, n (%) | <.001 | ||||
| Laparoscopy | 0 | 7 (.9) | 65 (8.6) | 72 (4.1) | |
| Laparotomy | 208 (100) | 761 (98.8) | 629 (83.1) | 1598 (92.1) | |
| Conversion to open (not scheduled) | 0 | 2 (.3) | 20 (2.6) | 22 (1.3) | |
| Liver mobilisation (planned conversion) | 0 | 0 | 42 (5.5) | 42 (2.4) | |
| Surgical time, mina | 240 (±81) | 281 (±72) | 263 (±72) | 271 (±73) | <.001 |
| Pringle maneuver, mina | 40 (±16) | 27 (±23) | 21 (±19) | 26 (±21) | <.001 |
| Perioperative transfusion, n (%) | 53 (25.5) | 149 (19.5) | 127 (16.9) | 329 (19.1) | .034 |
| Simultaneous colon resection, n (%) | 20 (9.6) | 64 (8.4) | 46 (6.1) | 130 (7.5) | .113 |
| Margin invasion (<1 mm), n (%) | 20 (9.6) | 142 (18.5) | 192 (25.8) | 354 (20.6) | <.001 |
| Post-op morbidity, n (%) | 88 (42.3) | 227 (29.5) | 296 (39.3) | 611 (35.3) | <.001 |
| Bile leak, n (%) | 22 (10.6) | 68 (8.8) | 51 (6.8) | 141 (8.2) | .144 |
| Grade C haemorrhage, n (%) | 1 (.5) | 6 (.8) | 13 (1.7) | 20 (1.2) | .141 |
| Liver failure, n (%) | 7 (3.4) | 60 (7.8) | 19 (2.5) | 86 (5) | <.001 |
| Surgical reintervention, n (%) | 8 (3.8) | 22 (2.9) | 22 (2.9) | 52 (3) | .754 |
| Post-op mortality, n (%) | 11 (5.3) | 24 (3.1) | 12 (1.6) | 47 (2.7) | .010 |
| Hospital stay, daysa | 12.8 (±9) | 11.2 (±8.2) | 10.3 (±9.9) | 11 (±9) | .002 |
| CT neoadjuvant to the LMs, n (%) | 27 (13) | 259 (33.7) | 520 (68.8) | 806 (46.5) | <.001 |
| CT adjuvant to the LMs, n (%) | 102 (49) | 519 (72.1) | 480 (73.3) | 1101 (69.6) | <.001 |
| Re-hepatectomies, n (%) | 15 (7.2) | 55 (7.1) | 81 (10.7) | 151 (8.7) | .035 |
CEA: Carcinoembryonic Antigen; CT: Chemotherapy; LM: Liver metastases. CRLM: ColoRectal Cancer Liver Metastases; Min: minute; MW: Microwave; OP: operation; PA: Pathological Anatomy. RF: Radiofrequency; SD: Standard Deviation.
Bold means variables with statistical significance.
In the last period, the patients were older, had a T4 stage of the primary tumour in a greater proportion, and there were more synchronous and bilobar LMs. Also, the CEA prior to resection and the size of the LM were smaller. The N stage of the primary tumour, the number of LMs, or extrahepatic disease at the time of hepatectomy did not vary significantly. Table 1
The evolution of the variables related to the surgical technique has been towards a decrease in the proportion of major and anatomical resections, with greater use of ablation associated with surgery. In parallel, we observed a lower need for perioperative transfusion, a lower need for hilar clamping, and progressively greater use of minimally invasive surgery. As an apparently negative point we see that in recent times there is a greater proportion of involvement of the surgical margin. Table 1
Although in general postoperative morbidity has remained stable over time, we can observe a discrete decrease in hospital stay and a more striking decrease in postoperative mortality. Breaking down the morbidity, liver failure has been lower in the last period, but the rest of the complications (biliary fistula, haemorrhage or surgical reintervention) have remained stable. The use of perioperative chemotherapy was significantly higher in the last period. Table 1
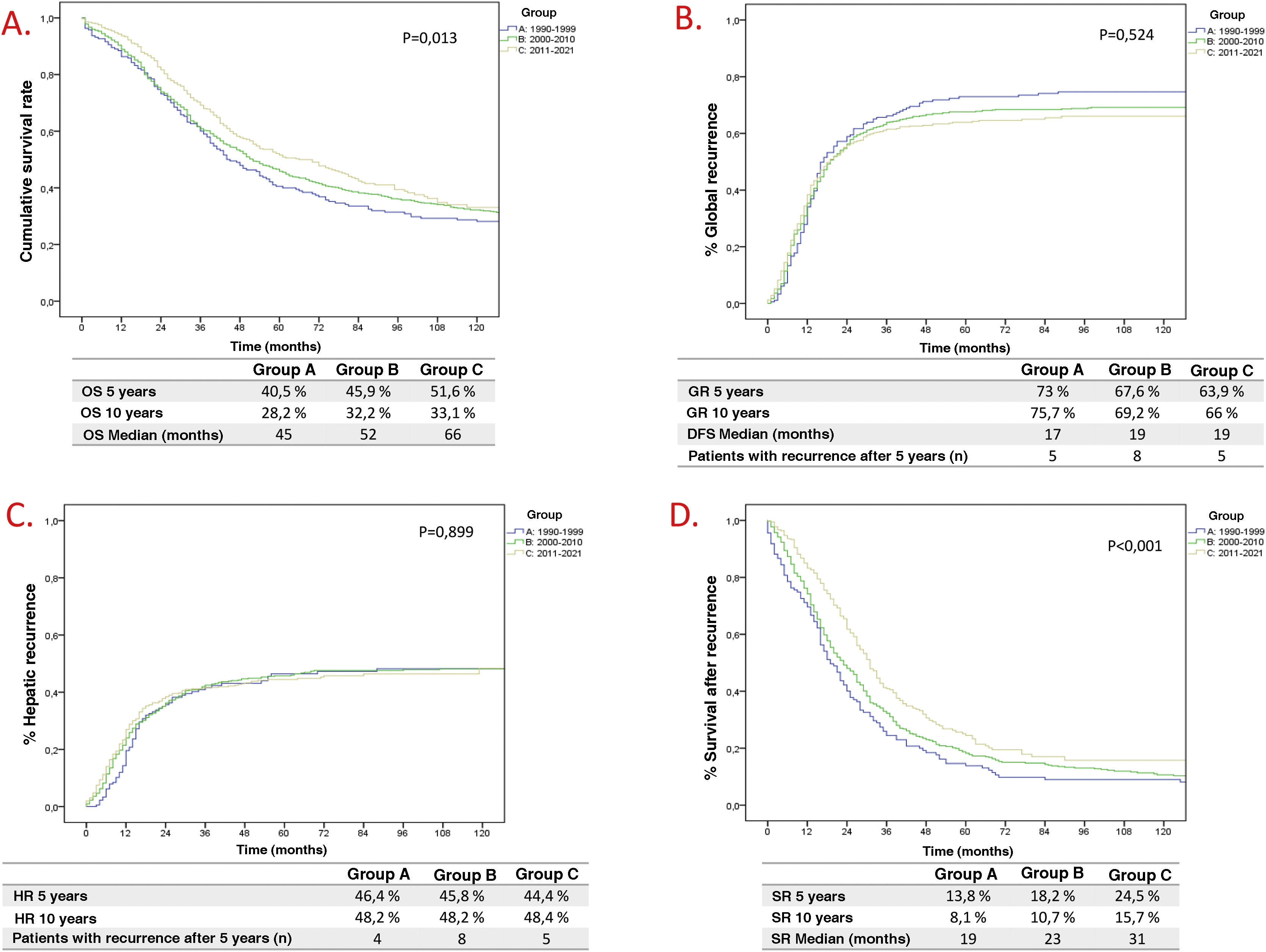
Overall survival (OS) has improved in each historical period (Fig. 1A), being 40.5% and 28.2% at 5 and 10 years in Group A, 45.9% and 32.2% in Group B, and 51.6% and 33.2% in Group C (p = .013). However, Global Recurrence (GR) and Hepatic Recurrence (HR) have remained stable (Fig. 1B and C). Five patients were identified in Group A, 8 in Group B and 5 in Group C, with a GR after 5 years of follow-up. The increase in overall survival despite a stable global and hepatic recurrence rate between periods is reflected by the increase in survival after recurrence (Fig. 1D).
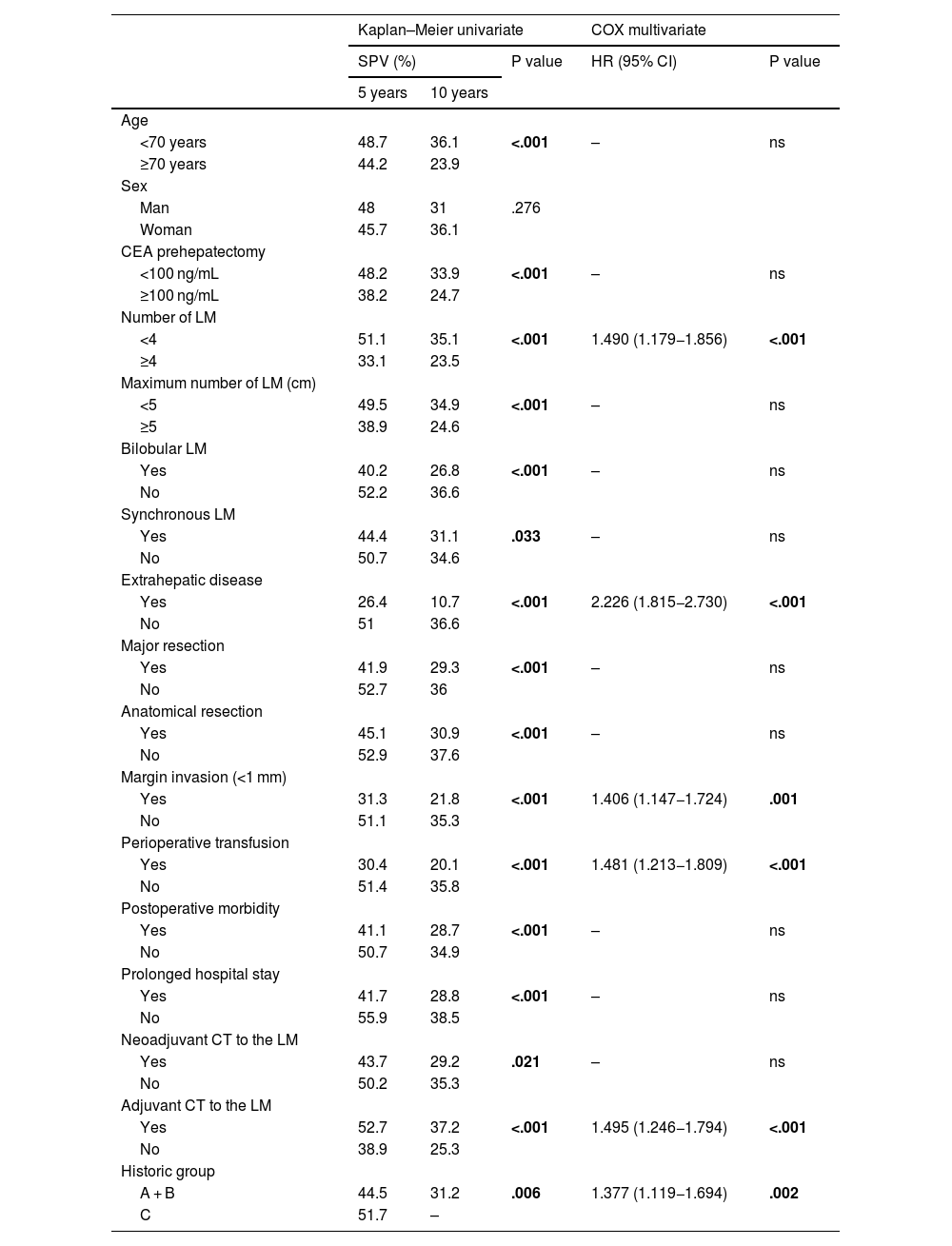
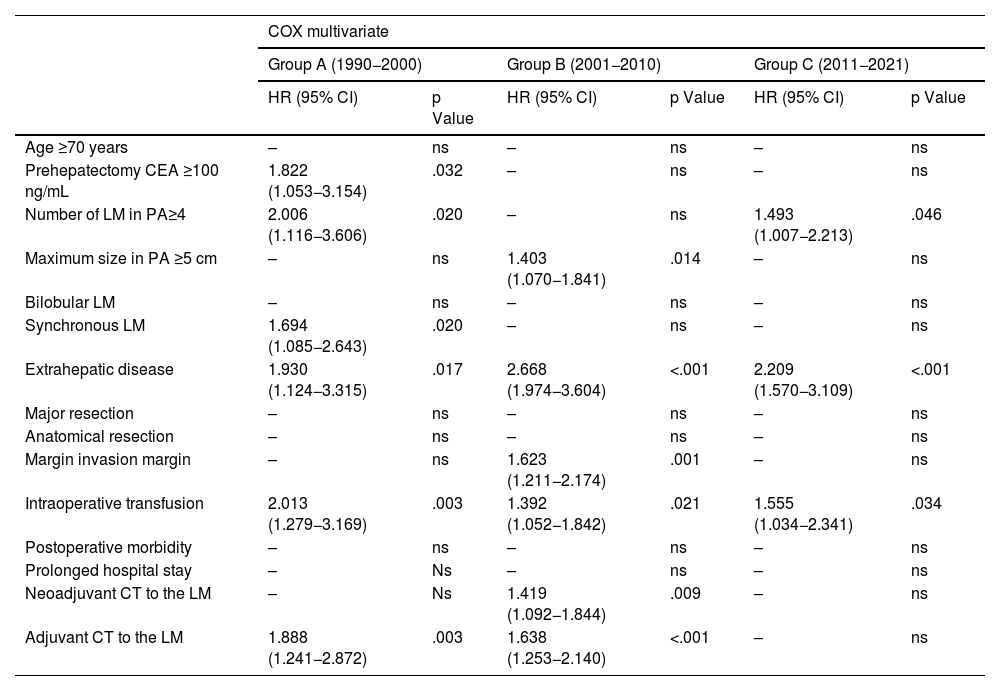
Of the negative prognostic factors for the 3 groups in the univariate analysis Table 2), only the number of LM ≥ 4, the presence of extrahepatic disease, invasion of the surgical margin and transfusion of blood products maintained statistical significance in the multivariate analysis. Adjuvant chemotherapy for metastases and belonging to the historical group C were positive prognostic factors. A multivariate analysis was carried out by historical subgroups (Table 3) and highlights that, in the last period (2011−2021), the only negative prognostic factors that maintain statistical significance are the number of LM ≥ 4, the presence of extrahepatic disease and perioperative transfusion. Compared to the 2 previous historical periods, in Group C, variables such as invasion of the surgical margin or perioperative chemotherapy have lost significance.
Long-term overall survival predictors: Kaplan-Meier univariate analysis and Cox multivariate analysis.
| Kaplan–Meier univariate | COX multivariate | ||||
|---|---|---|---|---|---|
| SPV (%) | P value | HR (95% CI) | P value | ||
| 5 years | 10 years | ||||
| Age | |||||
| <70 years | 48.7 | 36.1 | <.001 | – | ns |
| ≥70 years | 44.2 | 23.9 | |||
| Sex | |||||
| Man | 48 | 31 | .276 | ||
| Woman | 45.7 | 36.1 | |||
| CEA prehepatectomy | |||||
| <100 ng/mL | 48.2 | 33.9 | <.001 | – | ns |
| ≥100 ng/mL | 38.2 | 24.7 | |||
| Number of LM | |||||
| <4 | 51.1 | 35.1 | <.001 | 1.490 (1.179−1.856) | <.001 |
| ≥4 | 33.1 | 23.5 | |||
| Maximum number of LM (cm) | |||||
| <5 | 49.5 | 34.9 | <.001 | – | ns |
| ≥5 | 38.9 | 24.6 | |||
| Bilobular LM | |||||
| Yes | 40.2 | 26.8 | <.001 | – | ns |
| No | 52.2 | 36.6 | |||
| Synchronous LM | |||||
| Yes | 44.4 | 31.1 | .033 | – | ns |
| No | 50.7 | 34.6 | |||
| Extrahepatic disease | |||||
| Yes | 26.4 | 10.7 | <.001 | 2.226 (1.815−2.730) | <.001 |
| No | 51 | 36.6 | |||
| Major resection | |||||
| Yes | 41.9 | 29.3 | <.001 | – | ns |
| No | 52.7 | 36 | |||
| Anatomical resection | |||||
| Yes | 45.1 | 30.9 | <.001 | – | ns |
| No | 52.9 | 37.6 | |||
| Margin invasion (<1 mm) | |||||
| Yes | 31.3 | 21.8 | <.001 | 1.406 (1.147−1.724) | .001 |
| No | 51.1 | 35.3 | |||
| Perioperative transfusion | |||||
| Yes | 30.4 | 20.1 | <.001 | 1.481 (1.213−1.809) | <.001 |
| No | 51.4 | 35.8 | |||
| Postoperative morbidity | |||||
| Yes | 41.1 | 28.7 | <.001 | – | ns |
| No | 50.7 | 34.9 | |||
| Prolonged hospital stay | |||||
| Yes | 41.7 | 28.8 | <.001 | – | ns |
| No | 55.9 | 38.5 | |||
| Neoadjuvant CT to the LM | |||||
| Yes | 43.7 | 29.2 | .021 | – | ns |
| No | 50.2 | 35.3 | |||
| Adjuvant CT to the LM | |||||
| Yes | 52.7 | 37.2 | <.001 | 1.495 (1.246−1.794) | <.001 |
| No | 38.9 | 25.3 | |||
| Historic group | |||||
| A + B | 44.5 | 31.2 | .006 | 1.377 (1.119−1.694) | .002 |
| C | 51.7 | – | |||
CEA: Carcinoembryonic antigen; CT: Chemotherapy; LM: Liver metastasis; OP: Operation; PA: Pathological anatomy SV: Survival.
Bold means variables with statistical significance.
Predictors of survival by long-term historical periods: multivariate COX analysis.
| COX multivariate | ||||||
|---|---|---|---|---|---|---|
| Group A (1990−2000) | Group B (2001−2010) | Group C (2011−2021) | ||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age ≥70 years | – | ns | – | ns | – | ns |
| Prehepatectomy CEA ≥100 ng/mL | 1.822 (1.053−3.154) | .032 | – | ns | – | ns |
| Number of LM in PA≥4 | 2.006 (1.116−3.606) | .020 | – | ns | 1.493 (1.007−2.213) | .046 |
| Maximum size in PA ≥5 cm | – | ns | 1.403 (1.070−1.841) | .014 | – | ns |
| Bilobular LM | – | ns | – | ns | – | ns |
| Synchronous LM | 1.694 (1.085−2.643) | .020 | – | ns | – | ns |
| Extrahepatic disease | 1.930 (1.124−3.315) | .017 | 2.668 (1.974−3.604) | <.001 | 2.209 (1.570−3.109) | <.001 |
| Major resection | – | ns | – | ns | – | ns |
| Anatomical resection | – | ns | – | ns | – | ns |
| Margin invasion margin | – | ns | 1.623 (1.211−2.174) | .001 | – | ns |
| Intraoperative transfusion | 2.013 (1.279−3.169) | .003 | 1.392 (1.052−1.842) | .021 | 1.555 (1.034−2.341) | .034 |
| Postoperative morbidity | – | ns | – | ns | – | ns |
| Prolonged hospital stay | – | Ns | – | ns | – | ns |
| Neoadjuvant CT to the LM | – | Ns | 1.419 (1.092−1.844) | .009 | – | ns |
| Adjuvant CT to the LM | 1.888 (1.241−2.872) | .003 | 1.638 (1.253−2.140) | <.001 | – | ns |
CEA: Carcinoembryonic antigen; CT: Chemotherapy; LM: Liver metastasis; OP: Operation; PA: Pathological anatomy.
Surgery for liver metastases from colorectal cancer (CRC LM) remains the only potentially curative treatment.4,5,20–25 Although classically the indication for surgery was based on the liver tumour burden (number of lesions, size or distribution in the liver), currently resectability is defined as the possibility of achieving complete removal of the disease (R0) while maintaining sufficient liver volume,2,5,9,26 so that all patients with CRLM are potential candidates for resection.5,22,27 This concept has led to the emergence of surgical and complementary techniques to surgery that currently allow us to address LM that would have been considered unresectable 30 years ago.5,28–30 Given the constant evolution of the management of CRLM, we proposed this study with the objective of evaluating the postoperative and long-term results in our experience of more than 30 years, in a high-volume centre.
In our series, patients from the last 2 historical periods have an older age, more bilobar, synchronous LMs, extrahepatic disease, and are more frequently associated with a T4 stage of the primary tumour. That is, they are older patients with advanced disease, who have received more chemotherapy prior to surgery with its possible consequences (steatohepatitis, sinusoidal obstruction syndrome, etc.).31
Despite this, the incidence of liver failure has been significantly reduced, and a downward trend has been seen in the incidence of biliary fistula and the need for surgical reintervention (although without reaching significance). Postoperative mortality and hospital stay have obviously decreased in the last study period. This improvement in postsurgical results is possibly influenced by the evolution towards minimally invasive and parenchyma-conserving surgery, with a decrease in major resections and an increase in simultaneous ablative treatments. Probably the less aggressive surgical technique can explain the lower need for perioperative transfusion and Pringle maneuver. Less aggressive surgical techniques have been described as effective options in patients with bilobar disease, and together with an improvement in perioperative care, have contributed to maintaining stable postoperative morbidity despite the increase in the complexity of surgery and patients,10,32,33 with long-term results comparable to major hepatectomies.28–30
Similar to other historical series,10 there are two oncological characteristics that show differences in the last period: both the prehepatectomy CEA and the size of the LM are smaller. Kruger et al.10 justify this by the improvement in imaging techniques and follow-up after surgery of the primary tumour, allowing an early diagnosis of metastatic disease. In our series, because both the CEA and the size of the metastases are recorded after neoadjuvant chemotherapy, it is also likely that the chemotherapy treatment explains the reduction in the size of the LM and the CEA. In this context, the administration of CT has represented a selection mechanism for surgical resection, such that a good response to neoadjuvant CT translates into more favourable tumour biology.
In recent decades, overall survival after surgery for CRLM has increased, currently being close to 60% at 5 years,4,6,25,34–36 despite the fact that global and hepatic recurrence have remained stable (around 70 and 50% respectively).4,7,8,20,37 In our series, 5-year overall survival has risen from 40% in the 1990s to 51% today. The increase in survival has occurred despite the fact that the patients in the last period are older and have more advanced disease. Although recurrence after liver surgery remains high in the last period, possibly due to the greater severity of the oncological disease, survival after recurrence has improved significantly, which has practically doubled between the first and last period studied. One explanation would be better efficacy of current chemotherapy regimens, advances in indications and targeted therapies, improvement of imaging techniques for better staging and early diagnosis, and better surgical management of both global and hepatic recurrences. Surgery has become an indispensable tool in the treatment of hepatic recurrences, and is considered for extrahepatic recurrences in selected cases.22,23,38,39 This can be seen in our series with the increase in rehepatectomies due to LM recurrence in the last period.
In our series we found classic factors that are detrimental to overall survival, such as the number of LMs, the presence of extrahepatic disease, invasion of the surgical margin, perioperative transfusion, and not belonging to the last historical period (2011−2021), although these factors have varied between periods.
Various studies have suggested that invasion of the surgical margin is one of the most relevant negative prognostic factors21,40 In contrast, several recent reviews28,41 suggest that the margin <1 mm or even R1 (especially vascular R1) has less prognostic importance than the tumour biology itself, and that, in those patients with a good response to perioperative CT, the affected margin does not worsen prognosis.10,42 In our series, the involvement of the surgical margin has risen from 9.6% in the first era to 25.8% in the last, without this having translated into an increase in liver recurrence. A possible explanation is that the ablation or cautery techniques (bipolar with irrigation, Argon-beam, etc.) used during surgery allow the margin to be expanded “in situ”, eliminating the risk of local recurrence attributed to invasion of the surgical margin. Another explanation may be, as suggested by Andreou et al.,43 that the R1 margin is a surrogate marker of unfavourable tumour biology, since in R1 patients no more liver recurrence has been seen in the surgical margin than in the rest of the parenchyma. In line with these works29,42–44 in our series we see that margin invasion loses prognostic value in the last study period, probably because the majority of operated patients had shown a favourable response to CT, confirming that it is the tumour biology and not the invasion of the margin that has prognostic value. Extrahepatic disease and the presence of ≥4LM also reflect unfavourable tumour biology and are prognostic factors in the latest era.
It is currently accepted that the follow-up of patients operated on for CRLM should be for 10 years to establish a cure3–6,8,20,23,26,35,37,44–46 In our series there are 18 patients who have recurred after 5 years of follow-up.
This study has the limitations inherent to the retrospective nature of the analysis. Also, because the first study period corresponds to the 90 s, prognostic variables such as major complications according to Clavien-Dindo,4,6,15,35 ASA category,47 mutations in KRAS or BRAF35,48 are not available, and neither are histological growth patterns.4,42,49 As strengths of the work we can mention that it is a long series, of 1,736 hepatectomies, performed in a single centre, with a well-established multidisciplinary committee since the beginning of the study series.
ConclusionsThe profile of the patient operated on for CRLM has changed over time, with an increase in age, the percentage of synchronous and bilobar LM, the indication for perioperative chemotherapy, minor and non-anatomical resections, associated with an improvement in postoperative results with a reduction of mortality. Although survival has improved, it cannot be explained by a reduction in overall or hepatic recurrence, but rather by an improvement in survival after recurrence. This last aspect has a complex explanation influenced by multifactorial aspects. The involvement of the resection margin has lost prognostic value in the last decade.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Mils K, Lladó L, López-Domínguez J, Barrios O, Leiva D, Santos C, et al. ¿Hemos mejorado los resultados postoperatorios y a largo plazo de la hepatectomía por metástasis de cáncer colorrectal? Análisis de 1736 hepatectomías realizadas a lo largo de 3 décadas en un solo centro. Cir Esp. 2024. https://doi.org/10.1016/j.ciresp.2023.11.012