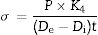This study has focused on the development of porous materials from representative Tunisian clay. The first step was the characterization of the clay through powder X-ray diffraction (XRD), thermal analysis and evaluation of its plasticity. With this data, an extrusion paste was prepared, mixing the clay with 26% water. This paste was extruded in the form of a tube and after a study of its thermal behavior; it was sintered at 850°C for 2h. The behavior of the prepared membranes was evaluated by measuring their porosity, permeability and mechanical strength mainly. The membranes thus obtained showed an acceptable porosity of about 36%, with a pore diameter of about 30nm and mechanical strength of 12MPa.
Este estudio se ha centrado en el desarrollo de materiales porosos a partir de arcillas representativas de Túnez. El primer paso consistió en la caracterización de la arcilla mediante difracción de rayos X (DRX), el análisis térmico y la evaluación de su plasticidad. Con estos datos, se preparó una pasta de extrusión, mezclando la arcilla con 26% de agua. Esta pasta se extruyó en forma de tubo y, tras un estudio de su comportamiento térmico, se consolidó a 850° C durante 2hrs. El comportamiento de las membranas preparadas se evaluó midiendo su porosidad, permeabilidad y resistencia mecánica fundamentalmente. Las membranas obtenidas mediante este procedimiento mostraron una porosidad aceptable, de aproximadamente el 36%, con un diámetro de poro de aproximadamente 30nm y una resistencia mecánica de 12MPa.
Considerable attention has been paid to the use of clay for the manufacture of ceramic membranes in the context of natural resources exploitation and local economy improvement [1–4].
Current research on clay focusing on ceramic materials has identified its diverse characteristics and fundamental properties for industrial processing (suitable for slip casting and easy shaping, as well as good mechanical strength) [5]. Tunisia is highly rich in clay deposits [6]. This abundant raw material requires low sintering temperatures, lower than that required for pure oxide materials.
Ceramic materials have very interesting characteristics that make them interesting for their use as filters [7,8], as their mechanical and thermal resistance and chemical stability.
Commercial membranes are indeed available in the market. Nevertheless, from a technical and economic point of view, ceramic membranes are still expensive for some applications, mainly due to the use of expensive materials such as alumina [9–12], zirconia [13,14], alumina-titania or mullite [15] and the required processing.
The objective of this work was to study clays from the quarry of Sidi El Bader, in the northwest of Tunisia, and the possibility of obtaining ceramic filters with it. The choice of this material is argued by its natural abundance, low cost, high plasticity and thermal behavior for the manufacture of ceramic membranes.
ExperimentalRaw materialThe quarry of Sidi El Bader is located in Tabarka City (northern Tunisia). Due to their abundance, the clays of Sidi El Bader are used in the field of ceramics. For this reason, they were selected as raw material in this study.
The mineralogical analyses were carried out by X-ray diffraction technique (XRD), using Philips X’Pert equipment with a Cu Kα radiation. The relative phase amounts were estimated by measuring the areas of the main diffraction peak using the Panalytical X’Pert Highscore software. Oriented aggregates were treated with ethylene glycol for 1h, solvated to detect expandable minerals, and heated at 500°C for 2h to differentiate chlorite from kaolinite. The chemical composition of powdered samples was determined by X-ray fluorescence, using a Panalytical Axios Dispersive XRF Spectrometer according to the conventional techniques. The particle size distribution of the as-received samples were obtained by laser scattering in aqueous suspension (Mastersizer S. Malvern, England). This study is important to know the particle size distribution and to estimate “a priori” the pore size in the membrane. Samples were dispersed using a standard surfactant (Dolapix CE 64. Zschimmerz-Swartz) and an ultrasonic bath treatment during 5min.
The Casagrande method, using the Spanish standard UNE 103-103-94, was selected for the determination of the Atterberg limits with an experimental error of ±3%.
The thermal analyses TDA–TGA were performed using SETSYS EVOLUTION (Setaram, France) equipment using a heating rate of 10°C/min and by using a-Al2O3 as inert reference.
The textural study and measurement of the specific surface area were carried out with a study of the N2 adsorption–desorption isotherm and application of the BET model. The equipment used was an Autosorb1 (Quantachrom, USA). The microstructure of the samples was studied through scanning electron microscopy mod. HP1 (Carl Zeiss, Germany).
Membranes preparation and characterizationThe preparation and characterization of ceramic membranes are the objective of the present paper. The paste for extrusion was prepared using 26wt% of water with the clay sample (Determined from Atterberg limits). In order to ensure good water distribution in the sample, the mixture was kept 12h in a closed plastic bag.
The ceramic membranes were prepared through extrusion to obtain tubular shaped products. A home made piston extruder (ICV – CSIC) was used. It consists of a pneumatic piston made up of a cylinder (Pneumax, Italy) with a piston diameter of 50mm and a stroke of 200mm. This cylinder was fed with compressed air pressure at 6kg/cm2. The membrane was sintered in a high temperature furnace from Agni GmbH (Germany). Sintering was carried out at different maximum temperatures using 2h as holding time and 5°C/min as heating and cooling rates. Mono-channels were prepared by extrusion. The dimensions of the membrane samples were the following: outer diameter=8mm; inner diameter=4mm.
Sintering behavior was studied up to 1500°C, through optical dilatometry using an EM301 Heating Microscope from Hesse Instruments (Germany), using 10°C/min as heating rate.
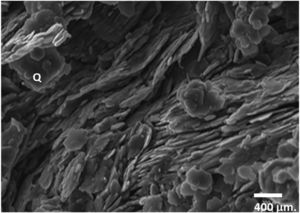
Porosity was evaluated using a mercury porosimeter Autopore II 9215 (Micromeritics, USA). The mechanical strength was evaluated through diametral compression tests. The samples used had a length of about 8mm. A universal testing machine with a cell of 5000N (Instron, UK) was used for this purpose. The equation describing the mechanical strength is as follows [16]:
where P is the fracture load, Di the internal diameter, De the external diameter given in mm, and t is the support length given in mm. K4 is a constant that depends upon the Di/De[17] relationship.The permeability of samples were tested using N2 obtained as permeate; the equipment consisted of a module in which the membrane (membrane module) is inserted [18]. This module was divided into two chambers, one of high pressure (P↑) and another of low pressure (P↓), both separated by a layer, or membrane to be characterized. Gas, introduced into the high-pressure chamber, tends to pass to the low-pressure chamber through the membrane. To introduce gas into the membrane module, two lines were set up: one to the high-pressure chamber (L1) and another to the low-pressure chamber (L2). Measurements were made under an atmospheric pressure up to 25bar. Flows ranged from 1 to 10l/min.
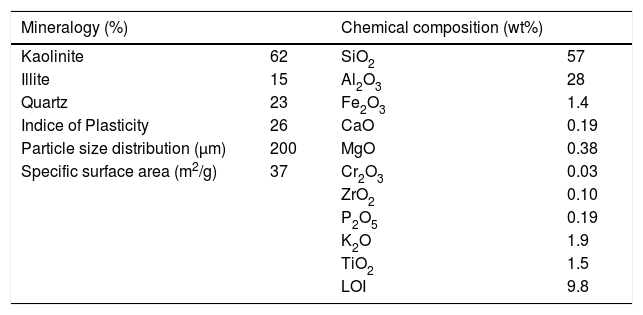
Results and discussionClay characterizationThe chemical analysis of the clay (Table 1) shows the dominance of SiO2 and Al2O3 (57 and 28wt%, respectively). The loss of ignition (LOI) is 9.8% attributed to the loss of H2O from clay minerals on the one hand, and to decarbonation on the other hand. The presence of other oxides such as Fe2O3, K2O, and TiO2 is also observed (under 1wt%); their amounts are relatively low and can be considered as acceptable in the elaboration of ceramic membranes. The studied sample has a high SiO2 content due to the presence of quartz, as shown by the diffraction peak (Fig. 1).
Mineralogical and chemical composition of clay. Some physicochemical characteristics.
| Mineralogy (%) | Chemical composition (wt%) | ||
|---|---|---|---|
| Kaolinite | 62 | SiO2 | 57 |
| Illite | 15 | Al2O3 | 28 |
| Quartz | 23 | Fe2O3 | 1.4 |
| Indice of Plasticity | 26 | CaO | 0.19 |
| Particle size distribution (μm) | 200 | MgO | 0.38 |
| Specific surface area (m2/g) | 37 | Cr2O3 | 0.03 |
| ZrO2 | 0.10 | ||
| P2O5 | 0.19 | ||
| K2O | 1.9 | ||
| TiO2 | 1.5 | ||
| LOI | 9.8 |
TiO2 and K2O amounts are related to anatase and illite contents, respectively. Fe2O3 proportion can be explained by isomorphic substitutions in the clay mineral structures (illite) or low iron oxide contents undetected in the diffraction peak.
The studied clay was composed mainly of kaolinite (62%), with minor amounts of illite (15%). The associate mineral is essentially quartz (23%). Indeed, an important quartz fraction (Table 1) was detected due to the regressive sea-level period (abundance of sandstone intercalations). This is in concordance with the high amount of SiO2 reported by the chemical analysis. Moreover, observation of the diffraction peak of the oriented sheets natural clay sample reveals the existence of quartz as the dominant impurity with refraction d101 at 3.34Å [19]. Kaolinite, characterized by refraction d001=7.14Å, does not change with ethylene glycol and disappears when heated to 500°C, while Illite always remains present under the effect of ethylene glycol or under the heating effect (Fig. 2).
The specific surface area SSA is 37m2/g calculated by the BET model from nitrogen adsorption measurement. According to mineralogical analysis, this value can be considered as part of both main components: specific surface values: Kaolinite (10–30) and Illite (100–140) as described in the literature [6].
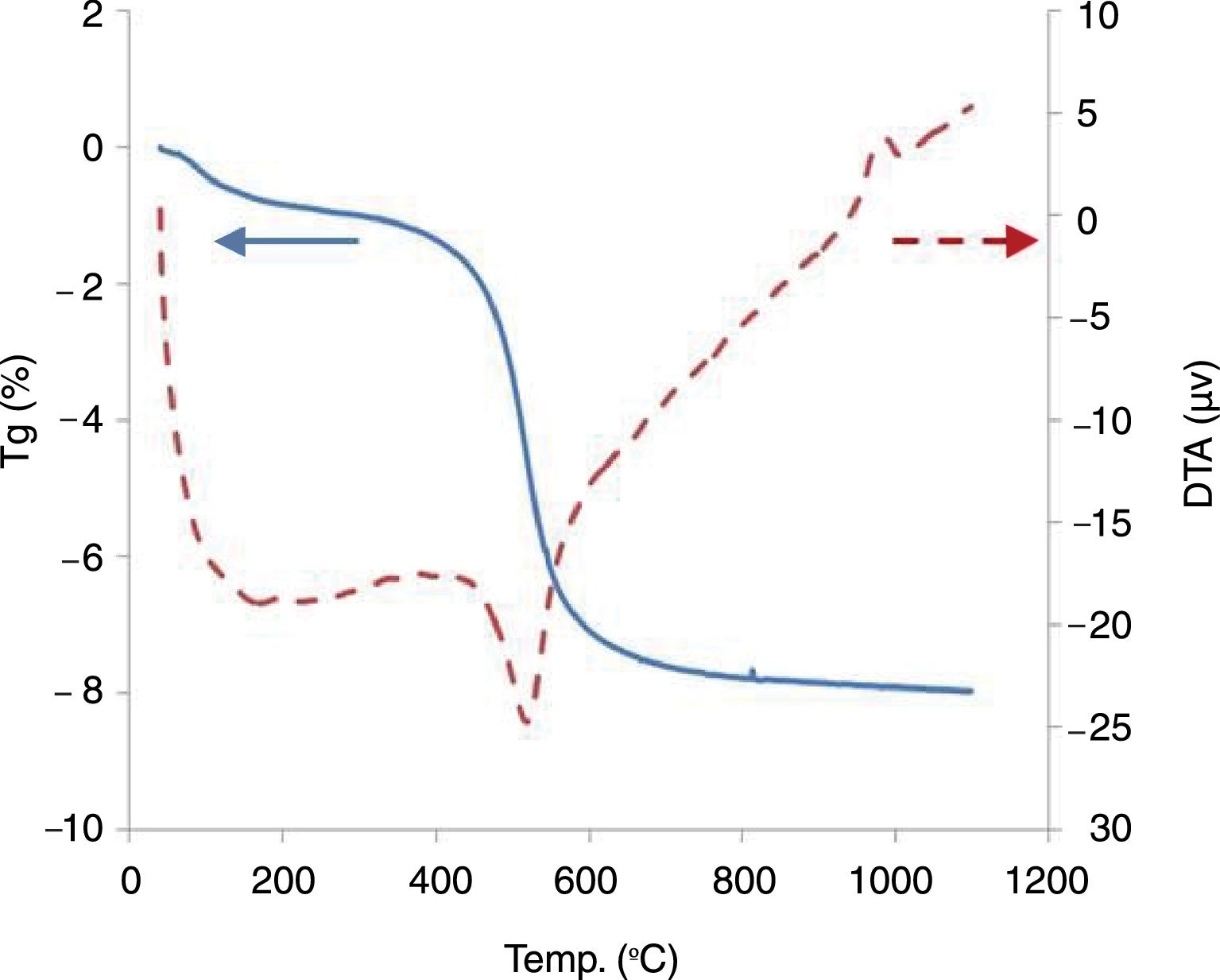
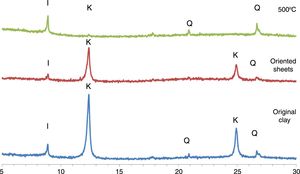
In the TDA–TGA experiments, two endothermic peaks were detected (Fig. 2). The first thermal effect, at 120°C, with loss of mass around 1.2% corresponding to the disappearance of hydratation water from the interlayer space. At 550°C, a second endothermic peak depicted the elimination of constitution water (dehydroxylation of kaolinite) [20].
At this stage, the mass loss was between 7 and 9%, which is in concordance with the LOI shown in Table 1.
A small exothermic effect without any weight change, observed around 950°C, is attributed to the beginning of mullite formation. The carbonates decomposition products react with the other existing oxides to form the new crystalline phases assigned to the reformation of mullite, anorthite, and the disappearance of metakaolin according to the mineralogical and chemical composition of the original mixture.
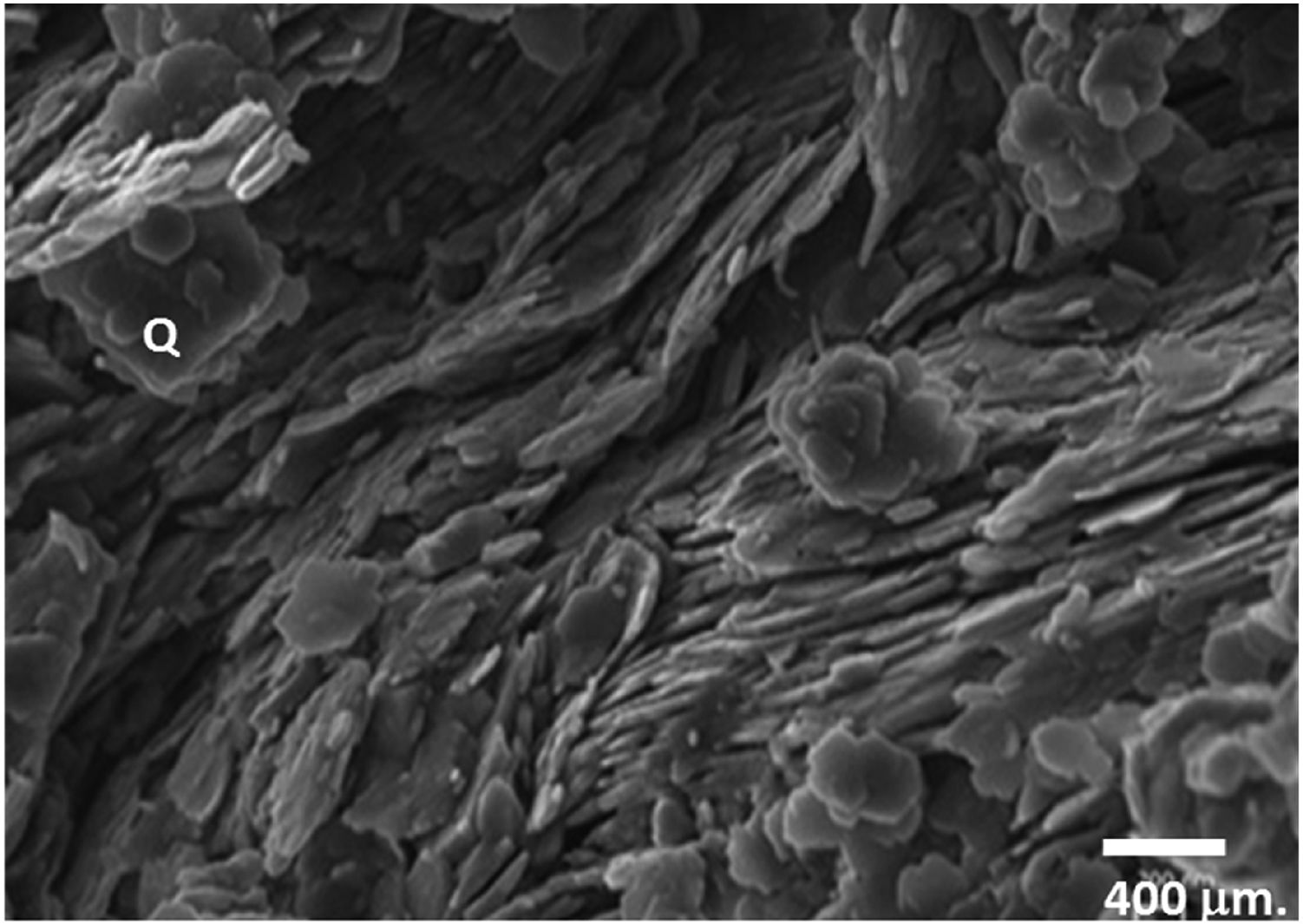
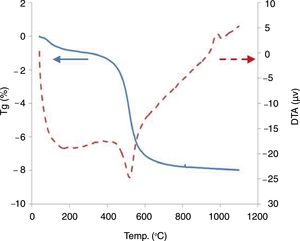
The observation of clay under a scanning electron microscope (Fig. 3) identified the presence of quartz and considerable amounts of kaolinite, thus showing pseudo hexagonal platelets with a thickness of a few micrometers.
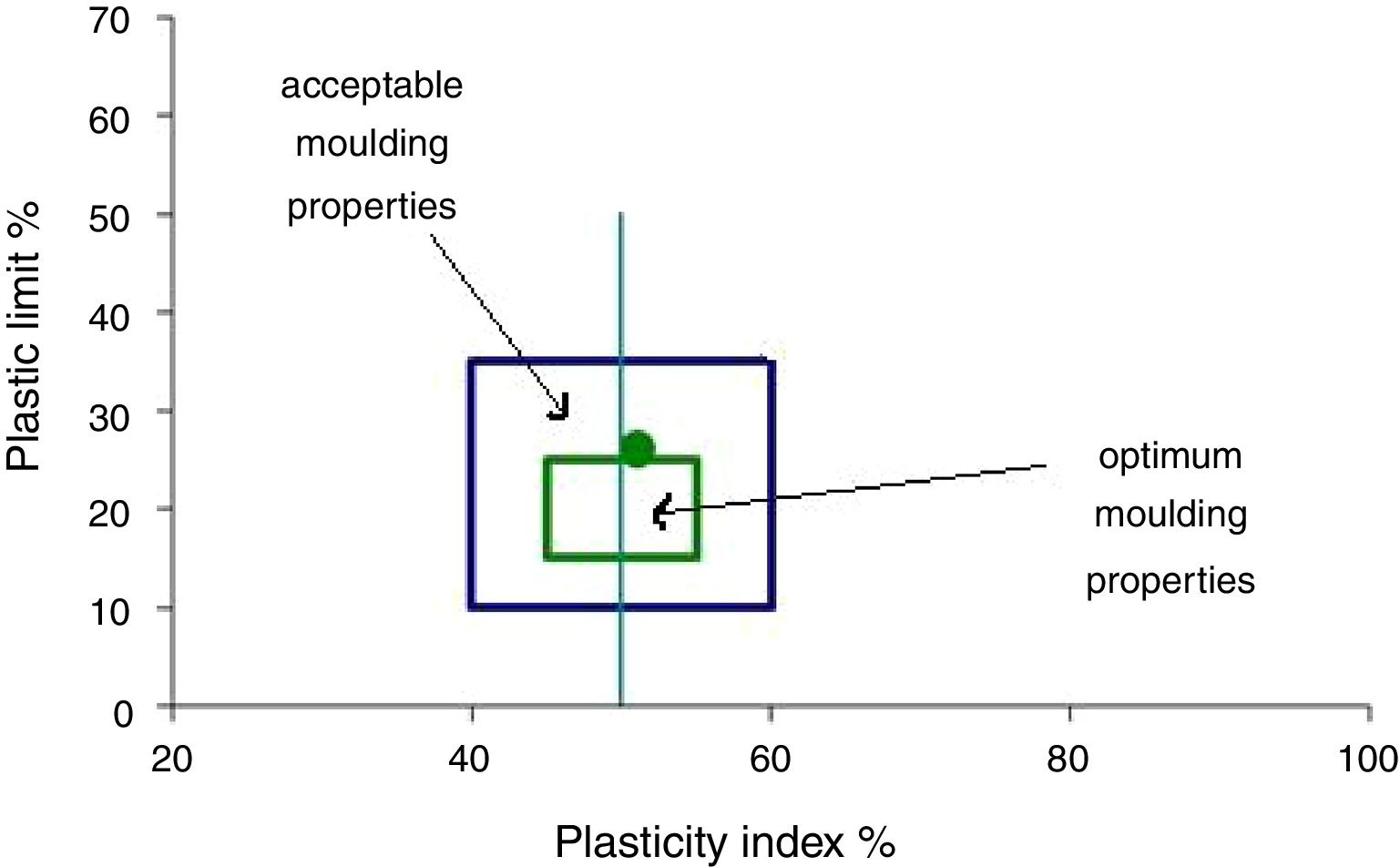
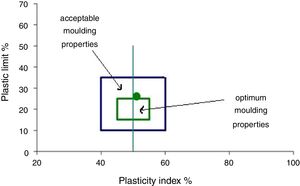
The mineralogical analyses are reasonably consistent with plasticity data according to the clay workability chart (Fig. 4). This method determines the quantity of water needed in the ceramic body to achieve a reliable extrusion process [21]. According to the Atterberg plasticity index around 26, the clay is in the category of Illitic–Kaolinitic clays and could be considered as a highly plastic behavior. For this kind of clay, plasticizer addition is not considered.
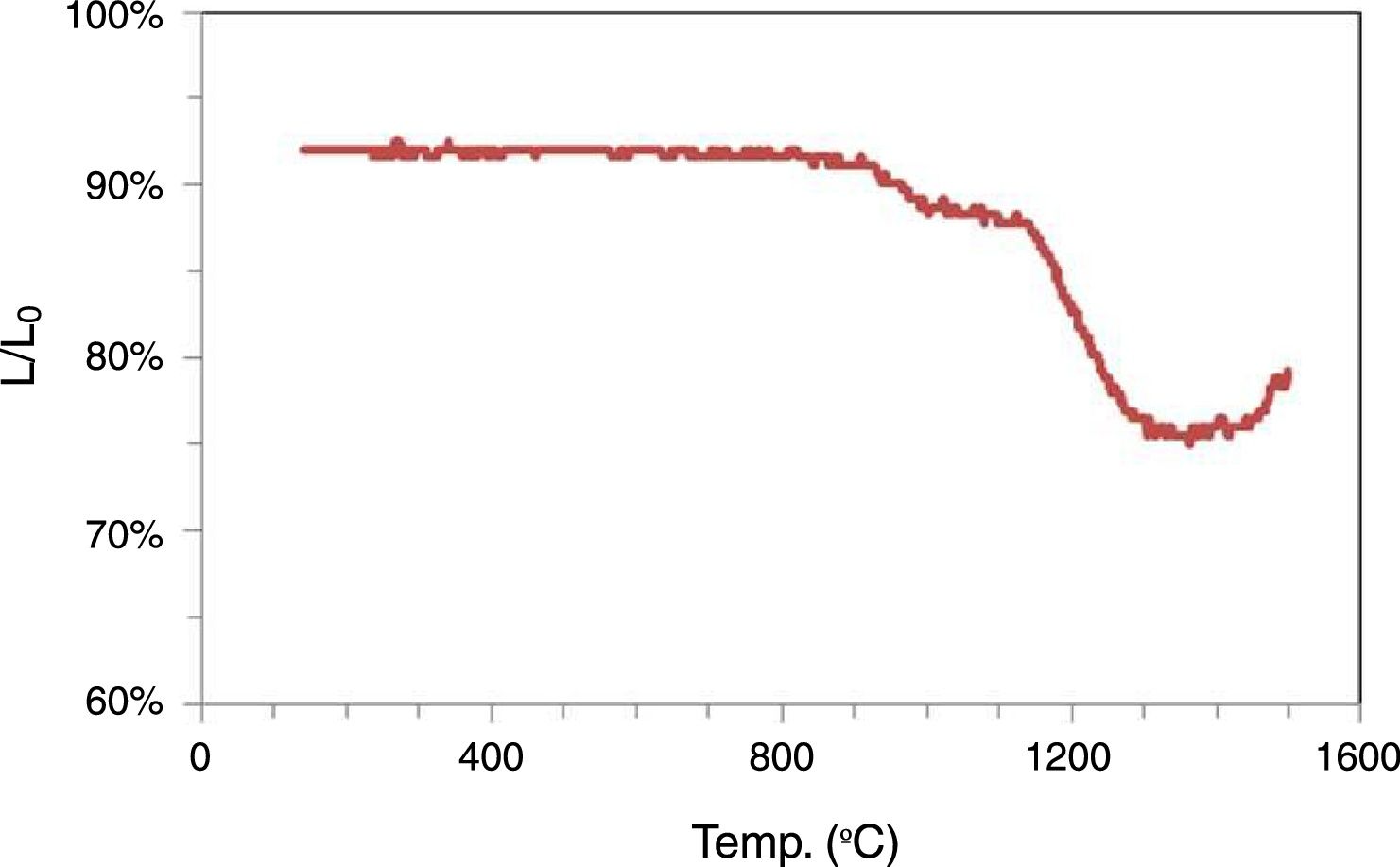
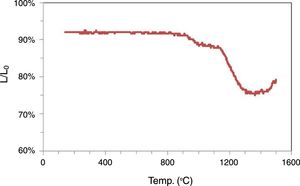
Membrane elaboration and characterizationThe optical dilatometry (Fig. 5) shows that shrinkage begins at 800°C reaching a maximum shrinkage of 14% approximately at 1300°C. After this temperature an expansion begins due to the expansion of gas in the closed pores. Pores are closed due to the increase of the amount of glassy phase. The shrinkage is a complex process with at least two effects. The first step is between 800°C and 1000°C, and the second above this temperature. The discontinuity between these two steps should be explained due to the beginning of Kaolinite decomposition and formation of mullite and silica. This reaction is expansive. Considering this behavior sintering temperatures between 700°C and 900°C were selected.
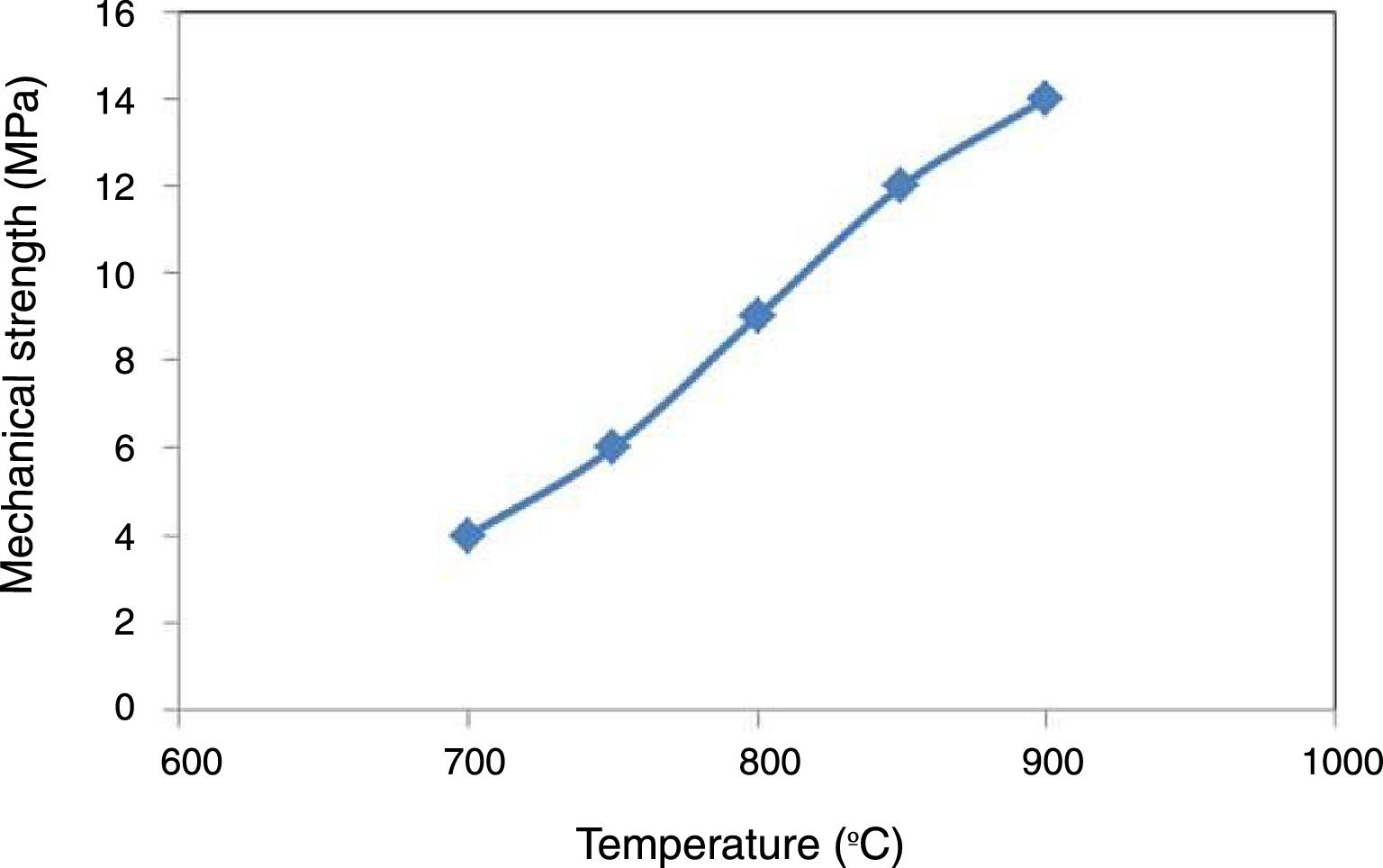
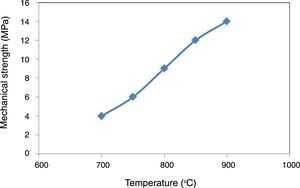
To understand the effect of sintering temperature on the ceramic membrane and its performance, the mechanical strength evolution of the shaped pipes was measured. The results are shown in Fig. 6. As expected, the higher the sintering temperature, the greater the mechanical strength. Mechanical strength at 900°C is more than 50% higher than at 800°C. This can be explained by the consolidation of clay material and the appearance of the first liquid coming from the decomposition of kaolinite and formation of mullite. These values are similar to the obtained by Ben Ali [22] and values collected in the review of Mestre et al. [23] for membranes kaolin based.
The sintering temperature is an important factor affecting the strength of the ceramic membrane, and the samples density should correspond to a parallel increase in the mechanical strength as shown in Table 2. It is well known [24,25] that porosity affects mechanical strength negatively.
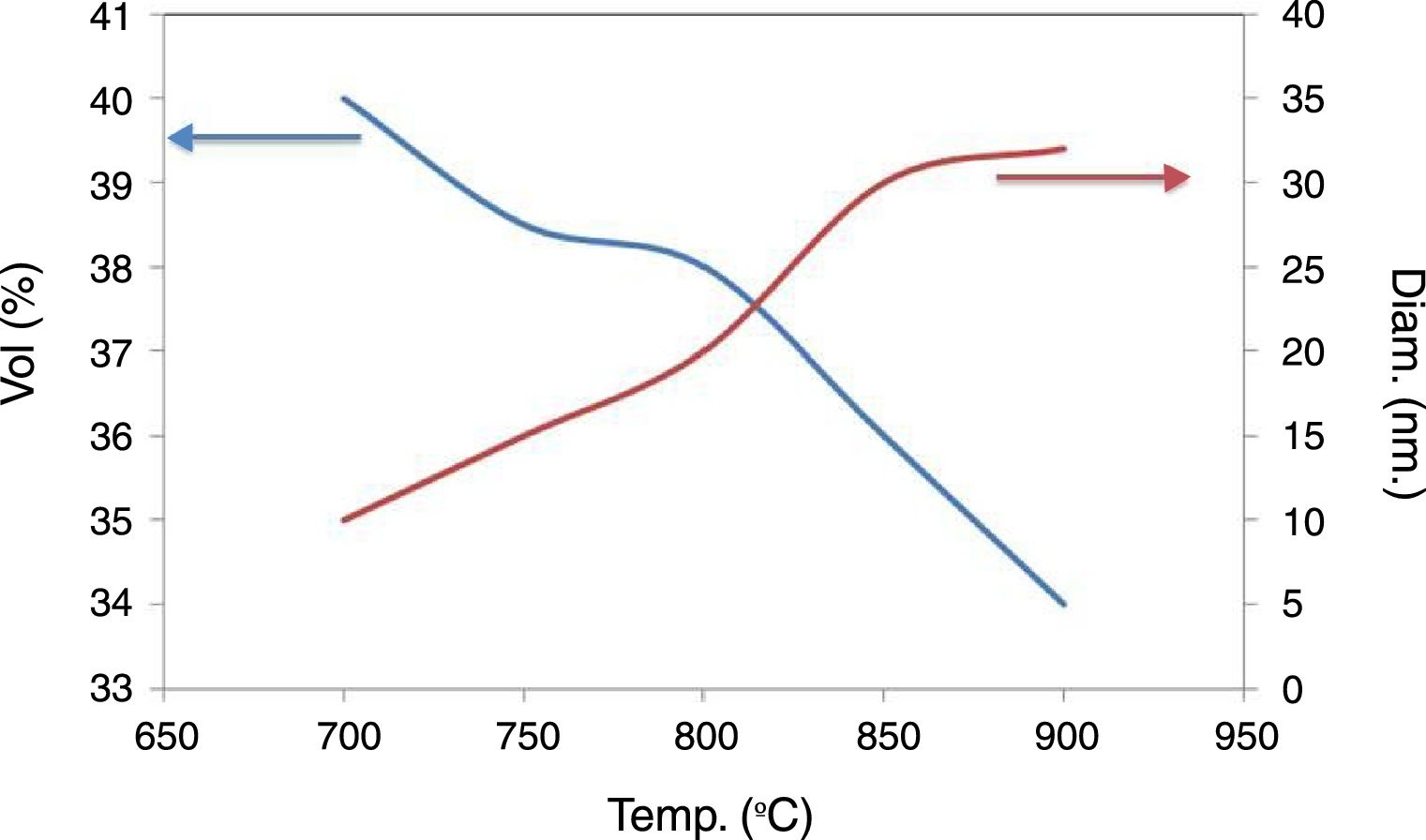
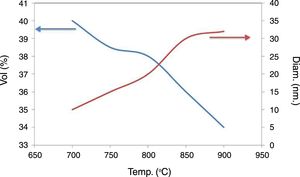
An increase in sintering temperature (Fig. 7) is accompanied with an increase pores diameter and a decrease in pores volume. It can be observed that porosity is in the range of tens of nanometers. This is due to the fact that during processing, the clays are exfoliated and organized, and the pores size approximates that of the sheets that make up the agglomerates of the clay.
The sintering temperatures of 700, 750 and 800°C lead to materials with a high pore volume and small diameter. However, their mechanical resistance is too low even at 800°C. The temperature of 850°C was chosen because the material has a larger pore volume than when it is treated at 900°C. It has 36% open porosity, measured by Hg porosimetry, with a pore size distribution centered on 30nm.
These level and sizes of porosities are similar to the obtained by Ben Ali [22]. It is noticeable that these volumes of porosity also are similar to the reported by Mestre et al. [23] but, in the present study without the use of porogen agents.
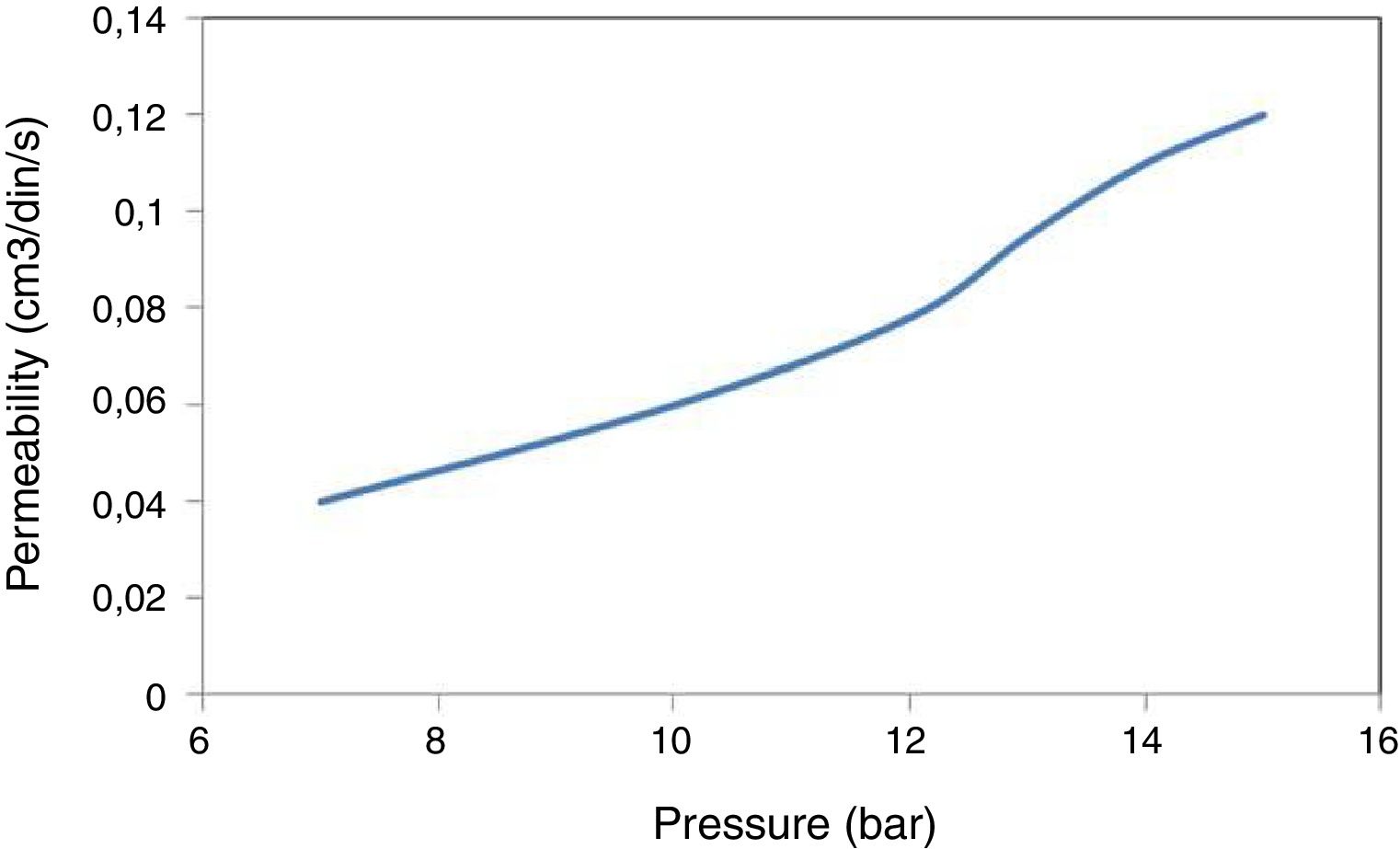
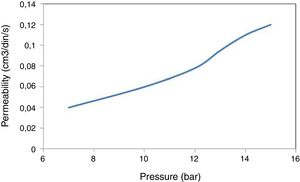
PermeabilityThe results of the gas permeability measurements are shown in Fig. 8. It can be observed that permeability depends upon the gas pressure. It can be seen that the variation of the flow as the pressure increases is quite small, typical of the low permeability of the membrane. It should be noted that at the highest pressures (15bar) the membranes cause a huge loss of pressure (approximately 9bar) between the two faces of the membrane. From these results it can be concluded that, although the total volume of porosity is sufficient, the small size of the pores gives rise to low gas permeability. Therefore, in case of trying to use these clays to obtain membranes, it would be necessary to design asymmetric systems, with a support with larger porosity and on depositing the membrane in small thicknesses and in this way improving the permeability.
ConclusionsThe current work has studied the possibility of producing ceramic membranes with tubular shape, by using natural clay from Tabarka (Tunisia). Based on the findings reported in this paper, we can highlight some conclusions:
- -
Mineralogical and X-ray diffraction analysis revealed a significant predominance of quartz and kaolinite in the clay.
- -
The behavior of the clay exhibits the characteristics of a plastic material, so it does not need to use plasticizers to improve this characteristic in pastes for extrusion.
- -
The single-channel membranes, obtained by extrusion, have been sintered at 850°C for 2h as the optimal thermal cycle.
- -
These materials have presented a mesoporous membrane character, with a pore size of 30nm and 36vol% of porosity with a mechanical strength of 12MPa.
- -
Its low permeability would advise an asymmetric system, with a support to which a porogenic additive would be added and subsequently the membrane itself would be incorporated on the surface.
This work was partially supported by the Ministry of Higher Education and Scientific Research of Tunisia and the Ministry of Economy and Competitiveness of Spain (MAT2013-48426-C2-1R).