Quadricuspid aortic valve (QAV) is a rare congenital malformation, attributed to abnormal mesenchymal proliferation that causes the subdivision of one of the three cushions forming the aortic valve. In some cases, coronary artery anomalies are present. We present the clinical case of a patient diagnosed with aortic regurgitation who lost follow-up due to the COVID-19 pandemic. The patient experienced a deterioration to NYHA functional class III. The echocardiogram identified a quadricuspid aortic valve, type F according to Hurwitz and Roberts and type I based on Nakamura et al., with thickened cusps and a coaptation defect resulting in severe regurgitation. Coronary angiography revealed an independent origin of the left anterior descending artery and the circumflex artery. Aortic valve replacement with a biological prosthesis was performed. The patient had a favorable outcome and remains in outpatient follow-up.
La válvula aórtica cuadricúspide (QAV) es una malformación congénita poco frecuente, atribuida a una proliferación mesenquimal anormal que causa la subdivisión de uno de los tres cojinetes que forman la válvula aórtica. En algunos casos se presentan anomalías de las arterias coronarias. Presentamos el caso clínico de una paciente diagnosticada con insuficiencia aórtica que perdió el seguimiento debido a la pandemia de COVID-19. La paciente experimentó un deterioro a clase funcional NYHA III. El ecocardiograma identificó una válvula aórtica cuadricúspide, tipo F según Hurwitz y Roberts y tipo I según Nakamura et al., con engrosamiento de los velos y un defecto de coaptación que provocaba insuficiencia severa. La coronariografía reveló un origen independiente de la arteria descendente anterior y la arteria circunfleja. Se realizó un reemplazo valvular aórtico con una prótesis biológica. La paciente tuvo una evolución favorable y se mantiene en seguimiento ambulatorio.
A quadricuspid aortic valve (QAV) is a rare congenital malformation. Its prevalence ranges from 0.013 to 0.043%. The first reported case was in 1847 during the autopsy of a patient with sudden death. Its etiology is associated with abnormal mesenchymal proliferation during aortic valve formation, leading to the formation of an accessory cusp. Between 18% and 32% of patients with QAV have an additional cardiac defect such as subaortic stenosis, atrial septal defect, ventricular septal defect, patent ductus arteriosus, or transposition of the great arteries. In 10% of cases, coronary artery anomalies or low implantation of the ostia are observed. A QAV can remain asymptomatic during childhood, and due to progressive hemodynamic stress with age, it may lead to valvular dysfunction, which commonly occurs between the fifth and sixth decade of life. 74.7% present with aortic valve regurgitation, and 8.4% present with stenosis. 96% of patients with QAV do not have aortic involvement. Surgical indication is based on the ESC/EACTS guidelines for aortic valve disease, using quantitative and qualitative data reported in the echocardiogram. Surgical management is the most common treatment for this type of pathology.1–8
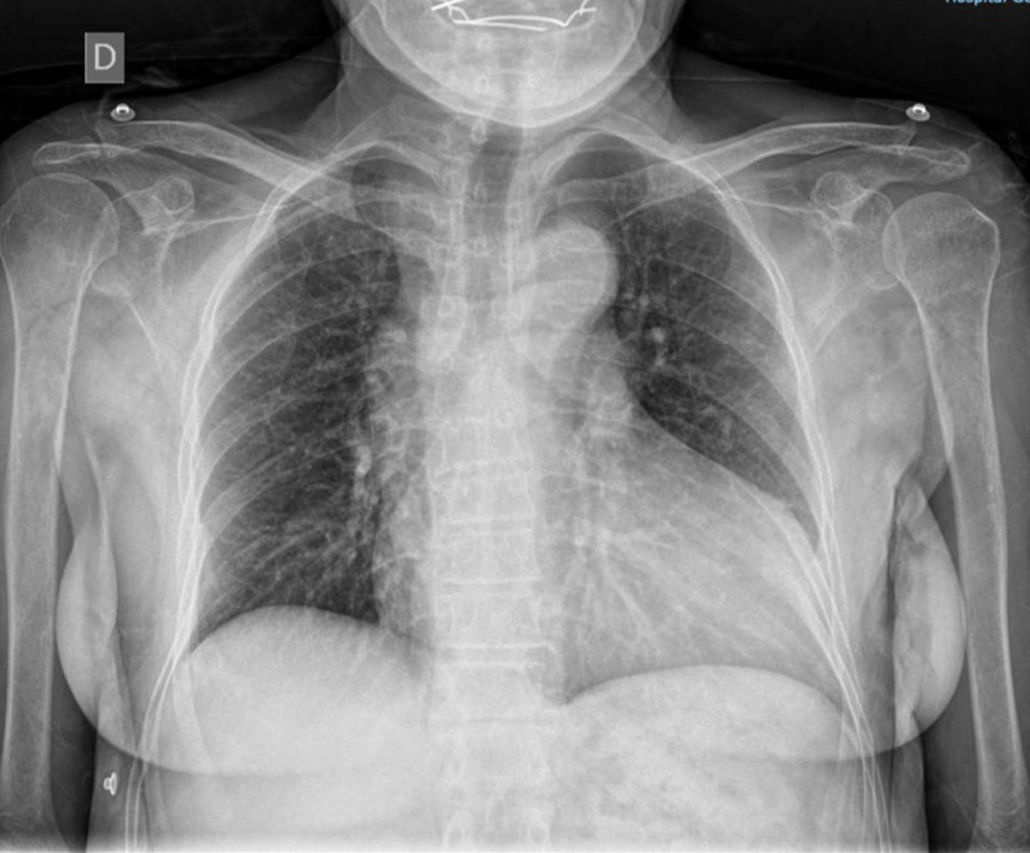
Case reportA 72-year-old female with hypertension. At the age of 69, she was diagnosed with mild aortic regurgitation, NYHA functional class I, and due to the COVID-19 pandemic, she lost follow-up. For the past eight months, she has reported a deterioration to NYHA class III, accompanied by chest pain, palpitations, and syncope. Physical examination revealed Müller's sign and a grade IV/VI holosystolic murmur at the aortic focus. The electrocardiogram showed sinus rhythm with criteria for left ventricular hypertrophy. The posteroanterior chest X-ray revealed widened hila, grade III cardiomegaly, and a prominent aortic knob (Fig. 1).
The transthoracic echocardiogram reported a dilated left ventricle with no motion abnormalities. LVEF of 51%. Quadricuspid aortic valve with sclerosis of its cusps, adequate opening, closure defect with a central jet that covers more than 2/3 of the left ventricular outflow tract. Vena contracta of 7mm. Aortic valve area of 2.4cm2, regurgitant volume of 79ml, indicating severe aortic regurgitation (Fig. 2).
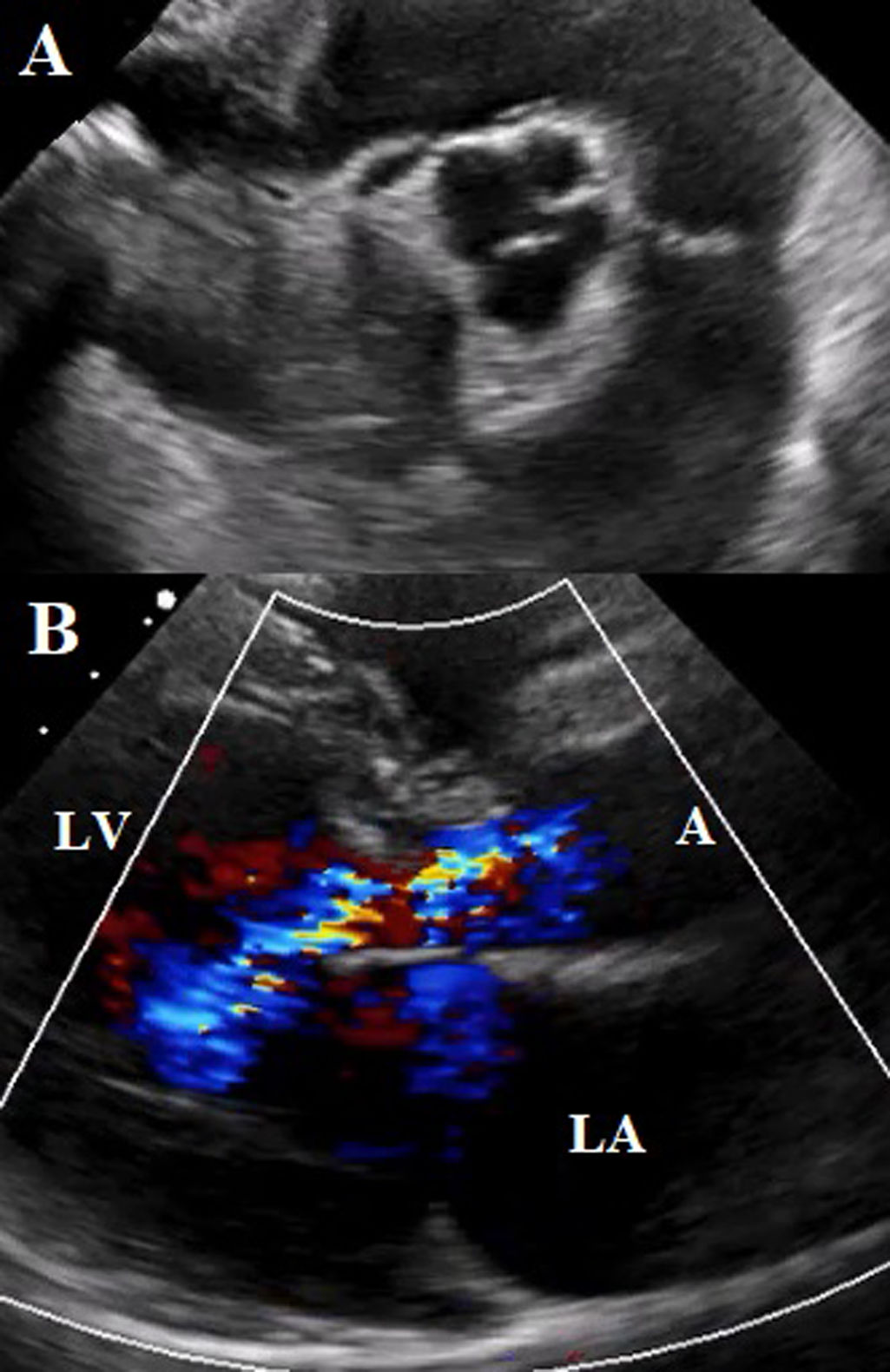
A transesophageal echocardiogram (TEE) was performed, showing a quadricuspid aortic valve with thickened cusps in their distal portion, right, left, and non-coronary cusps of the same size, with the presence of a small supernumerary cusp between the left and right coronary cusps. Aortic valve area by 3D of 2.5cm2. Central regurgitant jet reaching the apical portion of the left ventricle with an EROA of 0.44cm2 (Fig. 3).
(A) TEE mid-esophageal view in great vessels at 100 degrees. Showing quadricuspid aortic valve with thickened cusps. Commissural coaptation in an “X” shape in diastole. (B) 3D reconstruction of the quadricuspid aortic valve with a supernumerary cusp between the left and right coronary cusps.
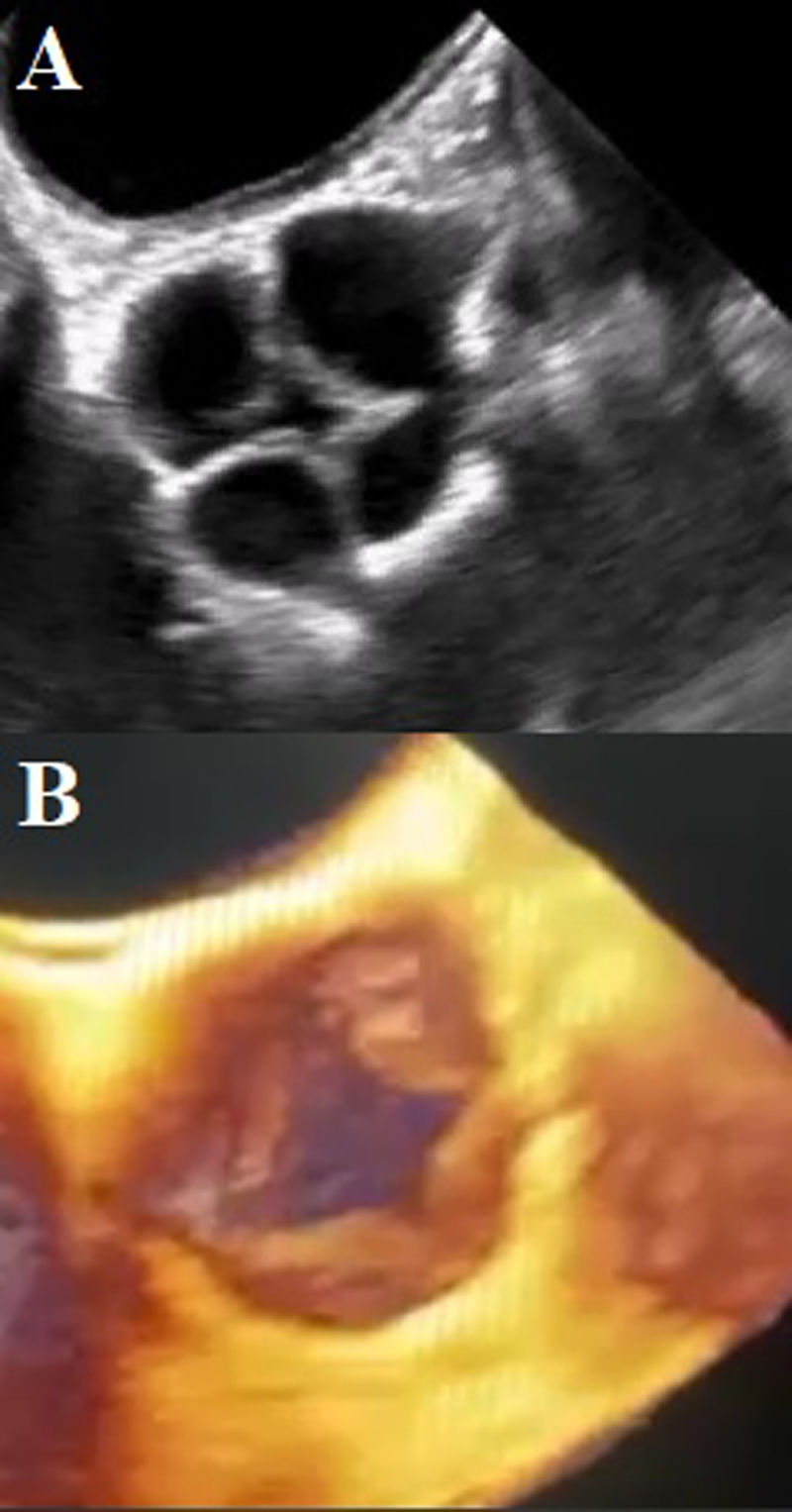
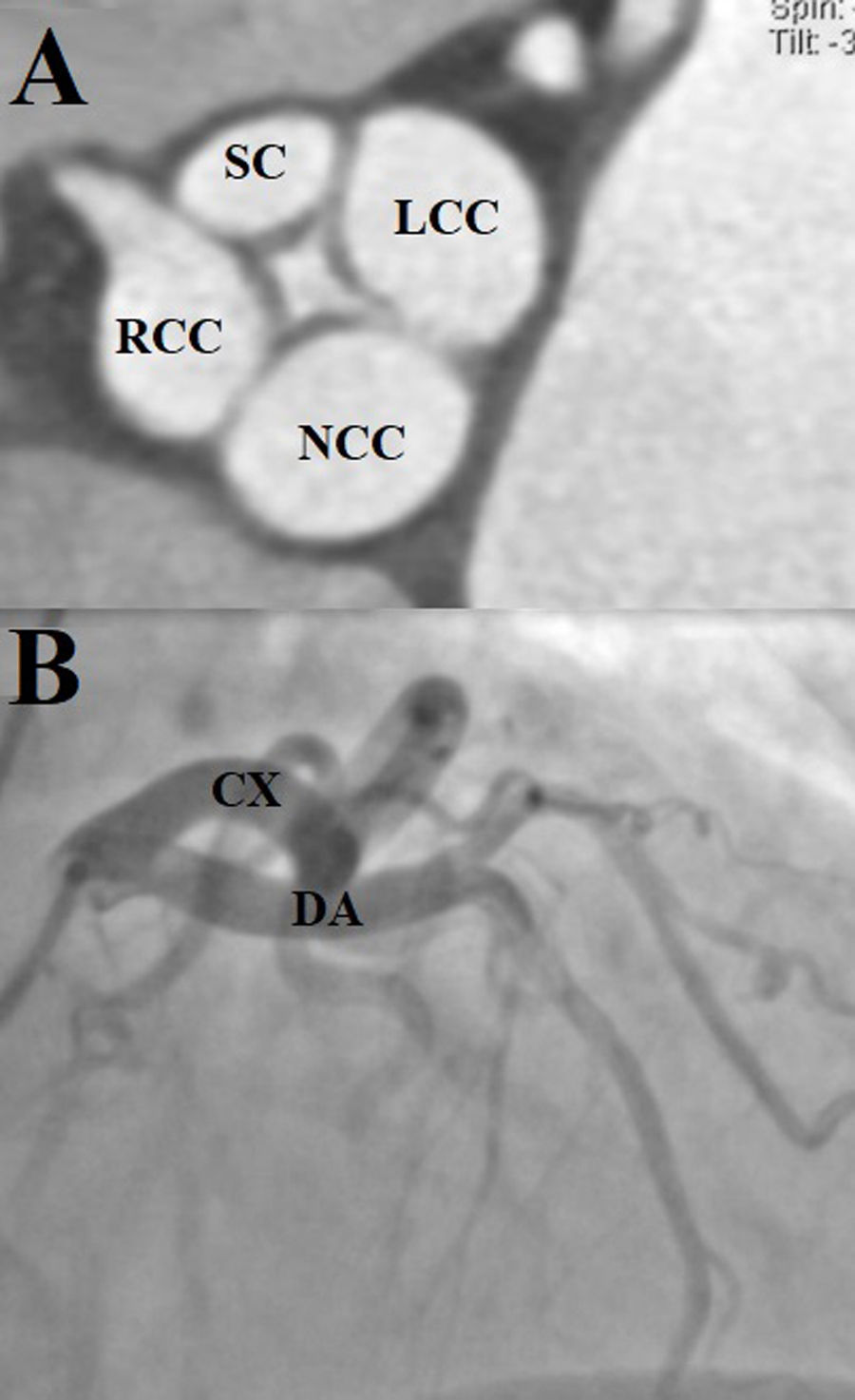
Dilated aortic complex with aortic root measuring 31mm, sinotubular junction 28mm, ascending aorta 35mm, complemented with contrast-enhanced tomography to assess the aorta, which provided clearer images of the aortic valve (Fig. 4A). Coronary angiography identified an independent origin of the left anterior descending artery and the circumflex artery. No atherosclerotic lesions were identified (Fig. 4B).
(A) Contrast tomography. Quadricuspid aortic valve. The supernumerary cusp (SC) is observed between the left and right coronary cusps (Nakamura type I). (LCC: left coronary cusps; RCC: right coronary cusps; NCC: non-coronary cusps). (B) Right anterior oblique projection with skull angulation, showing independent origin of the left anterior descending and circumflex arteries (DA: anterior descending artery; CA: circumflex artery).
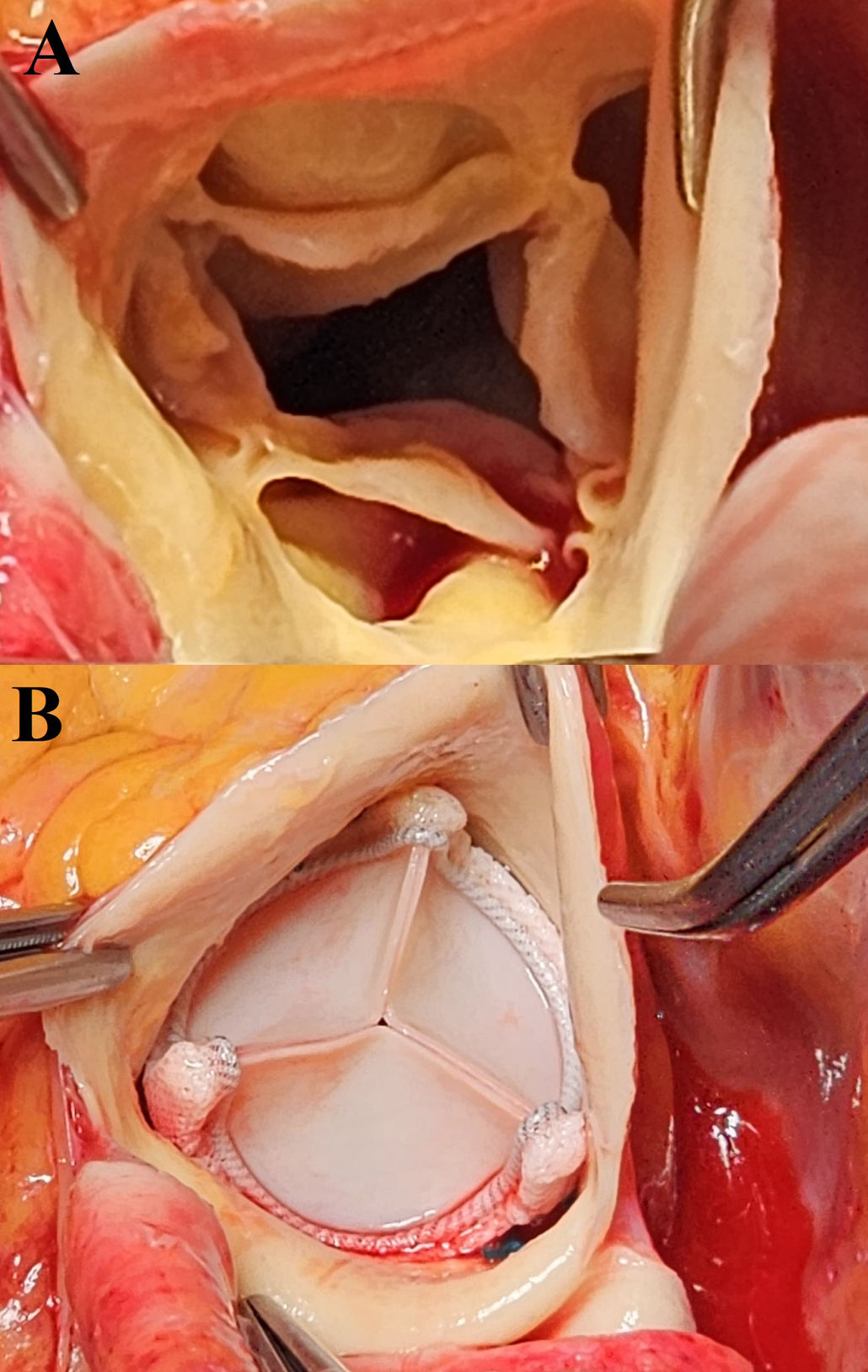
Considering the patient's age, valve replacement with a biological prosthesis was determined. Intraoperatively, a quadricuspid aortic valve with thickened cusps was observed. The aortic annulus was slightly thickened (Fig. 5A). A 21mm Edwards biological prosthesis was placed (Fig. 5B).
The surgery was uncomplicated, with an extracorporeal circulation time of 60min and aortic cross-clamp time of 50min.
DiscussionThe quadricuspid aortic valve (QAV) is a rare congenital malformation. The prevalence is almost similar between men and women. Bicuspid aortic valves are more common, followed by unicuspids and then quadricuspids.1,2,6
Between the fifth and ninth week of gestation, during the early stages of the septation of the truncus arteriosus and the formation of the aortic valve, mesenchymal cushions protrude into the aortic lumen to form the three semilunar cusps. Embryological studies have shown that abnormal mesenchymal proliferation causes subdivision or partition of one of the three cushions, leading to the formation of an accessory cusp and, therefore, a quadricuspid aortic valve.1,2,6,9
QAV is formed by four well-differentiated, thickened cusps with retraction of their edges, whose size and position may vary. True congenital QAVs are distinguished by the presence of nodules of Arantius at the center of the free edge of each of the four cusps, as was the case with our patient. Histological examination identified areas of edema, fibrosis, hyalinization, and foci of calcification, similar to what has been reported in the reviewed literature.1,4,10
No other cardiac malformation was identified in the patient, but an independent origin of the left anterior descending artery and the circumflex artery was observed.
Some adult patients with QAV may remain asymptomatic and require continuous clinical and echocardiographic follow-up, considering that valvular dysfunction is progressive over time. The patient was diagnosed with aortic regurgitation three years ago and remained asymptomatic until eight months prior to admission when she experienced a deterioration in functional class. Patients with large prolapsing cusps and those with small cusps are more prone to degeneration.1,2,4,6,8
Signs and symptoms present as congestive heart failure, peripheral edema, dyspnea, palpitations, fatigue, angina, and syncope. In our patient, syncope was a warning sign leading to timely intervention, suspecting ostium occlusion by a redundant cusp. The mechanism of regurgitation is due to the asymmetry of transvalvular flow caused by the increased number of cusps, generating turbulence and stress, favoring progressive fibrosis of the cusps and failure in coaptation. Its progression is slower compared to patients with bicuspid aortic valves. In this case, the dilation of the ascending aorta did not meet criteria for any surgical management.1,2,4
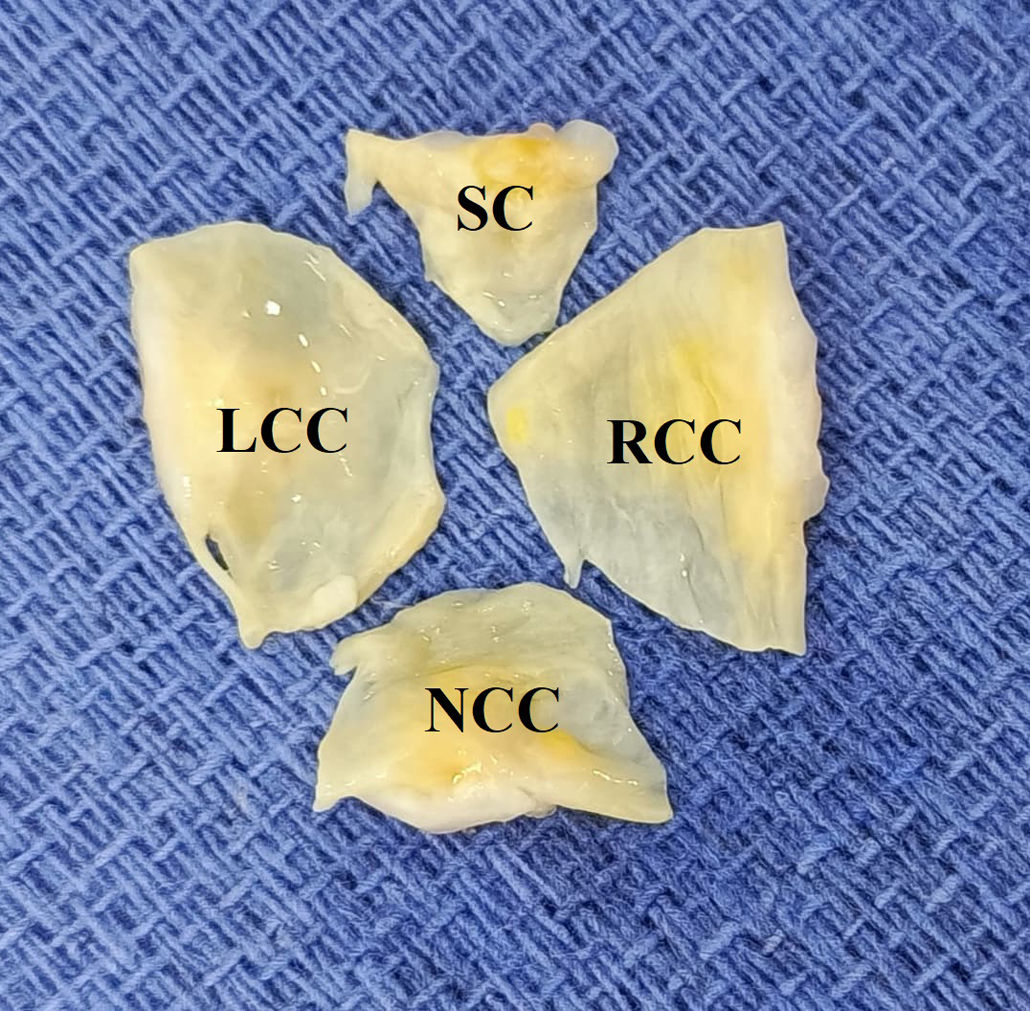
There are two classifications for QAV. The Hurwitz and Roberts classification categorizes based on the size of the cusps, establishing 8 types (A–H). Types A, B, and C represent 85% of the cases. Type F is very unusual. Nakamura et al. described a classification based on the position of the accessory cusp (I–IV), with type II (between the right coronary cusp and the non-coronary cusp) being the most common, with a prevalence of 30.9%.1,4,6 The QAV in our patient was type F by size and type I by position (Fig. 6).
For the diagnosis and valvular study, the transthoracic echocardiogram is the first-line choice as it allows the assessment of valvular function, the mechanism of dysfunction, additional defects such as ostial displacement, and characterization of the number of cusps. The classic pattern of QAV is visualized by commissural coaptation in an “X” shape in diastole. The morphology of QAV can vary in subtypes and may be distorted in elderly patients with calcified valves, making 3D reconstruction or transesophageal echocardiography useful for diagnostic support. Tomography can aid in evaluating the origin of the coronary ostia and aortic dimensions.1–4
In the particular case of QAV, if the patient is asymptomatic and there is a suspected risk of coronary ostial occlusion by valvular tissue, management should not be delayed due to the risk of sudden death. In the reported case, the surgery had a recommendation level of IB according to valvulopathy guidelines based on clinical and imaging data.1,5
Surgical management involves two scenarios: valve replacement with a prosthetic valve or conservative surgery, depending on age, symptoms, and cusp morphology. Valve replacement is more common since thickening and rolling of the cusps make repair difficult. Conservative surgery has gained ground in younger patients who wish to avoid valve-related risks such as bleeding, endocarditis, thromboembolism, and prosthetic valve dysfunction. There are two described techniques for QAV: tricuspidization, which involves resection of the supernumerary cusp along with commissuroplasty; and bicuspidization, which consists of plication and commissural closure of the QAV. For these last two techniques, a commonly described complication is complete atrioventricular block, as the accessory cusp lies over the membranous septum between the right and non-coronary cusps. In this patient, considering her age, valve replacement with a biological prosthesis was performed. Studies have evaluated overall survival between post-surgical and non-surgical groups without a significant difference. The long-term outcome was favorable not only for patients without aortic valve surgery but also for those who underwent aortic valve repair or replacement.1,6–8
The patient had a favorable outcome; signs and symptoms disappeared, and she was discharged with anticoagulation for ninety days. The follow-up echocardiogram showed a bioprosthesis in the aortic position with no qualitative or quantitative signs of dysfunction.
ConclusionQuadricuspid aortic valve (QAV) is a rare congenital defect. Its clinical manifestations commonly present with signs and symptoms of congestive heart failure. The echocardiogram remains the first-line study for its diagnosis. The type of surgical intervention is determined based on patient risk factors such as age, comorbidities, and QAV morphological characteristics. In this patient, valve replacement with a bioprosthesis was performed, and the subsequent clinical course was favorable. The presentation of this case adds our experience to the approximately 300 cases reported in the literature and contributes to the still poorly defined clinical aspects, natural history, diagnosis, and management guidelines of QAV.
CRediT authorship contribution statement- •
Rodrigo Cueva-Tutillo: Conceived and designed the analysis; Collected the data; Contributed data or analysis tools; Performed the analysis; Wrote the paper.
- •
Ignacio Salazar-Hernández: Collected the data; Contributed data or analysis tools.
- •
Octavio Flores-Calderón: Collected the data; Contributed data or analysis tools.
- •
Karen Ferreyro-Espinosa: Collected the data; Contributed data or analysis tools.
- •
Serafín Ramírez-Castañeda: Collected the data; Contributed data or analysis tools.
- •
Pamela Muñoz-Reyes: Collected the data; Contributed data or analysis tools.
The authors declare that a written informed consent was obtained for the publication of the case.
Declaration of generative AI and AI-assisted technologies in the writing processThe authors declare that they do not use IA for write this article.
FundingNo funding was received for this article.
Conflict of interestThe authors declare that they have no competing interests.