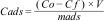A physicochemical characterization was performed on a pennate-type diatomite of lacustrine origin from the southern region of Peru. Diatomite treatments were conducted with the goal of applying this material in adsorption of cadmium, various physical and chemical modifications were carried out, including thermal treatment and modifications with hydrochloric acid, sulfuric acid, and sodium hydroxide. A composite with cellulose and sulfuric acid was also prepared, and finally, a chelating agent EDTA (Ethylenediaminetetraacetic acid) was added. The thermal modifications showed weakly bound hydroxyl groups on the surface, originating from adsorbed water, as well as strongly bound hydroxyl groups from silanol group, as observed in FTIR (Fourier Transform Infrared Spectroscopy) studies. Higher adsorption capacity of cadmium values were obtained in samples modified with 0.5M NaOH post thermal modification at 700°C. SEM analysis showed a uniform distribution of cadmium on the diatomite surface. However, modifications with cellulose and sulfuric acid, as well as EDTA, did not significantly increase cadmium adsorption.
Se realizó una caracterización fisicoquímica de una diatomita tipo pennada de origen lacustre de la región sur del Perú. Se realizaron tratamientos de diatomita con el objetivo de aplicar este material en la adsorción de cadmio, se efectuaron diversas modificaciones físicas y químicas, incluyendo tratamiento térmico y modificaciones con ácido clorhídrico, ácido sulfúrico e hidróxido de sodio. También se preparó un compuesto con celulosa y ácido sulfúrico, y finalmente, se agregó un agente quelante ácido etilendiaminotetraacético (EDTA). Las modificaciones térmicas mostraron grupos hidroxilo débilmente unidos en la superficie, originados del agua adsorbida, así como grupos hidroxilo fuertemente unido del grupo silanol, como se observó en estudios FTIR. Se obtuvieron mayores valores de capacidad de adsorción de cadmio en muestras modificadas con NaOH 0,5M posmodificación térmica a 700°C. El análisis SEM mostró una distribución uniforme de cadmio en la superficie de la diatomita. Sin embargo, las modificaciones con celulosa y ácido sulfúrico, así como EDTA, no aumentaron significativamente la adsorción de cadmio.
The presence of heavy metals such as cadmium, lead, copper, and zinc has been detected at levels exceeding permissible limits for safe drinking water [1]. A study on water and sediment samples from the Puyango-Tumbes River in Peru found elevated concentrations of arsenic, mercury, cadmium, copper, lead, and zinc, surpassing environmental protection limits set by the CCME (Canadian Council of Ministers of the Environment) [2]. These water sources are used for consumption in many regions, and health authorities, including the WHO (World Health Organization), have declared a cadmium concentration of 0.003mg/L as the limit for safe consumption [3,4]. The IARC (International Agency for Research on Cancer) has classified cadmium and its compounds as Group 2A, indicating probable carcinogenicity in humans. Moreover, the WHO states that skin is the primary organ affected by cadmium intoxication [4].
For water treatment, adsorption offers flexibility in design and operation. It is a reversible process, allowing the regeneration of adsorbents through desorption [5]. Ion exchange processes are particularly advantageous due to their high removal capacity, efficiency, and fast kinetics, making them widely used for removing heavy metals via ion exchange [6].
Diatoms are unicellular algae of microscopic size present in all aquatic habitats, and diatomites are siliceous sedimentary rocks composed of fossilized sedimented remains of diatoms [7]. Their inert nature and high surface area make diatomite a potential material for the removal of heavy metals from water [8]. Diatomite is abundant in Peru, in regions such as Tacna, Ayacucho, and Puno, but is not currently used for water treatment in Latin America [9].
Modifications to diatomite through temperature increments have demonstrated changes in the material's porosity [10]. Acid treatment of diatomite has been shown to improve mechanical properties, pore size, and the removal of elements such as iron and aluminum [11]. Modifications using cellulose and sulfuric acid as a carbonized impregnation compound have demonstrated high lead adsorption capacity [12], while the addition of EDTA to diatomaceous earth has achieved high zinc adsorption [13].
Experimental partThe diatomite used in the present study was collected from a deposit in Huamanga, Ayacucho, Peru. The sample was crushed, ground and sieved using a 100μm mesh, then washed with water, filtered and dried at 80°C for three hours prior to analysis.
Characterization of diatomite samplesElemental analysis has been carried out by ICP–MS (Inductively coupled plasma mass spectrometry). The use of calcium carbonate reduces the temperature and the treatment time necessary for the calcination of clays [14]. It has been described that the analysis of minerals rich in silica such as diatomites is complicated, requires considerable time and does not result in a complete extraction of the elements that compose it [15], however the treatment by fusion using sodium carbonate allows the extraction of most elements [16]. Therefore, the sample was fused with calcium carbonate as a fluxing agent and dissolved with 0.5M nitric acid. Analysis was performed using a Perkin Elmer Nexion 300D instrument. FTIR spectrophotometry was performed in the range of 500–4000cm−1 using a Thermo Scientific Nicolet iS10, Attenuated Total Reflectance (ATR) was used. Surface morphology was analyzed with SEM, equipped with energy-dispersive X-ray analysis (EDX), using a Thermo Scientific Axia instrument.
Diatomite modificationsTo evaluate the increase in the cadmium adsorption capacity, five types of surface modifications were carried out on the diatomites.
- a)
Thermal treatment: The thermal treatment of diatomite was carried out in two stages. The first stage consisted of heating the sample to temperatures of 110 and 180°C to study the presence of water on the surface of the diatomite, which would evaporate at 110°C and possible stronger hydrogen bonds which could even withstand temperatures of 180°C. In a second stage, calcination was carried out at 300, 500, 700 and 900°C in order to study the changes that the material undergoes, as well as a possible increase in the cadmium adsorption capacity.
- b)
Acid treatment: After heating to 700°C, the diatomite samples were submerged in sulfuric and hydrochlosimric acid solutions at concentrations of 0.25, 0.5 and 1.0M, for 2h at room temperature, magnetic stirrer Four model MS310 was employed. This treatment aims to generate an ionic exchange between alkaline and alkaline earth elements with protons, which in turn could be replaced by cadmium ions.
- c)
Basic treatment: After heating to 700°C, Diatomite samples were submerged in sodium hydroxide solutions at concentrations of 0.25, 0.5 and 1.0M for two hours at room temperature, magnetic stirrer was employed.
- d)
Cellulose/sulfuric acid: A study using cellulose and sulfuric acid as impregnation compounds and subsequent carbonization showed a high adsorption capacity for lead [12], so in the present study, after heating the diatomite sample to 700°C, cellulose and sulfuric acid were added, the proportions used regarding Cellulose: Diatomite were 8:1, 2:1 and 1:1. The mixtures were homogenized and carbonized with 20mL of concentrated sulfuric acid, for which they were maintained at 120°C for 4h. The mixtures were washed with deionized water several times, until the washing solution was clear, finally the samples were dried at 110°C for 2h.
- e)
EDTA treatment: A modification has been made by adding EDTA to diatomaceous earth, obtaining high zinc adsorption capacities [13], which is why in the present study this modification is applied to cadmium adsorption; after heating to 700°C, diatomite was suspended in EDTA solutions at concentrations of 10−4M, 10−2M, 10−1M, the mixture was stirred for 24h, then it was washed and separated by filtration, the samples were dried at 110°C for 2h.
For the measurement of the adsorption capacity, 0.05g of the natural diatomite, as well as the prepared modifications, were placed in contact with 100mL of cadmium solution at a concentration of 20mg/L. The solution was adjusted to pH 6.0 using HCl and NaOH, and the temperature was maintained at 25°C. Agitation was carried out at 150rpm for 24h to ensure equilibrium was achieved. The solution was filtered, and an aliquot was taken to analyze the final cadmium concentrations. The cadmium analysis was performed using flame atomic absorption, with a Perkin Elmer Analyst 400 instrument. The adsorption capacity of the diatomite (Cads), expressed in mg Cd/g, was calculated as follows:
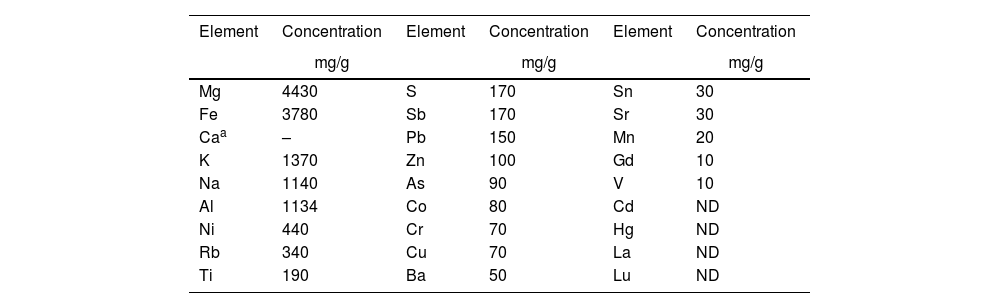
where Co (mg/L) is the initial cadmium concentration, Cf (mg/L) is the final cadmium concentration reached equilibrium, V (L) is the volume of the solution and mads (g) is the mass of diatomite. The tests were performed in triplicate, and the results are reported as the average, ensuring that the relative standard deviation was less than 10%.Results and discussionCharacterization of diatomite samplesElemental analysis results performed by ICP–MS are presented in Table 1, showing that magnesium, iron, calcium, potassium, sodium, aluminum, are the most abundant elements with concentrations in the range of 1100–4430ppm; A second group of elements in abundance are comprised of Ni, Rb, Ti, S, Sb, Pb, Zn with concentrations of 100–440ppm.
Elemental composition of the natural diatomite sample made by ICP-MS.
| Element | Concentration | Element | Concentration | Element | Concentration |
|---|---|---|---|---|---|
| mg/g | mg/g | mg/g | |||
| Mg | 4430 | S | 170 | Sn | 30 |
| Fe | 3780 | Sb | 170 | Sr | 30 |
| Caa | – | Pb | 150 | Mn | 20 |
| K | 1370 | Zn | 100 | Gd | 10 |
| Na | 1140 | As | 90 | V | 10 |
| Al | 1134 | Co | 80 | Cd | ND |
| Ni | 440 | Cr | 70 | Hg | ND |
| Rb | 340 | Cu | 70 | La | ND |
| Ti | 190 | Ba | 50 | Lu | ND |
The elemental composition of the diatomite samples aligns with the backgrounds reported in the literature, showing the presence of alkaline and alkaline-earth minerals in its composition [17].
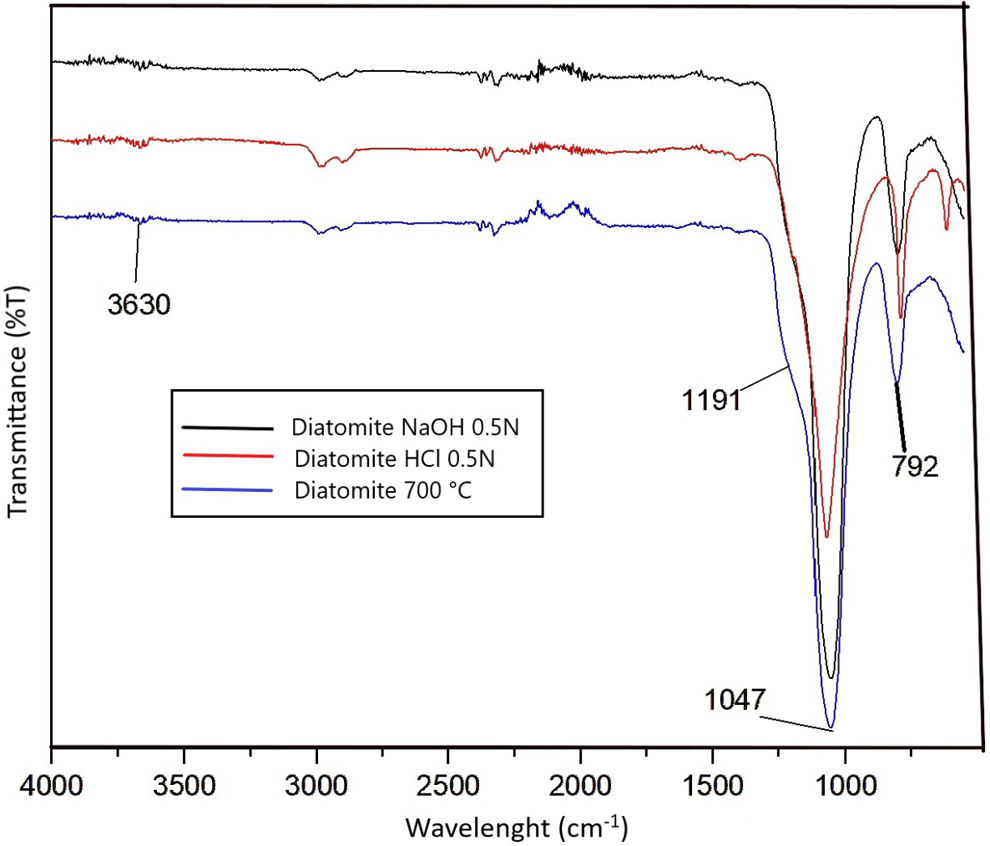
Fig. 1 shows the FTIR spectra of the natural diatomite sample, where bands at 1100cm−1 are clearly observed, with an associated shoulder at 1250cm−1 corresponding to the asymmetric bending movement of the SiOSi bond, and a band at 795cm−1 corresponding to the symmetrical bending of the same group. The broad band located at 3450cm−1 corresponds to the stretching vibrations of absorbed water, which imparts a hydrophilic behavior [18–20]. A peak is observed at 3650cm−1 (Fig. 1), corresponding to hydroxyl-bound silicon groups (Silanol groups), which can form hydrogen bridge bonds, a characteristic of amorphous siliceous materials such as diatomites [21].
Fig. 2 shows the SEM image of the natural diatomite at a magnification of 5000×, where the agglomeration of diatom units, 10–20μm in size, is appreciated vertical and a short transverse axis, corresponding to diatomites of the pennate type [14]. The EDX elemental analysis of natural diatomite (Table 3) indicates a composition of oxygen, silicon and aluminum 54.6%, 44.1% and 1.3% respectively.
Diatomite modificationHeat treatmentThe diatomite samples subjected to thermal treatment showed a color change, with the reddish hue intensifying as the treatment temperature increased (Fig. 3). This effect is likely due to the oxidation of ferrous ions to ferric ions, giving the sample a reddish tint. A noticeable reduction in the broad band located at 3450cm−1 is observed as the sample is heated to 110°C and more prominently at 180°C, which indicates the elimination of water molecules adsorbed on the sample surface. This reduction is also reflected in the peak at 1630cm−1, attributed to the stretching of the OH bonds in water [10]. The band at 3650cm−1 corresponds to an OH bond with a stronger attraction force due to its resistance to removal at high temperatures, likely associated with kaolinite (Fig. 1) [10].
The SEM image of the diatomite subjected to thermal modification at 700°C shows the diatom units with their initial oval shape (Fig. 4), which demonstrates that at this temperature the structure is still maintained with respect to the original sample.
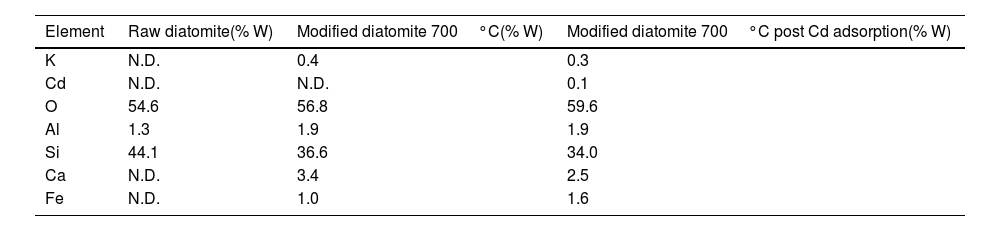
Table 3 presents the results of the surface elemental composition of the diatomite before and after being subjected to the thermal treatment at 700°C, the test was performed with the technique of Energy-dispersive X-ray spectroscopy (EDX), this technique does not require the dissolution of the sample and indicates the surface composition of the material, the explanation for the fact that diatomite after being subjected to 700°C has higher amounts of Ca and Fe than the starting material, would indicate that these elements were part of the internal structure of the diatomite and have been exposed on the surface of the diatomite after calcination and volatilization of various chemical species on the surface that did not allow the detection of these elements on the surface, additionally a significant increase in aluminum content is observed, possibly due to the same effect.
Acid and basic modificationThe FTIR spectrum shows that the peaks remain unchanged compared to the starting diatomite, indicating that the structure is not altered (Fig. 5).
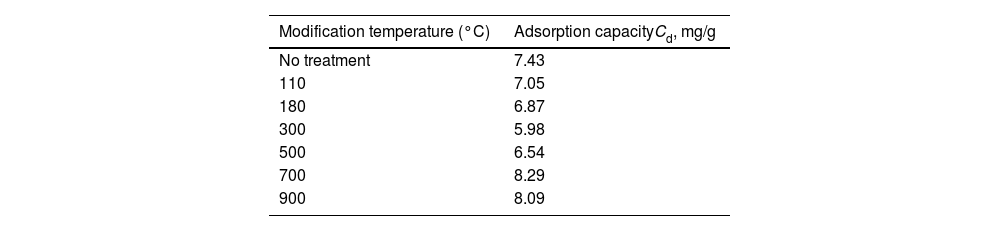
Adsorption tests of heavy metals in diatomitesCadmium adsorption on natural and thermally modified diatomiteTable 2 shows the results of cadmium adsorption capacity (Cads) for diatomite samples, with significantly higher adsorption observed in the modifications performed at 700°C and 900°C compared to the unmodified diatomite. This increase can be explained by the enhancement of active cation exchange sites and the volatilization of residues within the material's pores, which increases the material's surface area. Fig. 6 shows an EDX elemental mapping of the diatomite sample treated at 700°C after cadmium adsorption, indicating a uniform distribution of cadmium on the diatomite surface. Additionally, a uniform distribution of calcium and potassium ions was observed, suggesting that the material retains available ion exchange capacity.
The EDX cuantitative analysis results for the thermally treated diatomite sample after cadmium adsorption are shown in Table 3. A cadmium concentration of 0.1% was detected, which was not present in the starting diatomite nor in its thermally modified form. A significant reduction in potassium and calcium content, by 25% and 26%, respectively, was also observed compared to the diatomite before adsorption. This reduction suggests ion exchange between cadmium ions and potassium and calcium atoms.
EDX elemental analysis of raw diatomite, thermally treated diatomite at 700°C and after cadmium adsorption.
| Element | Raw diatomite(% W) | Modified diatomite 700°C(% W) | Modified diatomite 700°C post Cd adsorption(% W) |
|---|---|---|---|
| K | N.D. | 0.4 | 0.3 |
| Cd | N.D. | N.D. | 0.1 |
| O | 54.6 | 56.8 | 59.6 |
| Al | 1.3 | 1.9 | 1.9 |
| Si | 44.1 | 36.6 | 34.0 |
| Ca | N.D. | 3.4 | 2.5 |
| Fe | N.D. | 1.0 | 1.6 |
Reka et al. (2021), studied the thermal properties of diatomite and identified three phases during calcination: the first phase involved the loss of weakly adsorbed water, the second involved the calcination of organic matter, and the third phase involved the degradation of hydroxyl groups on the diatomite surface [22]. In the present study, natural diatomite showed some cadmium adsorption capacity, and thermal treatment increased this capacity. These findings are consistent with the study by Ediz (2010), which examined calcination parameters in diatomites and improved filtration properties [23].
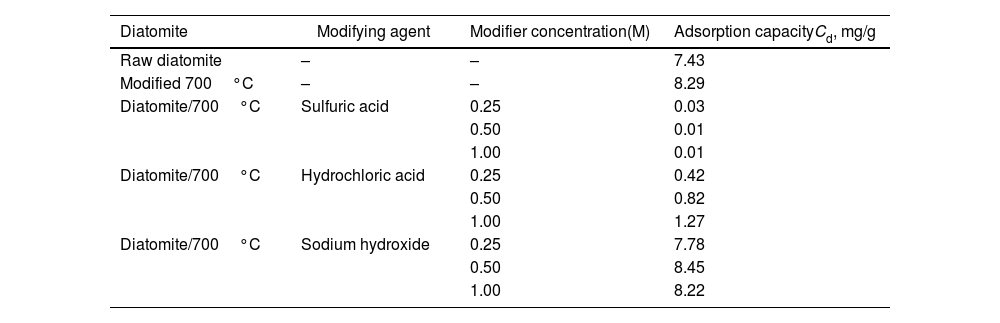
Cadmium adsorption on diatomite treated with acids and basesTable 4 shows the results of cadmium adsorption on diatomite samples modified with acid and base treatments. Although it has been reported that acid treatment of diatomite improves mechanical properties, increases pore size, and enhances the removal of metallic elements such as iron and aluminum [11], in this study, acid treatment drastically reduced cadmium adsorption capacity. For sulfuric acid treatment, the inactivation was almost complete. This effect is mainly due to the increase in positive charges on the diatomite surface, caused by protons, and a decrease in the material's ion exchange capacity. The hydrochloric acid modification also resulted in reduced adsorption, but it was still much lower than that of the unmodified diatomite. This result is concordant with others stdudies, Chang et al. (2020) [24], found a low adsorption capacity of diatomite at pH 5 to cadmium spite of negative charge at this pH. In another study the modification was opposite to this study, the acid modification was followed by thermal modification, the adsoption capacity was increassed. According the authors the acid modification removes differents oxides which could be interferences CaO, MgO, Na2O, FeO, Fe3O2, Al2O3 and etc. (Nurgain, A., 2020) [25], this effect could be explained because the acid treatment was done after thermal treatment, and it volatilized all groups would reaction and generate groups adsorbents to cadmium.
Adsorption capacity of diatomites modified with acid-basic treatment.
| Diatomite | Modifying agent | Modifier concentration(M) | Adsorption capacityCd, mg/g |
|---|---|---|---|
| Raw diatomite | – | – | 7.43 |
| Modified 700°C | – | – | 8.29 |
| Diatomite/700°C | Sulfuric acid | 0.25 | 0.03 |
| 0.50 | 0.01 | ||
| 1.00 | 0.01 | ||
| Diatomite/700°C | Hydrochloric acid | 0.25 | 0.42 |
| 0.50 | 0.82 | ||
| 1.00 | 1.27 | ||
| Diatomite/700°C | Sodium hydroxide | 0.25 | 7.78 |
| 0.50 | 8.45 | ||
| 1.00 | 8.22 |
On the contrary, the basic treatment significantly increased cadmium adsorption (Cads=8.45mg/g) for NaOH 0.5N, compared to the raw diatomite (Cads=7.43mg/g) and thermal modified diatomite (Cads=8.29mg/g), likely due to the increased negative charge on the material's surface caused by hydroxyl groups, enhancing the electrostatic attraction of cadmium.
Cadmium adsorption on diatomite treated with cellulose and sulfuric acidDespite the findings reported by Dobor et al. (2015) [12], who carried out a modification with cellulose and sulfuric acid to achieve a high adsorption capacity for lead, in the present study, this modification did not produce any significant increase in cadmium adsorption, according to authors, the type of organic material to produce activated carbon plays an important role to obtain a good adsorbent, furthermore, this could be the reason why a significant increase in adsorption capacity was not achieved. Yu Bian, (2015) [26], studied the adsorption of cadmium on activated carbon treated with nitric acid and concluded that the adsorption occurs mainly by two mechanisms, one by electrostatic attraction exerted by functional groups such as lactones, phenols and organic acids, and the second by cation exchange, which is why other tests are required considering the formation of these groups and the ion exchange capacity in order to increase the cadmium adsorption capacity.
Modification of EDTA-treated diatomiteDespite the results reported by Sosa et al. (2019) [13], who found good zinc adsorption capacities using diatomite modified with EDTA, in the present study, this modification did not result in a significant increase in cadmium adsorption compared to natural diatomite and thermal treated diatomite.
Unlike Sosa et al. (2019) [13], this procedure used diatomite previously calcined at 700°C to then apply EDTA treatment, calcination could cause EDTA adhesion to be much lower than EDTA adhesion when using uncalcined diatomite, therefore, cadmium adsorption capacity with EDTA-treated diatomite could be drastically affected.
ConclusionsThe diatomite collected from Huamanga, Ayacucho, Peru, is of the pennate type and contains kaolinite contamination. Alkaline and alkaline-earth elements are dispersed on its surface, facilitating ion exchange with cadmium. Adsorbed water was found on the surface, which was eliminated at temperatures below 200°C, although hydroxyl groups, strongly associated with hydroxyl-bound of silanol groups.
Thermal modification caused a change of color with to red, which intensity is proportional to the calcinated temperature and indicate the oxidation of ferrous to ferric ion present in the diatomite. The treatment temperature of 700°C generated a higher adsorption capacity compared to the starting material. This treatment also exposed elements like aluminum and iron on the surface, who was covered by interferents and were volatilized after thermal treatment. Chemical modification with sulfuric and hydrochloric acids significantly reduced cadmium adsorption capacity compared to the starting diatomite, this effect could be explained because the acid treatment was done after thermal treatment, and it volatilized all groups would reaction and generate groups adsorbents to cadmium.
The sodium hydroxide modification increased cadmium adsorption capacity, it could increasse the negative charge because hydroxyl groups. EDX elemental mapping showed that cadmium was uniformly adsorbed on the diatomite surface, with no morphological changes observed compared to the starting material. The incorporation of EDTA, as well as the modification with cellulose/sulfuric acid, did not increase cadmium adsorption capacity.
Conflict of interestsThe authors declare no conflict of interest.
This research was supported by the Universidad Nacional Mayor de San Marcos – RR N° 006081-2023-R/UNMSM and project number C23072031.