It is unknown whether cervical lymphadenectomy as a treatment for cutaneous squamous cell carcinoma of the head and neck (cSCCh&n) increases survival in elderly patients. The aim of this study is to determine whether this procedure has an influence on the survival of these patients, and whether the Short-Form Charlson Comorbidity Index (CCI-SF) can be used as an alternative to age in the surgeon's estimation of elderly patient mortality.
MethodsThe study population included all patients diagnosed with cSCCh&n consecutively treated between 2006 and 2011. Non-invasive, non-cutaneous carcinomas were excluded. Patients were grouped according to their age (<70, 70–79, 80–89, >90), CCI-SF (<3, ≥3) and presence (N1) or absence (N0) of cervical metastases. The dependent variable was the performance or not of cervical lymphadenectomy. A univariate survival analysis was performed according to the presence of metastases, a bivariate analysis for each of the independent variables according to the received treatment and a multivariate analysis.
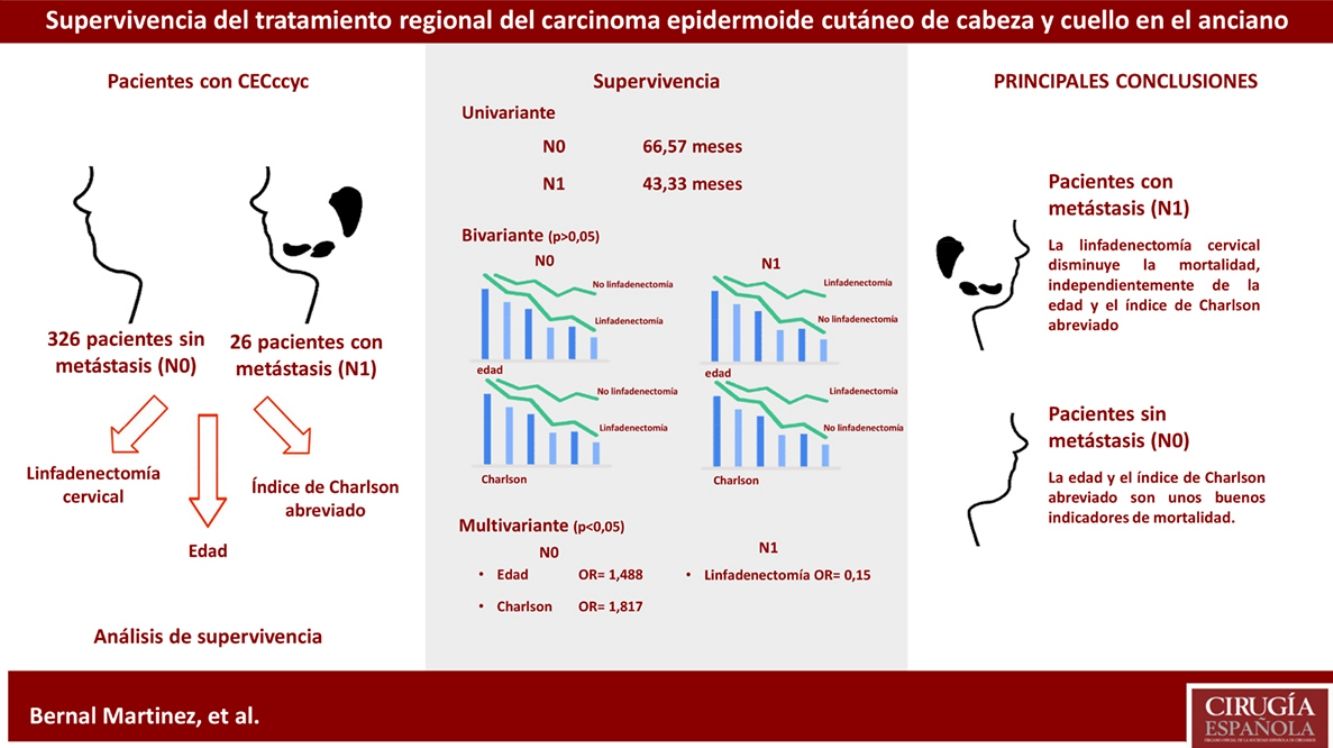
Results416 cases were included. The mean survival time was greater in the N0 group. For each of the groups based on the presence of metastasis, the differences in the mean survival time according to age and CCI-SF were not significant, regardless of the treatment received. The multivariate analysis showed the influence of age (P=.0001, OR=1.488, 95%CI=[1.318; 1.679]) and CCI-SF (P=.001, OR=1.817, 95%CI=[1.257; 2.627]) in the N0 group. In the N1 group only regional treatment has a positive influence on survival (P=.048, OR=0.15, 95%CI=[0.023; 0.981]).
ConclusionsCCI-SF and age are good mortality indicators in cSCCh&n N0 patients, but not so in cSCCh&n N1 patients. In cSCCh&n N1 patients, regional treatment has a positive influence on survival. Differences cannot be affirmed in the mean survival time of patients with cSCCh&n, based on the development of metastases and the treatment given. New studies will be necessary.
Se desconoce si la linfadenectomía cervical para el tratamiento del carcinoma espinocelular cutáneo de cabeza y cuello (CECccyc) aumenta la supervivencia en el paciente de avanzada edad. El objetivo de este estudio es determinar si esta terapia influye en la supervivencia de estos pacientes, y conocer si el índice abreviado de Charlson (ICa) puede utilizarse como alternativa a la edad en la estimación de la supervivencia.
MétodosLa población a estudio la componen todos los pacientes a quienes se diagnosticó CECccyc intervenidos consecutivamente entre 2006 y 2011. Se excluyeron los carcinomas no invasivos, no cutáneos. Se realiza una agrupación de los pacientes en función de la edad (<70, 70-79, 80-89, >90), ICa (<3, ≥3) y presencia (N1) o ausencia (N0) de metástasis cervicales. La variable dependiente es la realización o no de linfadenectomía cervical. Se efectúa un análisis de la supervivencia univariante según la presencia de metástasis; bivariante para cada una de las variables independientes según el tratamiento recibido; y multivariante.
ResultadosSe incluyen 416 casos. El tiempo medio de supervivencia es mayor en el grupo N0. Para cada uno de los grupos según la presencia de metástasis, las diferencias en el tiempo medio de supervivencia según la edad y el ICa no son significativas, independientemente del tratamiento recibido. El análisis multivariante muestra la influencia de la edad (p=0,0001, OR=1,488, IC95%=[1,318; 1,679]) y del ICa (p=0,001, OR=1,817, IC95%=[1,257; 2,627]) sobre los pacientes N0. Respecto a los pacientes N1, solo la variable tratamiento regional tiene una influencia positiva sobre la supervivencia (p=0,048, OR=0,15, IC95%=[0,023; 0,981]).
ConclusionesEl ICa y la edad son buenos indicadores de la mortalidad en pacientes CECccyc N0. No es así en pacientes CECccyc N1. En pacientes CECccyc N1 el tratamiento regional tiene una influencia positiva sobre la supervivencia. No se puede afirmar que existan diferencias en el tiempo medio de supervivencia de los pacientes con CECccyc en función de si han desarrollado metástasis o no y según el tratamiento recibido. Serán necesarios nuevos estudios.
Cutaneous squamous cell carcinoma (cSCC) is the second most frequent non-melanoma skin cancer.1 In Spain, the annual incidence rate is estimated at 86 per 100000 inhabitants.2 75%–90% of these lesions appear in the head and neck.3 cSCC is the most frequent cancer capable of metastasizing,4 which results in higher mortality rates. The 5-year survival of metastatic cSCC without treatment is less than 35%, and between 25 and 70% overall, depending on the bibliographic source consulted.4–8 Treatment of this tumor is radical excision, accompanied or not by cervical lymphadenectomy.1,8–12 The latter is performed in the presence of metastatic nodules, which are identified according to current tumor staging guidelines as N1.13,14 It is necessary to know the characteristics of the tumor and whether it presents metastasis at the time of treatment. Regional treatment involving cervical lymphadenectomy is an aggressive therapeutic procedure. Although it is performed routinely in carcinoma of the head and neck mucosa, there is greater reluctance to perform it systematically in cutaneous carcinomas, as predicting lymphatic drainage pathways of this type of skin lesions has been observed to be difficult. In addition, this therapeutic technique is not free of complications, and lymphadenectomy has a mortality rate of 1%.7
The aggressiveness of the technique raises concerns for surgeons who perform it, especially in seniors. In these patients, high mortality is assumed, and often due to causes other than the skin cancer itself. This phenomenon is known as ageism15,16 and consists of age-related discrimination of patients in clinical practice. In such instances, the therapeutic indication is determined by the subjectivity of the physician and not by scientific evidence. In contrast, the short-form Charlson Index is a predictive scale of mortality, independent of age, which has been validated internationally. A value equal to or greater than 3 on this scale indicates a probability of high short-term mortality.17,18 The aim of this paper is to analyze the influence of age, the short-form Charlson Index and treatment on the survival of senior patients with head and neck cSCC.
MethodsThis is an observational, retrospective analytical study including all cSCC treated surgically by the Plastic Surgery Department at the Miguel Servet University Hospital (Zaragoza, Spain) from 2006 to 2011. The inclusion criteria were: cSCC, surgical treatment, and histological confirmation of invasive carcinoma. The exclusion criteria were: non-invasive carcinomas (carcinomas in situ), non-cutaneous carcinomas, and tumors treated by other departments. An analysis was conducted in which regional treatment was selected as a dependent variable. The sample was divided into two groups, depending on the presence (N1) or absence (N0) of cervical metastases. As independent variables, age and the short-form Charlson Comorbidity Index were also selected. The sample was grouped again according to these variables, by age group (<70, 70–79, 80–89, >90) and according to the Charlson Index (<3, ≥3). The following study variables were recorded: patient gender, age at the time of surgery, death (yes/no) and time of death, main cause of death (regional cSCC, metastasis, etc.), short-form Charlson index, tumor size (high risk: ≥5cm; low risk: <5cm), tumor differentiation grade (high risk: Broders III; low risk: Broders I, II), resection margins (negative, affected), tumor location (high risk: face and auricle, low risk: others), invasion of deep extradermal structures (yes/no); TNM stage, presence of metastasis (yes/no), regional treatment (yes/no).
Statistical AnalysisA univariate survival analysis was performed: bivariate for each of the groups of independent variables according to whether the regional treatment was carried out or not; and multivariate. The following variables were included in the multivariate analysis: grouped age, short-form Charlson Index, gender, number of high-risk characteristics and regional treatment. The analysis was carried out in each of the groups, according to whether they developed metastasis (N1) or not (N0). To perform the bivariate survival analysis, mortality tables and the Wilcoxon test were used, and for the multivariate analysis, the Cox regression and the −2 log likelihood ratio test were used. Alpha=0.05 was considered statistically significant. All statistical analyses were conducted with the Statistical Package for Social Sciences, version 16.0 (SPSS Inc., Chicago, IL, USA).
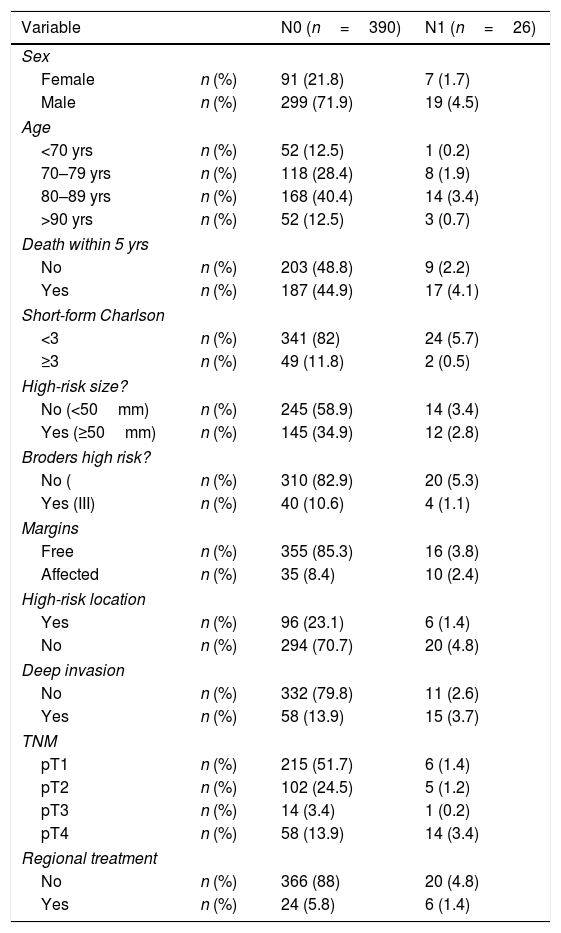
ResultsWe included 416 cases of cSCC. Table 1 reflects the general data of the population under study. Most of the metastases (25 out of 26) occurred in patients over the age of 70. Twelve cases were not treated according to current clinical guidelines; in 6 cases, it was followed when the patient did not present metastasis (N0), and in 6 it was not followed when they did (N1). The mortality of the surgery by regional treatment was 3.33%. The univariate survival analysis showed that the N0 group had a median survival of 66.57 months, and the N1 group survival was 43.33 months. When the bivariate analysis was conducted on the group that did not develop metastasis (N0)—390 cases—and on N1 patients—26 cases—, the results obtained are shown in Table 2. Both in the N0 group and in the N1 group, as age or the value of the abbreviated Charlson Index increased, the survival time was shorter, regardless of the treatment received. These differences were not statistically significant (Fig. 1).
General Characteristics of the Study Sample.
| Variable | N0 (n=390) | N1 (n=26) | |
|---|---|---|---|
| Sex | |||
| Female | n (%) | 91 (21.8) | 7 (1.7) |
| Male | n (%) | 299 (71.9) | 19 (4.5) |
| Age | |||
| <70 yrs | n (%) | 52 (12.5) | 1 (0.2) |
| 70–79 yrs | n (%) | 118 (28.4) | 8 (1.9) |
| 80–89 yrs | n (%) | 168 (40.4) | 14 (3.4) |
| >90 yrs | n (%) | 52 (12.5) | 3 (0.7) |
| Death within 5 yrs | |||
| No | n (%) | 203 (48.8) | 9 (2.2) |
| Yes | n (%) | 187 (44.9) | 17 (4.1) |
| Short-form Charlson | |||
| <3 | n (%) | 341 (82) | 24 (5.7) |
| ≥3 | n (%) | 49 (11.8) | 2 (0.5) |
| High-risk size? | |||
| No (<50mm) | n (%) | 245 (58.9) | 14 (3.4) |
| Yes (≥50mm) | n (%) | 145 (34.9) | 12 (2.8) |
| Broders high risk? | |||
| No ( | n (%) | 310 (82.9) | 20 (5.3) |
| Yes (III) | n (%) | 40 (10.6) | 4 (1.1) |
| Margins | |||
| Free | n (%) | 355 (85.3) | 16 (3.8) |
| Affected | n (%) | 35 (8.4) | 10 (2.4) |
| High-risk location | |||
| Yes | n (%) | 96 (23.1) | 6 (1.4) |
| No | n (%) | 294 (70.7) | 20 (4.8) |
| Deep invasion | |||
| No | n (%) | 332 (79.8) | 11 (2.6) |
| Yes | n (%) | 58 (13.9) | 15 (3.7) |
| TNM | |||
| pT1 | n (%) | 215 (51.7) | 6 (1.4) |
| pT2 | n (%) | 102 (24.5) | 5 (1.2) |
| pT3 | n (%) | 14 (3.4) | 1 (0.2) |
| pT4 | n (%) | 58 (13.9) | 14 (3.4) |
| Regional treatment | |||
| No | n (%) | 366 (88) | 20 (4.8) |
| Yes | n (%) | 24 (5.8) | 6 (1.4) |
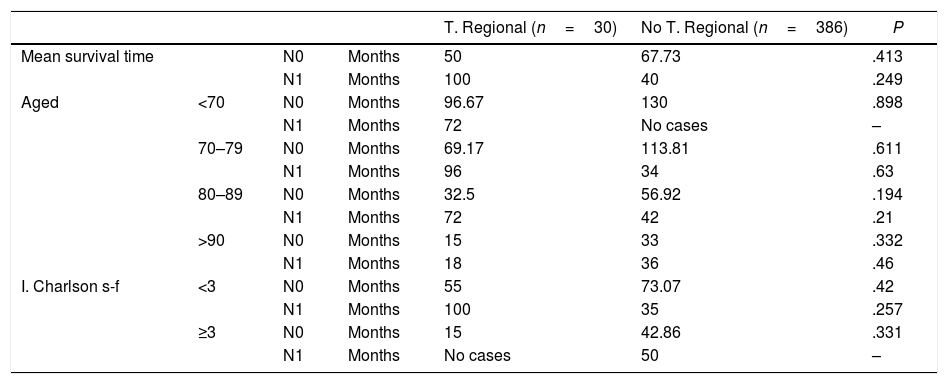
Survival Analysis.
| T. Regional (n=30) | No T. Regional (n=386) | P | ||||
|---|---|---|---|---|---|---|
| Mean survival time | N0 | Months | 50 | 67.73 | .413 | |
| N1 | Months | 100 | 40 | .249 | ||
| Aged | <70 | N0 | Months | 96.67 | 130 | .898 |
| N1 | Months | 72 | No cases | – | ||
| 70–79 | N0 | Months | 69.17 | 113.81 | .611 | |
| N1 | Months | 96 | 34 | .63 | ||
| 80–89 | N0 | Months | 32.5 | 56.92 | .194 | |
| N1 | Months | 72 | 42 | .21 | ||
| >90 | N0 | Months | 15 | 33 | .332 | |
| N1 | Months | 18 | 36 | .46 | ||
| I. Charlson s-f | <3 | N0 | Months | 55 | 73.07 | .42 |
| N1 | Months | 100 | 35 | .257 | ||
| ≥3 | N0 | Months | 15 | 42.86 | .331 | |
| N1 | Months | No cases | 50 | – |
In the case of patients who presented cervical metastasis (N1), the subgroup that received regional treatment with a Charlson Index higher than 3 and the subgroup of patients younger than 70 years of age in whom lymphadenectomy was not performed could not be analyzed because neither subgroups had sufficient cases. The multivariate analysis of the N0 group showed the following results: 390 cases, 39.5% censored, grouped age variables (P=.0001, OR=1.488, 95% CI=[1.318; 1.679], abbreviated Charlson Index (<3, ≥3) (P=.001, OR=1.817, 95%CI=[1.257; 2.627]); gender, number of high risk characteristics and regional treatment (P<.05). When applied to patients with N1 (26 cases, 30.8% censored), these were the results: regional treatment (P=.048, OR=0.15, 95%CI=[0.023; 0.981]), grouped age, Charlson Index, gender and number of high-risk characteristics (P<.05).
DiscussionWhen deciding whether to perform cervical lymphadenectomy in a senior patient, the surgeon may be influenced by the age of the patient before conducting such an aggressive maneuver.15 The logical reaction is to think that, because the patient is an older person, surgery may be harmful and the patient will probably as the result of some other cause.16 This is more understandable the greater the patient's age. In this instance, the therapeutic indication is influenced by subjective information, which entirely depends on the beliefs and opinions of the surgeon. In the survival analysis, and considering only the patients who did not develop metastases, the only variables that have an influence on patient survival are age (P=.0001) and the abbreviated Charlson Index (P=.001). Each has an OR of 1.488 and 1.817, respectively, which indicates that the probability of death increases with age, and when adding a Charlson Index greater than or equal to 3. Gender and the number of high-risk tumor characteristics do not generate significant differences in the survival of this patient sample. The same thing happens with regional treatment. This variable is included in the multivariate analysis of N0 patients to determine its validity. The results demonstrate, as expected, that cervical lymphadenectomy in a patient who has not developed metastasis has no influence on survival. These data should be interpreted with caution, because, although the sample is large, the analysis shows a high number of censored data (39.5%).
However, the same is not true in the group of patients with metastasized cervical lymph nodes (N1). In these patients, the multivariate analysis does not show that age or the Charlson index influence survival, and instead confirms the positive influence of the regional treatment (P=.048, OR=0.15), that is, that lymphadenectomy in these patients acts as a protective factor against mortality. Table 2 shows that patients with N1 who received regional treatment have a longer survival than those who did not (100 vs 40 months). These differences are not statistically significant (P=.249). The same occurs with patients who did not develop metastases, which in this case act as a control group. N0 patients who received regional treatment have a lower median survival than those who did not (50 vs 67.73). Likewise, these differences were not statistically significant (P=.413).
As age increased, the median survival in both groups decreased, regardless of the treatment received (Fig. 1). However, these differences were not statistically significant. Table 2 shows that the median survival was different in the N0 patient group, within each of the age groups but having received different treatments. In addition, as shown by the multivariate analysis, in this group of patients the treatment had no influence on survival (P<.05). It cannot be stated, therefore, that patients with N0 have longer survival rates depending on whether or not they undergo lymphadenectomy.
With the results of the bivariate survival analysis, this statement can be extrapolated to the N1 patient group, since their survival is not significantly greater according to the treatment received. However, the multivariate analysis confirmed the influence of treatment on the survival of N1 patients (P=.048). The mortality of N1 patients who had cervical lymphadenectomy was lower, but with these data it cannot be said that the survival time is longer or shorter than patients without cervical lymphadenectomy.
The Charlson Index has been shown to be a good indicator of mortality in patients who did not develop metastases (P=.001, OR=1.817), with an influence greater than age and whose OR was lower (1.488). This was not so in the group of patients with N1 tumors. This may have been due to the difference in the number of cases in each of the groups, and to the fact that N1 tumors have a more aggressive behavior, despite the treatment.
To our knowledge, there are no previous studies that analyze the utility of age or the short-form Charlson Index as indicators for the survival of patients with this pathology.
The study does not show that either the Charlson Index or age is a good indicator for survival in patients with cSCC and cervical nodules. Older patients are the most affected by metastasis of these tumors. In order not to fall into the practice of ageism, the decision should not be made to perform an aggressive and curative treatment on the patient while anticipating mortality because the patient is older. Diagnostic and therapeutic options should be contemplated, along with how these factors influence survival.
According to this study, regional treatment reduces the mortality of N1 cSCC, regardless of age and the value of the short-form Charlson Index. It does not demonstrate that treatment with cervical lymphadenectomy modifies the median survival time in these patients. Further studies, probably with larger samples, are necessary to present significant results. Age and the short-form Charlson Index are once again shown to be good indicators of mortality in patients with cSCC who do not develop cervical metastasis.
Conflict of InterestsNone of the authors have a conflict of interests to declare.
Please cite this article as: Bernal Martínez ÁJ, Fernández Letamendi N, Delgado Martínez J, Gómez-Escolar Larrañaga L, Reola Ramírez E, Puertas Peña J. Evaluación del tratamiento del carcinoma epidermoide cutáneo de cabeza y cuello en la edad avanzada. Análisis de la supervivencia. Cir Esp. 2018;96:577–582.