Short-term radiotherapy (STR) for rectal cancer (RC) has rarely been used in Spain. The aim of the present study is to describe oncological results after RTC and surgery for RC.
MethodsThis is a retrospective analysis of a consecutive series of patients treated with STR and surgery for RC (1999–2012). Epidemiological data, staging, complications of STR, STR-surgery interval, surgical approach, rate of anastomotic/perineal wound dehiscence, and pathological data (regression degree and staging) were collected. Global survival, disease free survival, local recurrence rate and incidence of toxicity, response and complications of combined treatment are reported.
ResultsOf 1229 patients treated, 209 patients received STR and surgery. The median follow-up was 6.2 years. Mean age was 68 years and 66% of the patients were men. A total of 88% were cT3-4 and 44% cN+17 (8.1%) patients had resectable synchronous metastases. Acute and chronic toxicity due to STR was <5%. In 75% of the cases the STR-surgery interval was <15 days, and in 9% >4 weeks. Seven patients (3.3%) presented complete response. Nine (4.3%) patients presented a local recurrence rate. Global survival at 5, 10 and 15 years was 67.8%, 49.2% and 37.5%, respectively. Disease free survival at 5, 10 and 15 years was 66.1%, 47.1% and 33%, respectively.
ConclusionsThe results compare favorably with multicentric historical series. STR offers certain advantages that could be increased by increasing the STR-surgery interval and/or interspersed with sequential chemotherapy.
La radioterapia preoperatoria corta (RTC) para el tratamiento del cáncer de recto (CR) ha sido poco utilizada en España. El objetivo del presente trabajo es describir los resultados oncológicos tras tratamiento con RTC y cirugía por CR.
MétodosEstudio retrospectivo que incluye una serie consecutiva de pacientes tratados por CR (1999-2012). Se recogieron datos epidemiológicos, estadificación, complicaciones de la RTC, intervalo RTC-cirugía, abordaje quirúrgico, tasa de dehiscencia de anastomosis o herida perineal e histológicos (grado de regresión y estadificación). Se analizan la supervivencia global, supervivencia libre de enfermedad, tasa de recurrencia local e incidencia de toxicidad, respuesta y complicaciones del tratamiento combinado con RTC y cirugía.
ResultadosDe 1.229 pacientes tratados, 209 pacientes recibieron RTC y cirugía. La mediana de seguimiento fue de 6,2 años. La edad media fue de 68 años y el 66% fueron hombres. El 88% eran cT3-4 y el 44% cN+. Un total de 17 pacientes (8,1%) tenían metástasis síncronas resecables. La toxicidad aguda y crónica por RTC fue inferior al 5%. En el 75% de los pacientes el intervalo RTC-cirugía fue inferior a 15 días y en el 9%, superior a 4 semanas. Fueron 7 los pacientes (3,3%) que presentaron respuesta completa. La mediana de supervivencia fue de casi 10 años. Nueve (4,3%) pacientes presentaron una recurrencia local. La supervivencia global a 5, 10 y 15 años fue del 67,8, 49,2 y 37,5%, respectivamente. La supervivencia libre de enfermedad a 5, 10 y 15 años fue del 66,1; 47,1 y 33%, respectivamente.
ConclusionesLos resultados se comparan favorablemente con las series históricas multicéntricas. La RTC ofrece ciertas ventajas que pueden ampliarse incrementando el intervalo RTC-cirugía o si se intercala con quimioterapia secuencial.
The preferred strategy for treating rectal cancer is currently a multidisciplinary approach that may include a combination of surgery, radiotherapy (RTx) and chemotherapy (CTx).1 Variability in surgical technique, a well-documented phenomenon, significantly affects the outcome of rectal cancer, particularly local recurrence rates. The implementation of circumferential total mesorectal excision (TME) has reduced the rates of pelvic relapse; furthermore, several multicenter trials in the late 1990s and early 21st century have demonstrated that preoperative RTx further reduces local recurrence rates, even in patients with TME.2,3
The biological effect of RTx, as well as the risk of acute or late-onset adverse effects, is related to dose, target volume, fractionation, beam energy or portal arrangements used for its administration. This means that RTx can be used for several purposes in the context of rectal cancer treatment.
There are 2 main ways of using RTx for resectable rectal cancer: short RTx (SRTx) and long-term chemoradiotherapy (CRTx). SRTx administers 25Gy in an accelerated and hypofractionated manner of 5G in 5 days (following a linear quadratic formula, this regimen equals a dose of 42Gy administered in 21 fractions of 2Gy4). With CRTx, 50.4Gy are administered, hyperfractionated into 1.8Gy in 28 sessions, concurrently with radiosensitizing CTx.
Both approaches have evolved in parallel: SRTx was developed mainly in Scandinavia, the Netherlands and Great Britain, while CRTx was implemented in the United States and in central and southern Europe, especially after the German study CAO/ARO/AIO-94.5 There are 2 randomized clinical trials comparing SRTx with CRTx6,7 and, although with certain nuances, none showed significant differences in terms of the frequency of local recurrence, metastasis or survival.
There is little information available about the use and results of SRTx in Spain, where CRTx is mostly used.
The main objective of the present study is to retrospectively analyze the overall survival (OS) and disease-free (DFS) survival as well as the gross and actuarial rates of locoregional recurrence and long-term metastasis in a single-center series of patients with rectal cancer treated with SRTx and radical surgery. The rate and type of acute and chronic complications of SRTx, degree of regression or tumor response to SRTx and rates of perineal wound or anastomotic dehiscence are the secondary objectives.
MethodsOurs is an observational study that follows the STROBE recommendations.
We retrospectively analyzed data entered prospectively into a SRTx-specific database designed and maintained by the hospital's Radiotherapy Department from the period between 1 January 1999 and 31 December 2012. These data were complemented with other data obtained retrospectively from the hospital tumor registry and from the registry of the Colorectal Surgery Division participating in the Viking project.
All patients studied had histologic confirmation of rectal adenocarcinoma prior to treatment. The lesion was considered rectal when the lower border of the tumor was located less than 15cm from the anal margin (measured by rigid rectoscopy) or below the S1–S2 junction (lateral image of the enema or magnetic resonance imaging [MRI]).
From 1999 to 2006, the patients were assessed in joint clinical sessions for digestive tumors with surgeons and oncologists. Starting in 2007, the patients were evaluated by a specific colorectal cancer multidisciplinary team created for this purpose.
For preoperative evaluation and staging, in addition to colonoscopy and possibly a radiocontrast enema, other tests included carcinoembryonic antigen (CEA) determination, chest X-ray, abdominopelvic computed tomography (CT), and endorectal ultrasound or pelvic MRI. Gradually, but definitively as of 2007, enemas were no longer used, the CT scans became thoraco-abdominopelvic and high-resolution MRI started to be used in all cases, with protocol-standardized reports. Endorectal ultrasound started to be used only for apparently initial or superficial tumors.
Between 1999 and 2006, SRTx was indicated for tumors that were clinically classified (by digital rectal examination or ultrasound/CT/MRI) as locally advanced and with clear and defined surgery prior to RTx, either by lower anterior resection or abdominoperineal resection. Starting in 2006, SRTx was gradually reserved for advanced upper rectal tumors, for equally advanced rectal tumors with no threatened mesorectal fascia, or for patients not candidates for CTx, and occasionally for tumors in the lower rectum with infiltration of adjacent structures. Likewise, SRTx was inserted in the “curative” strategy of patients with liver metastases and in individual cases, as agreed upon by the multidisciplinary team.
The SRTx was administered as 3-D conformal radiation therapy through one posterior and 2 lateral thoracic beams, with the patient in the prone position. The therapeutic target volume included the entire dorsal part of the pelvic cavity, from L4 to 3cm below the caudal edge of the tumor (depending on the height of the tumor), including in the lateral margins the internal iliac chains and the obturator lymph nodes.
Surgery was scheduled between 2 and 15 days after the completion of SRTx, with variations due to hospital organization or patient physiological conditions. On occasion, it was intentionally delayed for more than 4 weeks for several reasons (administrative, intercurrent illness, preoperative optimization, etc.). Surgery was performed with partial or total excision of the mesorectum depending on tumor height.
For SRTx, we recorded acute complications (gastrointestinal, urinary and dermal) as well as late-onset (pelvic pain or intestinal stenosis-fistulization) according to the definition of SOMA/LENT and the RTOG/EORTC toxicity grading scale.8
Data were recorded for the type of surgical approach, protective stoma, presence of clinical anastomotic dehiscence or the perineal wound in cases of abdominoperineal resection during post-op. Likewise, we registered the number of patients who received postoperative CTx and the number of cycles.
The pathology variables analyzed included: the degree of tumor differentiation; circumferential tumor extension, involvement of the circumferential radial margin (CRM) (when the tumor is less than 1mm from the mesorectal fascia); involvement of the distal tumor margin; lymph node, vascular or perineural infiltration; the number of resected or affected lymph nodes; and the degree of regression of Mandard. The Mandard regression grade was not collected between 1999 and 2004, so the films were reviewed by 2 pathologists specialized in digestive diseases.
Patient follow-up in the outpatient setting involved periodic office visits (every 3–4 months for the first 2 years, every 6 months until 5 years, and annually thereafter), imaging tests and tumor marker determination.
In the absence of a biopsy, locoregional recurrence was defined as new images suggestive of recurrence; distant recurrence was a tumor in any other area, including the inguinal region, retroperitoneal and iliac lymph nodes.
For the analysis of OS and DFS, we have reviewed the computerized integrated healthcare program of this community, which allows us to track the dates of the last office visit with Medical Oncology, Radiation Oncology, Colorectal Surgery Division or contact with any other medical center, either specialized or primary care. We recorded locoregional or distance recurrences and combinations of both.
Statistical AnalysisAnalyses were conducted with the STATA version 14.0 statistical software. Variables are reported using the most appropriate statistical analysis given their nature and scale of measurement: mean and standard deviation (or median and interquartile range) for quantitative variables, and absolute and relative frequencies in percentage for qualitative variables. The Kaplan–Meier procedure was used to estimate survival curves.
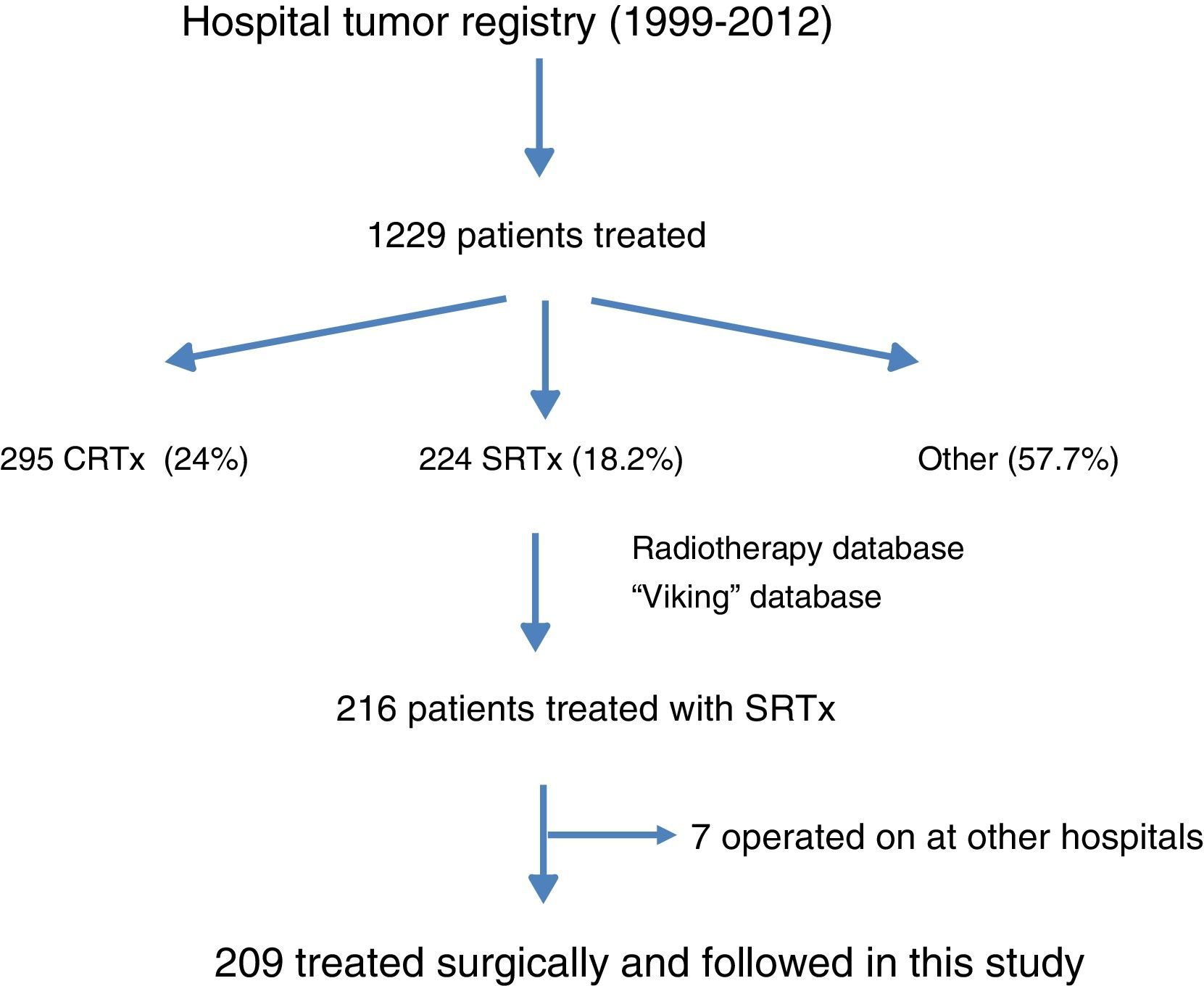
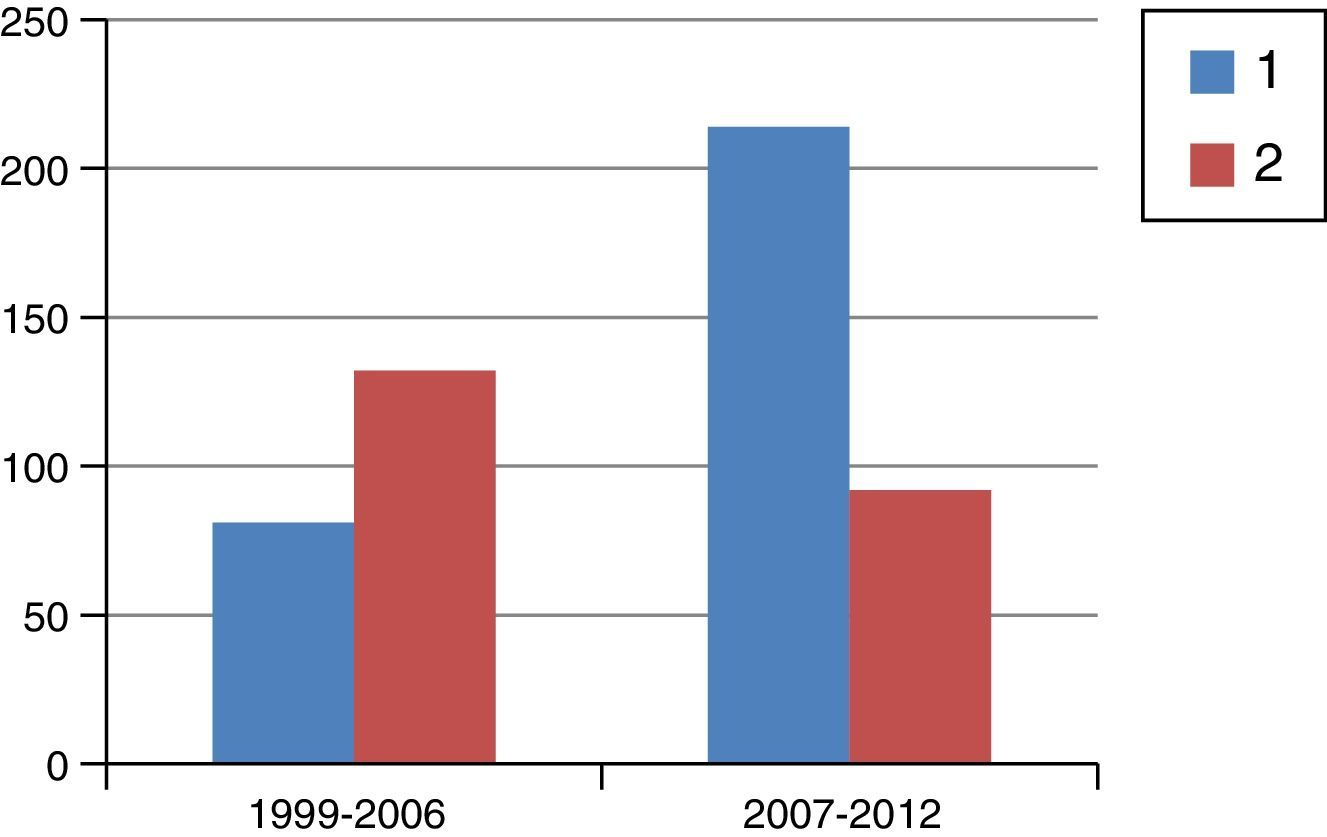
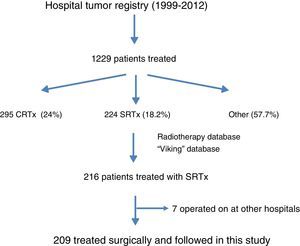
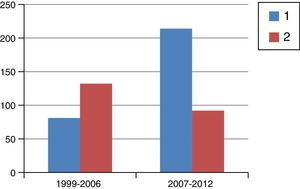
ResultsFig. 1 is a flowchart of patients included in this study. Between 1999 and 2012, 1229 patients with rectal cancer were treated at this hospital, 209 of whom received neoadjuvant SRTx and subsequent surgery. Fig. 2 shows the percentage of patients treated with CRTx and SRTx in the periods of time indicated. Between 1999 and 2006, 61% received SRTx; between 2007 and 2012, 30% received SRTx.
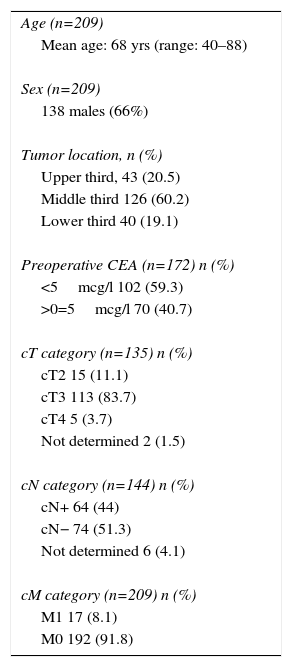
Table 1 shows the epidemiological and clinical data of the series. Out of these 209 patients, 138 (66%) were male. Mean age was 68 years (40–88), and 17 (8.1%) presented potentially resectable synchronous hepatic metastases (cM1). The tumor affected the middle rectum in most cases (60.2%). Out of the 135 patients who had pelvic MRI pretreatment staging, 88% were rmT3–4 and 44% had lymph nodes with suspected tumor infiltration; 40.7% presented CEA levels >5mcg/l.
Epidemiological and Clinical Characteristics.
| Age (n=209) |
| Mean age: 68 yrs (range: 40–88) |
| Sex (n=209) |
| 138 males (66%) |
| Tumor location, n (%) |
| Upper third, 43 (20.5) |
| Middle third 126 (60.2) |
| Lower third 40 (19.1) |
| Preoperative CEA (n=172) n (%) |
| <5mcg/l 102 (59.3) |
| >0=5mcg/l 70 (40.7) |
| cT category (n=135) n (%) |
| cT2 15 (11.1) |
| cT3 113 (83.7) |
| cT4 5 (3.7) |
| Not determined 2 (1.5) |
| cN category (n=144) n (%) |
| cN+ 64 (44) |
| cN− 74 (51.3) |
| Not determined 6 (4.1) |
| cM category (n=209) n (%) |
| M1 17 (8.1) |
| M0 192 (91.8) |
The cT category shown was obtained by magnetic resonance; the cN category shows the data obtained by magnetic resonance imaging, endoanal ultrasound or computed tomography.
Table 2 shows data related to SRTx toxicity, as well as data referring to the type of surgery, surgical approach and incidence of perineal wound or anastomotic dehiscence. Gastrointestinal, urinary or dermal complications of SRTx were uncommon (80% were classified as G0 of RTOG/EORTC) and persistent chronic pain occurred in 5.5% of patients. Ileal stenosis (0.6%) was recorded in only one case (with delayed SRTx). The time interval between the end of the SRTx and surgery was less than 2 weeks in 74.1%, while in 9% the delay was more than 5 weeks (delayed).
Complications of the SRTx, Surgery Performed, Anastomotic or Perineal Wound Dehiscence and Rate of Adjuvant Chemotherapy (1999–2012).
| Acute SRTx complications (n=165) n (%) |
| Gastrointestinal G0 134 (81.2) |
| G1 12 (7.3) |
| G2 18 (10.9) |
| G4 1 (0.6) |
| Dermatitis 4 (2.4) |
| Frequent urination/dysuria 7 (4.2) |
| Late-onset SRTx complications (n=163) n (%) |
| Pain 9 (5.5) |
| Ileitis/ileal stenosis 1 (0.6) |
| SRTx-to-surgery time interval (n=209) n (%) |
| Less than 15 days 155 (74.1) |
| Between 15 and 30 days 35 (16.7) |
| More than 4 weeks 19 (9.2) |
| Type of surgery (n=209) n (%) |
| Resection and anastomosis, 162 (77.5) |
| Abdominoperineal resection, 36 (17.2) |
| Total pelvic exenteration, 4 (1.9) |
| Hartmann procedure, 4 (1.9) |
| Local resection, 3 (1.4) |
| Surgical approach (n=200) n (%) |
| Laparotomy, 96 (48) |
| Laparoscopy, 104 (52) |
| Bowel diversion/definitive (n=209) n (%) |
| Temporary ileostomy, 86 (51.8) |
| No temporary ileostomy, 80 (48.7) |
| Definitive colostomy, 43 (20.6) |
| Anastomotic dehiscence (n=163) 7 (4.2%) |
| Dehiscence of perineal wound (n=36) 9 (25%) |
| Adjuvant chemotherapy (n=209) n (%) |
| Received CTx, 111 (53) |
| No CTx, 98 (46.8) |
CTx: chemotherapy; SRTx: short-cycle radiotherapy.
In 79.4% of the patients, a sphincter-preserving technique was performed, and 20.6% had a definitive colostomy. 51.8% of patients with reconstructive surgery had a diverting ileostomy. The rates of clinical anastomotic and perineal wound dehiscence were 4.2% and 25%, respectively. In this series, 53% of patients received subsequent adjuvant CTx.
Table 3 presents data from the pathology study. 70% presented ypT3-4 and 37.7% ypN+. The mean number of resected lymph nodes in the surgical specimens was 12.2 (4–37). A Mandard grade 1 or complete pathological response (pCR) was observed in 7 patients (3.6%), and in 110 patients (62.2%) the response was limited or nonexistent (Mandard 4–5). Out of the 7 cases with ypT0 pN0, 5 had undergone delayed SRTx, but in 2 cases complete pathological response was observed 2 weeks after the end of the SRTx. The CRM and distal margin were affected by the tumor in 8.1% and 1.9%, respectively.
Pathology Data: Histology and Post-SRTx Grade of Regression.
| Infiltration of intestinal wall (pT) (n=209) n (%) |
| T0 7 (3.3) |
| T1 8 (3.8) |
| T2 49 (23.4) |
| T3 138 (66) |
| T4 7 (3.3) |
| Number of isolated lymph nodes (n=207) |
| Median (range) 12.2 (4–37) |
| Affected lymph nodes (pN) (n=207) n (%) |
| pN0 129 (62.3) |
| pN1 44 (21.2) |
| pN2 34 (16.4) |
| Differentiation grade (n=197) n (%) |
| G I 88 (44.6) |
| G II 100 (50.7) |
| G III 9 (4.5) |
| Circumferential tumor involvement (n=137) n (%) |
| One-third, 53 (38.7) |
| Two-thirds, 46 (33.6) |
| Three-thirds, 38 (27.7) |
| Circumferential radial margin (n=185) n (%) |
| Not affected, 170 (91.8) |
| Affected, 15 (8.1) |
| Distal tumor margin (n=204) n (%) |
| Not affected 200 (98.0) |
| Affected 4 (2.0) |
| Lymphovascular–perineural infiltrate (n=35) n (%) |
| No 26 (80) |
| Yes 7 (20) |
| Mandard tumor regression grade (n=194) n (%) |
| Absence of tumor (Mandard 1) 7 (3.3) |
| Presence of isolated tumor cells (grade 2) 10 (5.1) |
| Presence of fibrosis and tumor cells (grade 3) 57 (29.5) |
| Greater tumor presence (Mandard 4 and 5) 120 (62.1) |
No patients were lost to follow-up. With an average follow-up of 6 years (3 months–16 years), a total of 9 patients (4.3%) had locoregional recurrence, 55 patients (26.3%) distance recurrence and 6 patients (2.9%) combined recurrences (locoregional and distance). At the time of analysis (November 2016), 107 patients were alive (51.1%), 7 (3.3%) with disease; 102 patients had died (48.8%), 51 of these (24.4%) due to disease recurrence.
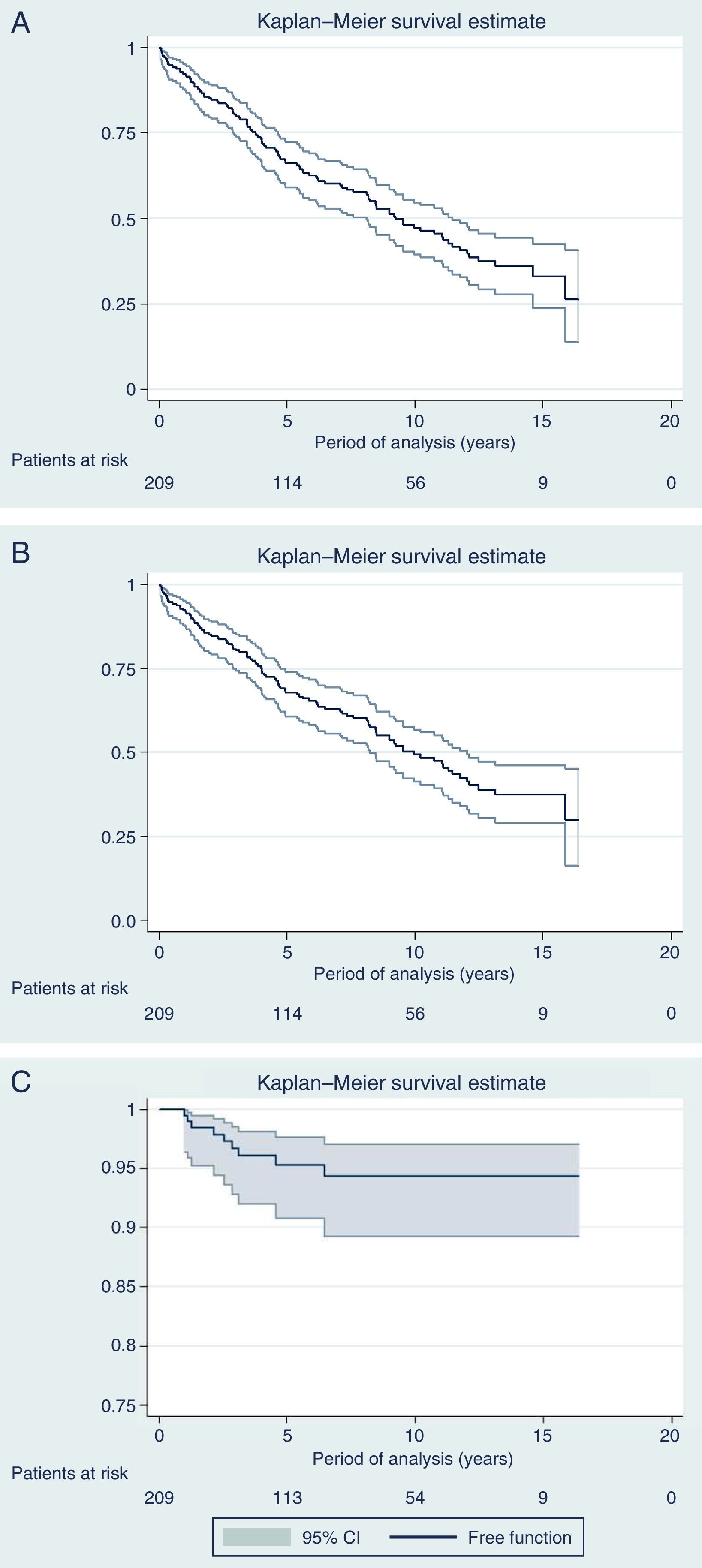
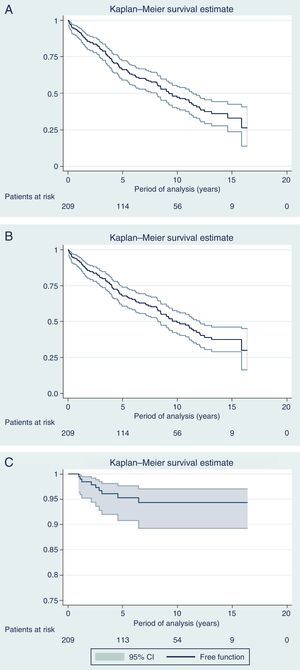
Fig. 3 shows the curves for OS, DFS and Kaplan–Meier local recurrence. Mean survival was almost 10 years. The 5-year OS was 67.8%, 10-year 49.2% and 15-year 37.5%. The 5-year DFS was 66.1%, 10-year 47.1% and 15-year 33%. The 5-year local recurrence rate was 4.7% (95% CI: 2.4–8.2).
DiscussionWith a median follow-up of more than 6 years, the actuarial 5-year local recurrence rate of 4.7% obtained in the present series is similar to the rate published in multicenter trials,2,3 with a mean survival rate of almost 10 years and OS rates of 68%, 50% and 38% at 5, 10 and 15 years, respectively, while the DFS was somewhat higher (66%, 47% and 33% at 5, 10 and 15 years, respectively). However, the present study covers 13 years, during which we have introduced modifications in the management of rectal cancer, including indications for RTx (SRTx vs CRTx), which are a result of the progress and evolution of our knowledge in this pathology.
Starting in 2007, we began to base therapeutic decisions on preoperative MRI images. The MERCURY multicenter study demonstrated that the measurement of transmural growth by MRI was equivalent to its pathological measurement within the surgical specimen, in addition to identifying extramural venous invasion and the risk of CRM involvement (<1mm).9 On the other hand, knowing the ability of CRTx to reduce the size and degrade the clinical stage of the tumor, the response to CRTx began to be identified as an important prognostic factor. Thus, as of 2007, and with the goal set on CRM, we began to indicate CRTx more frequently than SRTx, as can be seen in Fig. 2, which shows the evolution of both RTx modalities in our practice. Probably due to the exclusion of cases with threatened CRM for SRTx, from that date on and with pelvic exenteration in cases of CRM invasions, we obtained a histologic CRM involvement rate of 8.1%, half of that reported in the Dutch study.2,10
SRTx followed by immediate surgery (within one week after completion) leaves no interval for the reaction of the pelvic tissue to the radiation, which, together with the absence of concomitant CTx, causes the frequency of acute toxicities due to the administration of SRTx to be very low.11,12 The complications observed (<5%) consisted mainly of dysuria or pollakiuria; dermal and gastrointestinal toxicities were rare and, in any case, associated with the longer interval between RTx and surgery. Therefore, as expected, patient compliance with treatment was complete.
The incidences of clinical anastomotic and perineal wound dehiscence were 4.2% (>50% of cases with diverting ileostomy) and 25%, respectively. The effect of RTx (long or short) on the rate of dehiscence or anastomotic fistula is controversial. In the Dutch and Swedish clinical trials, no differences were found regarding anastomotic dehiscence among patients with surgery alone and patients with SRTx.2,3 However, increased perineal wound dehiscence after SRTx is well documented, especially when the SRTx-to-surgery interval is longer than 3 days or a delayed SRTx is not performed.2,4,12 The deleterious effect of RTx on genitourinary or gastrointestinal function is well known, but it was not specifically studied in the present study. However, there appear to be no significant differences in this regard between SRTx and CRTx.13,14
The histological response to SRTx begins to be observed 10 days after its completion.13,15 In 75% of our patients, the RTx-to-surgery period was less than 15 days and, consequently, the absence of tumor regression predominated in the surgical specimens studied (Mandard grades 4 and 5). However, in 7 patients (3.3% of the series total) a ypCR was observed after the SRTx; in 5 of these cases, the SRTx-to-surgery interval was longer than 4 weeks, which meant that 9.5% of cases with an interval longer than 4 weeks had a ypCR. These figures are similar to those obtained in the Stockholm III study,15 which examined the effect of increasing the interval between SRTx and surgery. In a clinical trial comparing the downstaging achieved by CRTx and delayed SRTx, it has been shown that the interval that would allow for the maximum response to SRTx would be 10–12 weeks.16
Unlike delayed CRTx and SRTx, histology evaluation after minimum-interval SRTx shows a surgical specimen with minimal or no alterations, which would facilitate the decision to administer adjuvant CTx.4,11,13 In the series presented, 54% of patients received adjuvant CTx, which contrasts with the practically systematic indication when performing CRTx.
The 2 traditional reasons for not recommending SRTx were: a) the absence of downstaging, which would not facilitate sphincter preservation and would not reduce the rate of CRM involvement; and b) the inability (due to toxicity) to administer simultaneous or concurrent CTx.
In our series, the percentage of reconstructive surgery was almost 80%, although, and especially since 2007, most of our patients with advanced lower rectal cancer received CRTx. It is true that in the Dutch and Swedish series the percentage of sphincter preservation was comparatively low. However, with an SRTx-to-surgery interval of 4–8 weeks in the Stockholm III study, a similar number of sphincter-preserving surgeries was achieved.15 In any case, the Polish trial comparing SRTx with CRTx reported no differences in sphincter preservation between the two groups.6
Therefore, downstaging and tumor reduction are not as important per se in resectable tumors if radical surgery is to be used instead of another strategy, like organ preservation. It should be mentioned that, in this latter case, increasing the SRTx-to-surgery interval to 8–10 weeks is the strategy used in the UK-TREK trial for initial rectal cancer (T1–2, N0).17
The development of local recurrence is explained today by the microscopic presence of tumor cells within one centimeter of the CRM and, although the rate of histologically affected CRM is lower when CRTx and delayed SRTx are used compared to non- interval SRTx, it is unclear whether one of these strategies is superior,10,13 even though it has been shown that SRTx only partially compensates for a positive CRM.2 Furthermore, CRTx would facilitate curative resection in cases where the surgeon considers the tumor to be unresectable or borderline (CRM involvement).
The argument that SRTx does not allow concurrent CTx can be refuted by pointing out that standard CRTx uses CTx at radiosensitizing or subsystemic doses; furthermore, as it has greater toxicity, compliance with complete therapy is lower than with SRTx. Attempts at increasing chemoradiosensitivity by adding other drugs, such as oxaliplatin or irinotecan, to the standard CRTx regimen have shown no benefits, yet a significant increase in toxicity.18
SRTx, on the other hand, can be combined with neoadjuvant CTx (before) or sequentially (after), which is relevant because metastases are currently the main cause of failure in the treatment of rectal cancer, not locoregional recurrence.
The strategy of using sequential CTx in patients with good initial response to RTx has shown promising results.18 SRTx adapts well to strategies with sequential CTx administration, and this is the focus of the ongoing phase III RAPIDO trial that uses SRTx followed by CAPOXx6 and radical surgery vs CRTx and radical surgery.19
Furthermore, SRTx with sequential CTx and posterior surgery is a strategy used in cases of resectable synchronous metastases, with a reduction in total treatment time, as the Dutch Colorectal Group study has shown.20 We performed a similar strategy in the 17 cases of the series that presented resectable synchronous metastases using the administration of neoadjuvant induction CTx and posterior SRTx followed by radical surgery.
In conclusion, according to a review of the literature, SRTx offers advantages in terms of the use of resources, costs, duration of treatment, low toxicity, high degree of compliance and allows the unchanged histology to be evaluated. In addition, by increasing the SRTx-to-surgery interval and the use of sequential CTx, the comparative disadvantages with conventional CRTx could be overcome.
We conclude by indicating that the oncologic results obtained in the recent series are similar to historical multicenter randomized studies about SRTx. However, we must consider that the study presented covers an extended period of time during which modifications have been implemented in the preoperative staging and selection indications for CRTx and SRTx.
Authorship/CollaborationsY. Saralegui and C. Placer designed the content of this study; M. Garmendia reviewed all the histology slides. M. Osorio and G. Elorza collected and interpreted the data. J.P. Ciria and A. Lacasta analyzed the data and critiqued the article. J.M. Enríquez-Navascués wrote the article, and J.P. Ciria approved the final version of the article.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Saralegui Y, Enríquez-Navascués JM, Ciria JP, Osorio M, Lacasta A, Elorza G, et al. Resultados de la radioterapia de ciclo corto seguida de cirugía radical en el cáncer de recto: estudio unicéntrico y observacional a largo plazo. Cir Esp. 2017;95:268–275.